ABSTRACT
This article reviews the evidence for theoretical models postulating that early adversity can set in motion a deleterious developmental cascade, involving changes in hypothalamic-pituitary-adrenal (HPA) axis activity that subsequently affect the development of self-regulation in early childhood. Focusing on the first five years of life, we describe studies showing that experiences of severe deprivation or maltreatment may both lead to a hypoactive HPA axis in children. However, it is too early yet to conclude what effects exposure to adverse conditions such as poverty or family instability have on young children’s HPA axis, with both low and high levels of cortisol having been observed under these circumstances. Both patterns of HPA axis dysregulation have been associated with impairments in executive functions, which are important for self-regulation. There is promising evidence that interventions targeting parenting behaviour have the potential to remediate adversity-related biological and behavioural alterations.
Considerable research has related early adverse experiences to problematic socio-emotional development, placing children at risk of developing behavioural, physical, and mental health problems (Appleyard et al., Citation2005; Kessler et al., Citation2010). In search of processes underlying this association, it has been suggested that alterations in neurobiological systems and impairments in self-regulation act as mechanisms through which adversity increases the risk for pathology (McCrory et al., Citation2010; Moffitt et al., Citation2011). While extensive knowledge about neurobiological and behavioural consequences of childhood adversity stems from investigations with school-aged children, adolescents, and adults, less is known about the impact of adversity in early childhood. However, in-depth knowledge on early developmental pathways in the face of adversity is needed to inform intervention efforts. Focusing on the first five years of life, we will review evidence for theoretical models postulating that early adversity can set in motion a deleterious developmental cascade, involving changes in hypothalamic-pituitary-adrenal (HPA) axis activity that subsequently affect the development of self-regulation.
During the first years of life, children make great strides in self-regulation. While regulation success critically depends on the caregiver in infancy, the child progresses to more internally regulated behaviours from the second year of life on. In toddlerhood, a broad range of adaptive skills like self-control, autonomy, and compliance emerge as a function of increasing self-regulatory abilities which continue to develop during the preschool years (Calkins, Citation2007). The first years of life are further marked by maturational changes in one of the major stress systems which regulates circulating levels of glucocorticoid hormones, the HPA axis (Watamura et al., Citation2004). While studies indicate that both self-regulation and HPA axis functioning can be disturbed by adversity (e.g., Sturge-Apple et al., Citation2017), less is known about their interplay in shaping developmental pathways.
According to Koss and Gunnar (Citation2018), experiences of early adversity include ‘the absence of stimulation needed for typical development and the presence of harmful or threatening stimulation’ (p. 327). In line with this broad definition, the construct of early adversity can take many forms. On the one hand, it includes adverse experiences that affect the child directly, as when caregivers engage in maladaptive parenting. On the other hand, it encompasses conditions which more indirectly impact the child, like growing up in poverty (see Hughes et al., Citation2017). It is important to note that direct and contextual (or indirect) adversities often co-occur which makes it difficult to disentangle them. For instance, children exposed to maltreatment are more likely to come from poor families (McGuinness & Schneider, Citation2007). However, all forms of early adversity have in common that they challenge the child’s coping resources, leading to chronic stress (Pechtel & Pizzagalli, Citation2011).
Given that studies have commonly focused on either direct or contextual adversity, the first part of this review will examine adversity-related alterations in children’s HPA axis functioning structured along this distinction. The question will be addressed as to whether different forms of early adversity have different stress signatures, impacting development through differential effects on HPA axis functioning. Next, we will discuss how these HPA axis alterations could affect the development of self-regulation. The review will end with a brief perspective on current interventions and limitations.
Early adversity disrupts functioning of the HPA axis
Although prior research clearly demonstrates that experiences of adversity are important risk factors for psychopathology (Kessler et al., Citation2010), we have only a limited understanding of the psychobiological processes underlying this association. What is widely viewed as one important mechanism through which early adversity ‘gets under the skin’ is disruption in the development of stress-response systems (Koss & Gunnar, Citation2018). Notably, the Allostatic Load Model (ALM; McEwen, Citation1998) has guided research designed to understand how stress-response systems may serve to mediate the effects of childhood adversity on psychopathology. Central to this theory is the notion of allostasis, which denotes the body’s ability to achieve stability through physiological changes in stress-mediating systems (McEwen & Wingfield, Citation2003). Among others, physiological changes comprise the secretion of glucocorticoids, catecholamines, and inflammatory cytokines. In the short term, these adjustments are adaptive and have protective effects because they prepare an individual to meet the energy demands associated with a stressful event. However, chronic environmental stressors can lead to a sustained allostatic state reflected in altered activity of stress mediators. Over longer time intervals, this results in wear and tear of the body, termed allostatic overload, and pathologies are likely to develop (McEwen, Citation1998; McEwen & Wingfield, Citation2003).
Children growing up under adverse conditions are frequently or even chronically exposed to environmental stressors, which requires frequent responses of the stress systems (Koss & Gunnar, Citation2018). Researchers have devoted much attention to the HPA axis, and a wealth of studies has been published on salivary cortisol, an easy-to-assess and non-invasive measure of HPA activity. Under normal conditions, cortisol is released with a pronounced diurnal rhythm, characterized by high levels in the morning followed by a decline over the day. Superimposed on this diurnal rhythm, activity of the HPA axis results in cortisol increases when the organism is confronted with a stressor (Kudielka & Kirschbaum, Citation2005; Spiga et al., Citation2014).
Although mounting a stress response to an adverse event is critical and adaptive, it is thought that multiple stressful events over time – as in chronic stress – lead the HPA axis to become increasingly overactive in providing metabolic resources to cope with threats. This may result in an allostatic state characterized by elevated circulating levels of cortisol (McEwen & Wingfield, Citation2003). In chronically stressed individuals, however, reduced morning cortisol levels have repeatedly been observed (Miller et al., Citation2007). This is why it has been suggested that mechanisms have evolved to downregulate the HPA axis under conditions of chronic activation in order to protect the body from damage caused by the toxic effects of stress hormones (Fries et al., Citation2005). These mechanisms include potent negative feedback circuits acting at different levels in the HPA axis. Thus, both heightened and lowered cortisol levels have been found in chronically stressed individuals. To explain this, it has been hypothesized that chronic stress initially elevates cortisol output, but as time passes, secretion can rebound to below normal concentrations (Miller et al., Citation2007). However, another explanation could be that different types of chronic stress, as in different forms of early adversity, differentially affect HPA axis functioning. For the purpose of this review, we will term high and low basal levels of cortisol as indicative of a ‘hyperactive’ and ‘hypoactive’ HPA axis, respectively.
Direct adversity
During the first years of life, caregivers provide a strong social regulator of HPA axis activity (Gunnar & Donzella, Citation2002). Thus, disturbances in caregiving, as through engagement in harsh parenting or maltreatment, can disrupt normal regulation of this neuroendocrine system (Gunnar & Quevedo, Citation2007). While the construct of child maltreatment broadly indicates adverse experiences with caregivers, a common distinction is made between abuse and neglect (English, Citation1998). Abuse tends to involve repeated exposures to threatening behaviours perpetrated towards the child (Barnett et al., Citation1993), whereas neglect reflects lack of necessary care from the primary caregiver, including failure to adequately meet the child’s physical and/or emotional needs (Hildyard & Wolfe, Citation2002).
In a sample of mothers identified during pregnancy as at risk for future child maltreatment, Bugental et al. (Citation2003) examined ‘subtle’ types of maltreatment. They showed that in 18-month-old toddlers, those who encountered frequent maternal emotional unavailability had elevated midmorning levels of cortisol. This finding is consistent with a recent investigation in which maternal unresponsiveness was a stable predictor of children’s elevated cortisol levels, assessed at ages two, three, and four (Suor et al., Citation2015).
Providing evidence for detrimental effects of more severe forms of maltreatment, Cicchetti et al. (Citation2011) showed that compared to non-maltreated children, maltreated children whose families were involved with the Child Protective Services (CPS) progressively evinced lower levels of morning cortisol at assessments which took place at 19, 26, and 38 months of age. However, it should be noted that at the initial assessment at 13 months, no significant differences in morning cortisol were observed among groups. This could suggest that maltreatment-related alterations in HPA axis activity were not yet present or not as pronounced as from the second assessment on.
In order to study the impact of ongoing neglect, researchers have focused on children from those institutions which provided a very low quality of care. In these institutions, children experienced inadequate nutrition, emotional deprivation, and a lack of cognitive stimulation (Gunnar et al., Citation2001). Studies with institutionalized children are fairly uniform in revealing patterns indicative of a hypoactive HPA axis in toddlerhood. A first study (Carlson & Earls, Citation1997) reported that institutionalized two-year-olds who experienced severe deprivation had lower morning cortisol levels and blunted diurnal patterns compared to home-reared children. The blunted diurnal pattern resulted from flatter slopes in morning to bedtime values which were largely determined by the low height of the morning cortisol value. Studying 7- to 30-month-old institutionalized children, Kroupina et al. (Citation2012) found low wake-up cortisol levels in 82% of the children.
As an alternative to institutional care, children may be placed in foster care when the home environment is considered harmful for their development. One study found lower waking cortisol levels and flatter slopes from waking to bedtime in CPS-involved foster children up to 2.5 years of age compared to non-foster children from low-risk environments (Bernard et al., Citation2010). Given that a third group of CPS-involved children who continued to live with their birth parents exhibited the flattest slope of diurnal cortisol production, the authors suggested that foster care may have a regulating influence on cortisol levels among children with maltreatment experiences.
A study of Dozier, Manni, et al. (Citation2006) revealed that low levels of cortisol do not uniformly characterize diurnal HPA axis functioning in foster children. In this study, salivary samples were obtained from 20- to 60-month-old foster children for whom the primary reason for removal had been neglect (86%), and from a comparison group composed of children from non-maltreating families. 38% and 18% of foster children showed low and high patterns of cortisol production, respectively, compared with only 14% and 11% of non-maltreated children. Thus, foster children exhibited higher incidences of atypical cortisol patterns, with patterns indicative of a hypoactive HPA axis being observed more often than patterns indicative of a hyperactive HPA axis.
Testing the hypothesis that specific maltreatment experiences may have differential effects on HPA axis functioning, Bruce et al. (Citation2009) examined morning cortisol levels of preschool-aged foster children. Foster children who experienced more severe physical neglect demonstrated low cortisol levels, whereas foster children who experienced more severe emotional maltreatment had high cortisol levels. When compared to a group of low-income, non-maltreated children, foster children were more likely to have low morning cortisol levels.
In sum, the presented studies suggest that severe deprivation as experienced by children in institutions with low quality of care, and maltreatment as experienced by CPS-involved children, may both lead to a hypoactive HPA axis in early childhood. This hypoactivity seems to be characterized by low levels of cortisol in the morning, which determine the flatter slopes observed throughout the day, resulting in a blunted pattern of HPA axis activity. Although evidence more strongly relates neglect rather than abuse to HPA hypoactivity (Bruce et al., Citation2009), it still remains unclear whether both forms of maltreatment have different or relatively similar effects on HPA axis functioning in early childhood. Despite the fact that neglect is often the primary reason why children are being removed from their homes, this question is particularly difficult to solve given that these children likely have experienced a broad range of adverse conditions simultaneously before their displacement, including abuse, poverty, and/or family instability (Grogan-Kaylor, Citation2000; Jamora et al., Citation2009).
In contrast to severe neglect, studies have shown an association between maternal unresponsiveness and high cortisol levels in early childhood. Both neglect and maternal unresponsiveness seem to reflect the absence of positive stimulation but to a different degree of severity. Thus, it could be that parental behaviour which carries the potential for stress (as maternal unresponsiveness) increases circulating levels of cortisol, while neglect as experienced by children during institutionalization or in maltreating families is that severe that it leads to downregulation of the HPA axis.
Contextual adversity
There are adverse conditions not as extreme as maltreatment, but still challenging for the child, bearing the potential to impact typical development. Examples of such adversities include growing up in poverty, experiencing household chaos, or family instability – conditions which are likely to indirectly challenge the child through a chain of mechanisms encompassing distress among the caregiver, which in turn can impact the quality of caregiver–child interactions (Grant et al., Citation2003). Further evidence for the hypothesis that parental behaviour mediates the effects of contextual adversity on child outcomes stems from a large literature demonstrating that high-quality parenting serves as a powerful buffer of stress for children (Gunnar, Citation2017). Within the context of adversity, sensitive and responsive parenting may help children to cope with the stressful events and interactions that regularly occur in their environments.
Given its broad meaning, researchers have studied different experiences as representative of the construct of early adversity. For instance, Ouellet-Morin et al. (Citation2009) investigated the effects of family adversity on six-month-old infants’ morning cortisol levels by creating a cumulative index of seven perinatal and postnatal risk factors, including maternal smoking during pregnancy, low birth weight, low family income, low maternal education, single parenthood, young motherhood, and maternal hostile behaviours. Although it is reasonable to assume that accumulation of risk, rather than the experience of one risk factor alone, would be related to disruptions in HPA axis functioning (see Zalewski, Lengua, Kiff et al., Citation2012), this study did not observe differences in cortisol secretion between twins exposed to low versus high family adversity. While it is possible that not enough time had elapsed for alterations in HPA axis activity to emerge as a function of adversity, it could also be that the individual adversity indicators, relating to different experiences for the child, affected infants’ HPA activity in different ways.
Building on the work of Ouellet-Morin et al. (Citation2009), Saridjan et al. (Citation2010) looked at each of the formerly investigated risk factors separately. Based on collecting five saliva samples over the day from 12- to 20-month-old toddlers, they reported that children of low-income families as well as children of mothers experiencing high levels of parenting stress showed a higher cortisol secretion over the day. The slope, a measure of the diurnal cortisol decline, was not associated with any risk factor.
A different analytical approach was taken by Suor et al. (Citation2015) to consider that specific types of family adversity might differentially shape trajectories of HPA axis functioning across early childhood. In their longitudinal study, morning cortisol levels of children from a low-income family background were assessed at ages two, three, and four years. Using growth-mixture modelling, three patterns (elevated, moderate, and low) of basal HPA activity were identified. At age two, children displayed categorically different homoeostatic ‘set points’ of basal cortisol activity which remained relatively stable across the two years of the study. Intriguingly, both elevated and low cortisol patterns were predicted by exposure to greater levels of family instability, characterized by disruptive and unstable caregiving conditions. In contrast, inter-partner violence and socioeconomic status did not predict cortisol profiles.
Studies examining specifically the impact of poverty on HPA axis functioning in preschoolers have yielded mixed results. Zalewski, Lengua, Kiff et al. (Citation2012) found lower income to be related to lower morning but not evening cortisol levels or diurnal slope in 36-month-old children. In contrast, another study found that families with a lower income-to-needs ratio, indicating extreme poverty, had children with higher levels of cortisol (Blair et al., Citation2005). This discrepant finding may be explained by differences in the study population, as Blair et al. (Citation2005) investigated low-income families only. Rather than being contradictory, findings could suggest that the consequences of different ‘degrees’ of poverty may differentially affect the child’s HPA axis functioning.
Given that the presented studies are not entirely comparable due to differences in study design, age groups, cortisol assessments, and specific types of adversity, it is too early yet to conclude whether exposure to contextual adversity is associated with high or low levels of cortisol in early childhood. Consistent with the hypothesis that chronic stress can lead to hyper- and hypoactivation of the HPA axis, both cortisol patterns were associated with family instability and poverty. As variations in the association between contextual adversity and HPA axis activity likely relate to other factors such as parental behaviour (Johnson et al., Citation2018), more research on potential differential effects of positive parenting, such as warmth and responsiveness, and negative parenting, such as harshness and hostility, could shed light on the pattern the child’s HPA axis may adopt.
Open questions and future directions
Taken together, there is no clear connection between specific types of early adversity and HPA axis hyper- or hypoactivity in the first five years of life. Based on the available evidence at this point, it seems possible that conditions which comprise stressful experiences for the child like family instability, parenting distress, and maternal unresponsiveness lead to HPA axis hyperactivity. Certainly, this does not preclude the occurrence of lowered cortisol levels at a later stage. Studies on children in low-quality institutions, CPS-involved children who continued living with their families, and CPS-involved children placed in foster care are fairly uniform in demonstrating low levels of cortisol. Although it is still unclear how experiences of neglect and abuse contribute to this pattern, one might speculate that experiencing extreme developmental stressors more likely leads to downregulation of the HPA axis.
It also seems possible that high cortisol levels appear under ongoing adversity (as in studies on contextual adversity) and low levels after cessation of adversity (as in studies on foster children). However, the finding of HPA axis hypoactivity both in institutionalized children who continued to suffer from deprivation (Carlson & Earls, Citation1997) and in CPS-involved children who continued living with their maltreating birth parents (Cicchetti et al., Citation2011), challenges this notion.
Intriguingly, both patterns indicative of a hyper- and hypoactive HPA axis were detected among same-age and socioeconomically similar children within the context of adversity. These findings raise multiple questions: For those children whose HPA axis is hypoactive, was this pattern preceded by hyperactivation? And for those whose HPA axis is hyperactive, will a transition to hypoactivation occur later on? Only a few longitudinal studies have investigated the direct link between adversity-related chronic hyperactivation of the HPA axis and a consequent downregulation, with a transition suggested to occur during childhood (Doom et al., Citation2014) or adolescence (Kaess et al., Citation2017). However, given the repeated finding of adversity-related HPA hypoactivity in the first years of life (e.g., Carlson & Earls, Citation1997), downregulation of the HPA axis can occur much earlier than previously proposed.
The literature does not provide a clear answer to these questions yet, highlighting the need for studies on early adversity which span multiple developmental periods. Such longitudinal studies should begin the first assessment ideally in infancy and apply a pattern-based approach to examine individual HPA axis functioning and its development in the face of adversity. To develop a comprehensive picture of HPA axis functioning, at least three assessments of cortisol from morning to bedtime should be conducted, from which the diurnal slope and total diurnal secretion can be calculated. Due to intra-individual cortisol variability in the first years of life (De Weerth & van Geert, Citation2002), it is advisable to carry out sampling for a period of two to three days. Beyond that, assessments of cortisol stress reactivity provide insight into an individual’s reaction upon confrontation with a stressor. In order to gain an objective measure of chronic stress exposure during the past three months, researchers may further opt for hair cortisol analysis (Russell et al., Citation2012). In addition, specifying relevant factors related to the child (e.g., age/developmental period, gender, temperament, genotype) and to adversity (e.g., type, duration, severity, and recency) might shed light on HPA axis functioning and potential transitions therein.
From HPA axis-dysregulation to impaired self-regulation
Although we do not know under what conditions early adversity leads to hyper- or hypoactivity of the HPA axis, there is sufficient evidence to conclude that the HPA axis runs the risk of becoming dysregulated in early childhood. Changes in stress physiology, in turn, may have an impact on many areas of children’s lives due to their impact on children’s basic ability to self-regulate.
Self-regulation, broadly defined as the ability to control emotions and behaviours (Calkins et al., Citation2016), critically depends on executive functions (EFs), as this set of higher-order cognitive processes is involved in all top-down aspects of self-regulation (Nigg, Citation2017). Previous research has shown that adverse experiences are associated with deficits in self-regulation during childhood (Evans & Kim, Citation2013; Shields et al., Citation1994). An impaired development of the ability to regulate emotions and behaviour in early life is in a way detrimental in that it directly contributes to behaviours such as expressed frustration, wilful non-compliance, and tantrums that disrupt the child’s functioning in the situation in which they occur. Ongoing failures to self-regulate contribute to behavioural problems during childhood, as they limit opportunities to learn adaptive skills in social interactions (Calkins, Citation2007). Over time, this has detrimental consequences for the individual, as impairments in self-regulation during childhood have been shown to increase the risk for psychopathology later in life (Moffitt et al., Citation2011).
While research points out the detrimental effects of adversity on children’s cognitive functions supporting self-regulation (e.g., Hostinar et al., Citation2012), little is known about the mechanisms underlying this association. Recently, theories have been proposed that describe how stress physiology may act as a primary mechanism relating experiences of early adversity to lowered cognitive abilities (Blair & Raver, Citation2012; Raymond et al., Citation2018). The idea behind these theories is that early in life, glucocorticoids as main actors of stress-response systems impact the development of brain structures and neural activity that underlie self-regulation. Adversity-related structural and functional alterations have been observed in the hippocampus, the amygdala, and the prefrontal cortex (PFC; for reviews, see McCrory et al., Citation2010; Raymond et al., Citation2018) – brain regions which are dense in glucocorticoid receptors (Lupien et al., Citation2009). The PFC plays a major role in the control of many aspects of behaviour, cognition, and emotion (Miller & Cohen, Citation2001; Ochsner & Gross, Citation2005). Given its involvement in response inhibition and selective attention, the PFC supports effortful control (EC), a self-regulatory mechanism referring to the ability to suppress a dominant response to perform a subdominant response (Kochanska et al., Citation2000; Nigg, Citation2017; Rothbart & Bates, Citation1998). Impairments in the development of the PFC may thus underlie deficits in children´s capacity to self-regulate.
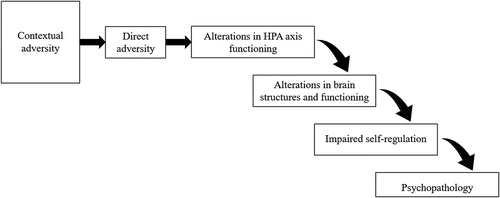
Building on previous work (Blair & Raver, Citation2012; Raymond et al., Citation2018), we outline one possible pathway through which experiences of adversity in early life can lead to psychopathology over time: Beginning throughout the first years of life, experiences of adversity shape functioning of the child’s developing HPA axis. Although we have described the effects of direct and contextual adversity separately, it is likely that contextual adversity exerts influence on HPA axis functioning through increasing experiences of direct adversity. To give an example, poverty as a contextual adversity likely increases the caregiver’s distress which can lead to insensitive parenting practices that directly affect the child (Grant et al., Citation2003). Over time, alterations in HPA axis functioning impair development and functioning of brain structures which are important for self-regulation. Impairments in self-regulation, in turn, increase the risk for psychopathology in the long term (see ).
Figure 1. Model of the developmental cascade discussed in this review, in which experiences of early adversity increase risk for psychopathology
Addressing the hypothesis that the HPA axis serves as a key mediator between adversity and cognitive functioning in early childhood, Blair et al. (Citation2011) conducted a seminal study including a predominantly low-income population-based sample of 1,292 children. They showed that a higher level of cortisol was related to lower EF ability at age three. In addition, cortisol levels partially mediated the association between parenting quality and later EFs: Highly supportive parenting apparently resulted in lower levels of cortisol, which in turn accounted for improvements in children’s EFs over time.
Similarly, Sturge-Apple et al. (Citation2017) examined cortisol levels and cognitive abilities in children living within economically impoverished environments. They focused on ‘hot’ EC, assessed at four years of age with a task that called for suppressing an emotionally charged response in the context of a potential reward. Results revealed that higher family instability predicted elevated morning cortisol levels, as well as decreased hot EC. Further, the association between family instability and children’s hot EC was mediated by morning cortisol levels, revealing that higher basal cortisol was associated with lower hot EC.
Based on the same participants as in the study of Sturge-Apple et al. (Citation2017), Suor et al. (Citation2015) showed that cortisol deviations in both directions can be detrimental for cognitive functioning. The observed patterns of elevated and lowered cortisol were both associated with lower child global cognitive ability at age four. In support of the adverse effects of low cortisol levels, Zalewski et al. (Citation2012) found that a blunted cortisol pattern, characterized by low morning and evening cortisol levels, was related to lower EC in preschoolers. This also accords with a recent investigation (Koss et al., Citation2016) in which hypocortisolism (indexed by low early morning cortisol, a flat diurnal slope, and a blunted stress response) mediated between early adversity and teacher-reported attention and externalizing problems during kindergarten.
While high levels of cortisol have frequently been viewed as exerting neurotoxic effects on brain structures underlying cognitive functions, low levels of cortisol may also be problematic, as they may be insufficient to marshal metabolic resources necessary for optimal learning and memory consolidation (Suor et al., Citation2015). Further, basal cortisol levels are required for normal functioning of the sympathetic nervous system and the immune system (Fries et al., Citation2005; Sorrells et al., Citation2009), implying that deviations of cortisol in both directions may be detrimental to health.
Taken together, the presented findings provide support for the idea that early adversity sets the stage for impairments in children’s cognitive functions underlying self-regulation through calibration of the HPA axis. While evidence points to impairments in behavioural aspects of self-regulation such as EC (Sturge-Apple et al., Citation2017), it remains unclear whether HPA axis functioning mediates the effect of early adversity on emotion regulation. Unfortunately, no early childhood study to date has combined trajectories of HPA axis activity and cognitive functioning with neuroimaging to identify whether differences in cortisol levels are associated with structural differences, for instance, in the PFC, which in turn lead to reduced cognitive abilities. It thus remains an open question whether HPA axis activity acts as a key biological mechanism in shaping cognitive abilities that underlie self-regulation, or whether it serves as a marker for other processes that lead to impairments.
Implications for interventions
As outlined throughout this review, adversity-related alterations in HPA axis activity and self-regulatory capacity can already emerge in the first years of life, underscoring the need for early intervention. The literature provides promising evidence of the potential malleability of development through interventions targeting parenting behaviour. Considering that experiential and biological influences on development are highly intertwined, supporting parents to maintain high levels of responsiveness and warmth can be expected to improve the child’s regulation of stress physiology with cascading influences on self-regulation (Blair & Raver, Citation2012).
Some research demonstrates that parenting interventions are successful in ‘normalizing’ children’s cortisol levels, resulting in post-treatment cortisol levels that approximate a low-risk comparison group. This has been shown in foster children (Dozier, Peloso et al., Citation2006), children at risk for neglect (Bernard et al., Citation2015), and maltreated children (Cicchetti et al., Citation2011) younger than five years of age. Thus, there is initial support for a process by which changes in caregiving behaviour lead to changes in HPA axis functioning that should be conducive to self-regulation. Indeed, studies on parenting training have provided evidence of positive effects on aspects of child self-regulation, including attention and emotion regulation (Landry et al., Citation2006). Given that no early childhood study has simultaneously assessed HPA axis activity and self-regulation skills post-intervention, it remains a key question whether intervening in maladaptive caregiving yields linked improvements both in children’s stress physiology and self-regulation abilities.
Limitations
Although the developmental cascade described in this review portrays an important pathway through which experiences of early adversity increase risk for psychopathology, other pathways are likely as well. Intriguingly, recent theories have outlined a pathway associating adversity-related HPA axis alterations to changes in brain development that predict cognitive dysregulation underlying psychopathology (Raymond et al., Citation2018). Given that this pathway gains importance in later development, our model can be seen as complementary, providing an enhanced understanding of adversity-related processes in the first years of life.
While our review focuses on direct and indirect adversity in early childhood, prenatal factors like maternal stress, substance use, or malnutrition during pregnancy have also been associated with altered HPA axis functioning in children (Love et al., Citation2012). However, only few studies have investigated how pre- and postnatal adversity interact to predict emerging HPA axis alterations in the first years of life. Another important issue for future research is to examine the role of (epi-)genetic factors in affecting an individual’s likelihood of developing impairments in HPA axis functioning and self-regulation after exposure to childhood adversity.
Summary
In the present review, we outlined evidence for theoretical models postulating that early adversity can set in motion a deleterious developmental cascade leading to an increased risk for psychopathology. Studies focusing on the first five years of life have pointed to both heightened and lowered cortisol levels in children exposed to various forms of adversity. Both types of atypical HPA axis functioning have been associated with impairments in cognitive functions that underlie self-regulation. However, intervention research points to the potential malleability of developmental pathways in the face of adversity. In order to refine interventions, more research is needed to understand how environmental and individual factors contribute to the child’s risk of developing HPA axis alterations in the face of adversity.
Disclosure statement
The authors declare that they have no relevant or material financial interests relating to the research described in this paper.
References
- Appleyard, K., Egeland, B., van Dulmen, M. H. M., & Sroufe, L. A. (2005). When more is not better: The role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 46(3), 235–245. https://doi.org/10.1111/j.1469-7610.2004.00351.x
- Bernard, K., Butzin-Dozier, Z., Rittenhouse, J., & Dozier, M. (2010). Cortisol Production Patterns in Young Children Living With Birth Parents vs Children Placed in Foster Care Following Involvement of Child Protective Services. Archives of Pediatrics & Adolescent Medicine, 164(5), 5. https://doi.org/10.1001/archpediatrics.2010.54
- Bernard, K., Dozier, M., Bick, J., & Gordon, M. K. (2015). Intervening to enhance cortisol regulation among children at risk for neglect: Results of a randomized clinical trial. Development and Psychopathology, 27(3), 829–841. https://doi.org/10.1017/S095457941400073X
- Blair, C., Granger, D. A., & Razza, R. P. (2005). Cortisol Reactivity Is Positively Related to Executive Function in Preschool Children Attending Head Start. Child Development, 76(3), 554–567. https://doi.org/10.1111/j.1467-8624.2005.00863.x
- Blair, C., Granger, D. A., Willoughby, M., Mills-Koonce, R., Cox, M., Greenberg, M. T., Kivlighan, K. T., & Fortunato, C. K., & the FLP Investigators. (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood: Cortisol and cognition. Child Development, 82(6), 1970–1984. https://doi.org/10.1111/j.1467-8624.2011.01643.x
- Blair, C., & Raver, C. C. (2012). Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist, 67(4), 309–318. https://doi.org/10.1037/a0027493
- Bruce, J., Fisher, P. A., Pears, K. C., & Levine, S. (2009). Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology, 51(1), 14–23. https://doi.org/10.1002/dev.20333
- Bugental, D. B., Martorell, G. A., & Barraza, V. (2003). The hormonal costs of subtle forms of infant maltreatment. Hormones and Behavior, 43(1), 237–244. https://doi.org/10.1016/S0018-506X(02)00008-9
- Calkins, S. D. (2007). The emergence of self-regulation: Biological and behavioral control mechanisms supporting toddler competencies. In C. A. Brownell & C. B. Kopp (Eds.), Socioemotional development in the toddler years: Transitions and transformations (pp. 261–284). Guilford Press.
- Calkins, S. D., Perry, N. E., & Dollar, J. M. (2016). A biopsychosocial model of self-regulation in infancy. In L. Balter & C. S. Tamis-LeMonda (Eds.), Child Psychology: A Handbook of Contemporary Issues: Third Edition, (pp. 3–20). New York: Psychology Press. https://doi.org/10.4324/9781315764931
- Carlson, M., & Earls, F. (1997). Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences, 807(1), 419–428. https://doi.org/10.1111/j.1749-6632.1997.tb51936.x
- Barnett, D., Manly, J. T., & Cicchetti, D. (1993). Defining child maltreatment: The interface between policy and research. In D. Cicchetti & S. L. Toth (Eds.), Advances in applied developmental psychology: Child abuse, child development, and social policy (pp. 7-74). Norwood, NJ: Ablex.
- Cicchetti, D., Rogosch, F. A., Toth, S. L., & Sturge-Apple, M. L. (2011). Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Development and Psychopathology, 23(3), 789–800. https://doi.org/10.1017/S0954579411000307
- De Weerth, C., & van Geert, P. (2002). A longitudinal study of basal cortisol in infants: Intra-individual variability, circadian rhythm and developmental trends. Infant Behavior and Development, 25(4), 375–398. https://doi.org/10.1016/S0163-6383(02)00141-8
- Doom, J. R., Cicchetti, D., & Rogosch, F. A. (2014). Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. Journal of the American Academy of Child & Adolescent Psychiatry, 53(11), 1206–1215. https://doi.org/10.1016/j.jaac.2014.08.006
- Dozier, M., Manni, M., Gordon, M. K., Peloso, E., Gunnar, M. R., Stovall-McClough, K. C., Eldreth, D., & Levine, S. (2006). Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment, 11(2), 189–197. https://doi.org/10.1177/1077559505285779
- Dozier, M., Peloso, E., Lindhiem, O., Gordon, M. K., Manni, M., Sepulveda, S., Ackerman, J., Bernier, A., & Levine, S. (2006). Developing evidence-based interventions for foster children: An example of a randomized clinical trial with infants and toddlers. Journal of Social Issues, 62(4), 767–785. https://doi.org/10.1111/j.1540-4560.2006.00486.x
- English, D. J. (1998). The extent and consequences of child maltreatment. The Future of Children, 8(1), 39–53. https://doi.org/10.2307/1602627
- Evans, G. W., & Kim, P. (2013). Childhood poverty, chronic stress, self-regulation, and coping. Child Development Perspectives, 7(1), 43–48. https://doi.org/10.1111/cdep.12013
- Fries, E., Hesse, J., Hellhammer, J., & Hellhammer, D. H. (2005). A new view on hypocortisolism. Psychoneuroendocrinology, 30(10), 1010–1016. https://doi.org/10.1016/j.psyneuen.2005.04.006
- Grant, K. E., Compas, B. E., Stuhlmacher, A. F., Thurm, A. E., McMahon, S. D., & Halpert, J. A. (2003). Stressors and child and adolescent psychopathology: Moving from markers to mechanisms of risk. Psychological Bulletin, 129(3), 447–466. https://doi.org/10.1037/0033-2909.129.3.447
- Grogan-Kaylor, A. (2000). Who goes into kinship care? The relationship of child and family characteristics to placement into kinship foster care. Social Work Research, 24(3), 132–141. https://doi.org/10.1093/swr/24.3.132
- Gunnar, M. R. (2017). Social buffering of stress in development: A career perspective. Perspectives on Psychological Science, 12(3), 355–373. https://doi.org/10.1177/1745691616680612
- Gunnar, M. R., & Donzella, B. (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. https://doi.org/10.1016/S0306-4530(01)00045-2
- Gunnar, M. R., Morison, S. J., Chisholm, K., & Schuder, M. (2001). Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology, 13(3), 611–628. https://doi.org/10.1017/S095457940100311X
- Gunnar, M. R., & Quevedo, K. M. (2007). Early care experiences and HPA axis regulation in children: A mechanism for later trauma vulnerability. Progress in brain research, 167, 137–149. https://doi.org/10.1016/S0079-6123(07)67010-1.
- Hildyard, K. L., & Wolfe, D. A. (2002). Child neglect: Developmental issues and outcomes. Child Abuse & Neglect, 26(6–7), 679–695. https://doi.org/10.1016/S0145-2134(02)00341-1
- Hostinar, C. E., Stellern, S. A., Schaefer, C., Carlson, S. M., & Gunnar, M. R. (2012). Associations between early life adversity and executive function in children adopted internationally from orphanages. Proceedings of the National Academy of Sciences, 109(Suppl. 2), 17208–17212. https://doi.org/10.1073/pnas.1121246109
- Hughes, K., Bellis, M. A., Hardcastle, K. A., Sethi, D., Butchart, A., Mikton, C., Jones, L., & Dunne, M. P. (2017). The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health, 2(8), e356–e366. https://doi.org/10.1016/S2468-2667(17)30118-4
- Jamora, M. S., Brylske, P. D., Martens, P., Braxton, D., Colantuoni, E., & Belcher, H. M. E. (2009). Children in foster care: Adverse childhood experiences and psychiatric diagnoses. Journal of Child & Adolescent Trauma, 2(3), 198–206. https://doi.org/10.1080/19361520903120491
- Johnson, A. B., Mliner, S. B., Depasquale, C. E., Troy, M., & Gunnar, M. R. (2018). Attachment security buffers the HPA axis of toddlers growing up in poverty or near poverty: Assessment during pediatric well-child exams with inoculations. Psychoneuroendocrinology, 95, 120–127. https://doi.org/10.1016/j.psyneuen.2018.05.030
- Kaess, M., Whittle, S., Simmons, J. G., Jovev, M., Allen, N. B., & Chanen, A. M. (2017). The interaction of childhood maltreatment, sex, and borderline personality features in the prediction of the cortisol awakening response in adolescents. Psychopathology, 50(3), 188–194. https://doi.org/10.1159/000456549
- Kessler, R. C., McLaughlin, K. A., Green, J. G., Gruber, M. J., Sampson, N. A., Zaslavsky, A. M., Aguilar-Gaxiola, S., Alhamzawi, A. O., Alonso, J., Angermeyer, M., Benjet, C., Bromet, E., Chatterji, S., de Girolamo, G., Demyttenaere, K., Fayyad, J., Florescu, S., Gal, G., Gureje, O., Hu, C.-Y., … Williams, D. R. (2010). Childhood adversities and adult psychopathology in the WHO world mental health surveys. The British Journal of Psychiatry, 197(5), 378–385. https://doi.org/10.1192/bjp.bp.110.080499
- Kochanska, G., Murray, K. T., & Harlan, E. T. (2000). Effortful control in early childhood: continuity and change, antecedents, and implications for social development. Developmental Psychology, 36(2), 220–232. https://doi.org/10.1037/0012-1649.36.2.220
- Koss, K. J., & Gunnar, M. R. (2018). Annual research review: Early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry, 59(4), 327–346. https://doi.org/10.1111/jcpp.12784
- Koss, K. J., Mliner, S. B., Donzella, B., & Gunnar, M. R. (2016). Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology, 66, 31–38. https://doi.org/10.1016/j.psyneuen.2015.12.018
- Kroupina, M. G., Fuglestad, A. J., Iverson, S. L., Himes, J. H., Mason, P. W., Gunnar, M. R., Miller, B. S., Petryk, A., & Johnson, D. E. (2012). Adoption as an intervention for institutionally reared children: HPA functioning and developmental status. Infant Behavior and Development, 35(4), 829–837. https://doi.org/10.1016/j.infbeh.2012.07.011
- Kudielka, B. M., & Kirschbaum, C. (2005). Sex differences in HPA axis responses to stress: A review. Biological Psychology, 69(1), 113–132. https://doi.org/10.1016/j.biopsycho.2004.11.009
- Landry, S. H., Smith, K. E., & Swank, P. R. (2006). Responsive parenting: Establishing early foundations for social, communication, and independent problem-solving skills. Developmental Psychology, 42(4), 627–642. https://doi.org/10.1037/0012-1649.42.4.627
- Love, O. P., McGowan, P. O., & Sheriff, M. J. (2012). Maternal adversity and ecological stressors in natural populations: The role of stress axis programming in individuals, with implications for populations and communities. Functional Ecology, 27(1), 81–92. https://10.1111/j.1365-2435.2012.02040.x
- Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. https://doi.org/10.1038/nrn2639
- McCrory, E., Brito, S. A. D., & Viding, E. (2010). Research Review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry, 51(10), 1079–1095. https://doi.org/10.1111/j.1469-7610.2010.02271.x
- McEwen, B. S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. https://doi.org/10.1111/j.1749-6632.1998.tb09546.x
- McEwen, B. S., & Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43(1), 2–15. https://doi.org/10.1016/S0018-506X(02)00024-7
- McGuinness, T. M., & Schneider, K. (2007). Poverty, child maltreatment, and foster care. Journal of the American Psychiatric Nurses Association, 13(5), 296–303. https://doi.org/10.1177/1078390307308421
- Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202. https://doi.org/10.1146/annurev.neuro.24.1.167
- Miller, G. E., Chen, E., & Zhou, E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. https://doi.org/10.1037/0033-2909.133.1.25
- Moffitt, T. E., Arseneault, L., Belsky, D., Dickson, N., Hancox, R. J., Harrington, H., Houtsa, R., Poultonc, R., Robertsd, B. W., Rossa, S., Searse, M. R., Thomsong, W. M., & Caspi, A. (2011). A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences, 108(7), 2693–2698. https://doi.org/10.1073/pnas.1010076108
- Nigg, J. T. (2017). On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 58(4), 361–383. https://doi.org/10.1111/jcpp.12675
- Ochsner, K. N., & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–249. https://doi.org/10.1016/j.tics.2005.03.010
- Ouellet-Morin, I., Dionne, G., Pérusse, D., Lupien, S. J., Arseneault, L., Barr, R. G., Tremblay, R. E., & Boivin, M. (2009). Daytime cortisol secretion in 6-month-old twins: Genetic and environmental contributions as a function of early familial adversity. Biological Psychiatry, 65(5), 409–416. https://doi.org/10.1016/j.biopsych.2008.10.003
- Pechtel, P., & Pizzagalli, D. A. (2011). Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology, 214(1), 55–70. https://doi.org/10.1007/s00213-010-2009-2
- Raymond, C., Marin, M.-F., Majeur, D., & Lupien, S. (2018). Early child adversity and psychopathology in adulthood: HPA axis and cognitive dysregulations as potential mechanisms. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 85, 152–160. https://doi.org/10.1016/j.pnpbp.2017.07.015
- Rothbart, M. K., & Bates, J. E. (1998). Temperament. In Handbook of child psychology: Social, emotional, and personality development Vol. 3. 5th (pp. 105–176). John Wiley & Sons Inc.
- Russell, E., Koren, G., Rieder, M., & Van Uum, S. (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. https://doi.org/10.1016/j.psyneuen.2011.09.009
- Saridjan, N. S., Huizink, A. C., Koetsier, J. A., Jaddoe, V. W., Mackenbach, J. P., Hofman, A., Kirschbaum, C., Verhulst, F. C., & Tiemeier, H. (2010). Do social disadvantage and early family adversity affect the diurnal cortisol rhythm in infants? The generation R study. Hormones and Behavior, 57(2), 247–254. https://doi.org/10.1016/j.yhbeh.2009.12.001
- Shields, A. M., Cicchetti, D., & Ryan, R. M. (1994). The development of emotional and behavioral self-regulation and social competence among maltreated school-age children. Development and Psychopathology, 6(1), 57–75. https://doi.org/10.1017/S0954579400005885
- Sorrells, S. F., Caso, J. R., Munhoz, C. D., & Sapolsky, R. M. (2009). The stressed CNS: When glucocorticoids aggravate inflammation. Neuron, 64(1), 33–39. https://doi.org/10.1016/j.neuron.2009.09.032
- Spiga, F., Walker, J. J., Terry, J. R., & Lightman, S. L. (2014). HPA Axis-Rhythms. Comprehensive physiology, 4, 1273–1298. https://doi.org/10.1002/cphy.c140003
- Sturge-Apple, M. L., Davies, P. T., Cicchetti, D., Hentges, R. F., & Coe, J. L. (2017). Family instability and children’s effortful control in the context of poverty: Sometimes a bird in the hand is worth two in the bush. Development and Psychopathology, 29(3), 685–696. https://doi.org/10.1017/S0954579416000407
- Suor, J. H., Sturge‐Apple, M. L., Davies, P. T., Cicchetti, D., & Manning, L. G. (2015). Tracing differential pathways of risk: Associations Among family adversity, cortisol, and cognitive functioning in childhood. Child Development, 86(4), 1142–1158. https://doi.org/10.1111/cdev.12376
- Watamura, S. E., Donzella, B., Kertes, D. A., & Gunnar, M. R. (2004). Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Developmental Psychobiology, 45(3), 125–133. https://doi.org/10.1002/dev.20026
- Zalewski, M., Lengua, L. J., Fisher, P. A., Trancik, A., Bush, N. R., & Meltzoff, A. N. (2012). Poverty and single parenting: Relations with preschoolers‘ cortisol and effortful control. Infant and Child Development, 21(5), 537–554. https://doi.org/10.1002/icd.1759
- Zalewski, M., Lengua, L. J., Kiff, C. J., & Fisher, P. A. (2012). Understanding the relation of low income to HPA-axis functioning in preschool children: cumulative family risk and parenting as pathways to disruptions in cortisol. Child Psychiatry & Human Development, 43(6), 924–942. https://doi.org/10.1007/s10578-012-0304-3
