ABSTRACT
Background: This user experience study evaluated the suitability of single-use versus multi-dose follitropin alfa pen injectors for self-administration by women undergoing fertility treatment.
Methods: Twenty-four fertility patients and 19 specialist nurses were recruited in four European countries to assess their use of Bemfola (a single-use pen), Gonal-f®, and Ovaleap® (multi-use pens). Participants completed usability tests in which their performance in assembling and administering doses of each pen was assessed against defined critical steps for ensuring safe and correct administration.
Results: Critical error rates among nurses were 4%, 40%, and 49% for Bemfola®, Ovaleap®, and Gonal-f®, respectively; and among patients were 7%, 16%, and 38%. The most frequently reported critical errors occurred with the multi-use pens and were incorrect/lack of priming and failure to check the dose window prior to setting a new dose. The need to ‘top up’ doses from a new pen or cartridge when a pen contained insufficient dose also caused errors. The single-use pens did not cause these errors. Overall, 63% of nurses and 67% of patients had most confidence in Bemfola® for correct dosing and self-administration.
Conclusions: Single-use pens require fewer preparation and administration steps than multi-use pens and are associated with fewer critical handling errors.
1. Introduction
Follicle-stimulating hormone (FSH) is a pituitary glycoprotein hormone that plays a key role in regulating reproductive function in both males and females. During controlled ovarian stimulation for assisted reproductive technologies (ART), several different medications and dosing regimens are often used [Citation1]. The medications involved include gonadotropin treatment in the form of FSH, which is self-injected daily, usually over a period of 9 to 11 days [Citation2,Citation3]. FSH is available in many different forms and administered through different systems, such as vials and syringes; disposable, single-use, pre-filled pens; multi-use pre-filled pens; and re-useable pens requiring insertion of cartridges [Citation4–7]. Self-administration of FSH with these different delivery systems can be confusing and stressful for patients, potentially leading to the possibility of dosing errors during the treatment cycle [Citation8]. One of the most common reasons for treatment discontinuation during ART is the high overall burden of care, and this can be attributed to factors related to the patient, clinic, or the actual treatment [Citation9].
Recombinant human FSH (r-hFSH) formulations have been in widespread use since the 1990s [Citation10]. Recently, biosimilar versions of r-hFSH have been developed, providing high quality, economically attractive alternative choices for physicians and patients [Citation10].
Noncompliance to hormonal treatment regimens represents a critical obstacle to reaching therapeutic goals [Citation11–14]. The self-injection of gonadotropins during fertility treatment cycles is often limited by factors such as the patient’s fear of injection, fear of using injection devices incorrectly, and fear of incorrect dosing; importantly, these fears and the reality of how accurately doses are delivered can be related to the device itself [Citation15]. It has been shown previously that fewer steps required to prepare a pen injector and perform an injection might reduce the potential for handling errors. This in turn may increase the ease of use and thereby reduce treatment-related anxiety [Citation16].
Accordingly, easy-to-use devices may positively influence patient compliance. The convenient and simple handling of a pen injector device specifically designed for use by patients during fertility treatment would be expected to increase adherence to the prescribed treatment regimen and therefore lead to a higher success rate of hormonal treatment [Citation17]. Indeed, recognition of the impact of human factors on reducing risk and potential dosing errors has in the past led to the re-designing of injection pens to improve their safety for handling by patients [Citation18].
The first available pen injector formulation of r-hFSH alfa was Gonal-f® (follitropin alfa), developed by Merck KGaA, Germany (originally Serono S.A.), and registered by the European Medicines Agency (EMA) in 1996. The product is available in ready-to-use, multi-use pens in four dose sizes (150IU, 300IU, 450IU, and 900IU). The pen has the ability to fine-tune doses in 12.5IU increments and has a display window allowing the patient to read the dose, along with a graduated scale on a transparent cartridge.
The first follitropin alfa biosimilar launched in Europe was Bemfola® [Citation2,Citation17], developed by Finox Biotech (Switzerland), a member of the Gedeon Richter group, in collaboration with Ipsomed, a leading designer and manufacturer of pens and auto-injection systems. The product is delivered in a novel, innovative injector pen system for which it received the Red Dot Design Award in 2011, and is designed as a pre-filled, ready-to-use, single-use, disposable pen available in five different doses (75IU/0.125 ml, 150IU/0.25 ml, 225IU/0.375 ml, 300IU/0.50 ml, and 450IU/0.75 ml). It has been assessed by the EMA to have no clinically relevant difference in efficacy and safety profile compared with Gonal-f® [Citation2,Citation17]. Each dosage is differentiated by a different color on the dosage knob of the pen, making it easily identifiable for the user. The Bemfola® pen also allows a precise, fine-tuned dosing adjustment in 12.5IU and 25IU increments with a bi-directional dose dial. The pen is intended for self-injection by patients who have been adequately trained by a qualified healthcare professional, such as a nurse or physician.
Other features of the Bemfola® single-use pen are volume- and injection-control mechanisms, facilitated by visual aids such as colored bars indicating the injection volume. The clearly legible selected dose and a click signal after successful completion of the injection – which confirms the full delivery of the dose – help to avoid dosing errors, which in turn may improve therapy compliance. If patients require a lower dose than the maximum injection volume, another safety feature is an in-built lock, which prevents re-use of the pen device in order to eliminate the possibility of redosing. Despite the single-use nature of Bemfola®, and the possible perception of potential drug wastage and associated costs of unused portions of the pens, a UK-based multicenter analysis of nearly 5,000 FSH treatment cycles showed that if Bemfola® had been used rather than a multi-use formulation, there would have been a potential saving of 61 to 104IU per cycle; across nearly 5,000 cycles this amounted to a reduction in drug wastage of 367,800IU, equivalent to a saving of over £100,000 in total drug costs [Citation19].
Ovaleap® (Theramex Ireland Ltd), is another follitropin alfa biosimilar, originally developed by TEVA, approved in Europe in 2013 and launched in 2016 [Citation20,Citation21]. Like Bemfola®, it has the same approved indication, and a comparable efficacy and safety profile to Gonal-f® [Citation20,Citation21]. Ovaleap® is administered via a re-useable self-injection pen, in which a cartridge of follitropin alfa injection must be loaded before use. The cartridge is available in three dose sizes (300IU, 450IU, and 900IU), all provided in the same physical dimensions and with color coding to differentiate the different cartridge doses. The pen is also able to adjust in 12.5IU increments, has a large dosing window with legible numbers, while a clear cartridge holder lets the patient see how much medication is inside. A dial-back feature allows correction if the dose is set incorrectly, and the injection is administered via the press of a side-button.
The goal of this study was to assess and compare the features of these three different pen injector formulations of follitropin alfa – Bemfola® Pen, Gonal-f® Pen, and Ovaleap® Pen – in a user experience investigation involving patients and nurses. These pens were chosen as they are the most comparable devices in terms of available strengths and doses usually administered, whilst they differ in design and features, and thus allow a valid comparison to be made. Specifically, the Bemfola® pen is a single-use pen whereas the Gonal-f® and Ovaleap® pens are re-useable pens, which can be used on multiple occasions over consecutive days of treatment, using a fixed cartridge system or replaceable cartridges, respectively. The primary objective of this user experience investigation was to evaluate the impact of human factors on the use of Bemfola®, Gonal-f®, and Ovaleap® follitropin alfa pen injectors by patients and nurses, with special focus on understanding the suitability of multi-use versus single-use pens for self-administration by patients during fertility treatment.
2. Methods
2.1. Study design
This study was carried out by reviewing the Instructions for Use (IFU) and dosing steps for each product and identifying potential critical errors and associated risks that could lead to administration errors. According to the International Electrotechnical Commission (IEC) [Citation22] a ‘use error’ is defined as ‘a user action or lack of user action while using the medical device that leads to a different result than that intended by the manufacturer or expected by the user’. Recent publications [Citation23,Citation24] have described the standard types of error that can occur during the use of injector pens and devices. With reference to these guidelines and in consultation with a multi-disciplinary group – which included specialist nurses, an external agency with expertise in user testing, and experts in the field of ART with previous experience in critical error determination – the critical steps in which it was considered an error or omission could pose a safety risk or a dosing risk for patients were determined.
Nurses and patients each completed a usability test in which their performance in assembling and administering doses of each of the pens was assessed against the criteria defined in the IFU critical steps. After completion of the usability tests, the nurses and patients were asked a series of questions to assess their experiences of the handling of each of the pens, during which they were asked to rank the pens in order of preference.
2.2. Participants
During routine clinical use of a follitropin alfa pen there are two main classes of users, both of which were included in this user experience testing: fertility patients who are undergoing controlled ovarian stimulation and who will use the pens to induce multiple follicular stimulation; and specialist fertility nurses who train and supervise patients in the use of the pens.
Women with typical characteristics of fertility patients and having a range of ages, educational backgrounds, and personal situations were recruited via the use of ART clinics. All patients recruited had to be a minimum of 18 years of age and have already had a consultation by a healthcare professional who had discussed the need for ART. At the time of testing, they were completely naïve to all pen systems containing gonadotrophins for fertility treatment. All nurses recruited into the testing were to be experienced fertility nurses, with a maximum age of 60 years, who regularly trained patients in the use of the various medications available for the ART process. Additionally, they had to have a minimum of 2 years’ experience working in the fertility environment, be employed by a site that carried out a minimum of 300 treatment cycles per year, and undertook a minimum of 10 training sessions per month on fertility devices. All participants were native language speakers in their country or had a good level of understanding of the local language. Participants provided written, informed consent. All simulations were video recorded with the participants’ permission.
Evaluations were carried out in a simulated, non-interventional manner (i.e. no actual patient injections were performed), and therefore did not require ethics committee approvals in any of the countries that participated.
Participants were recruited in cities from four European countries (Berlin, Madrid, Paris, and London) during February and March 2020. The interviews were conducted by experienced and appropriately trained research professionals at each of the country locations. All testing was done in the local language, and all questionnaires were completed on-site by the interviewers.
2.3. Materials
The materials used for the testing were commercially available samples of each of the three follitropin alfa pens and were provided in dose sizes suitable for preparing the doses specified in the test. Gonal-f® pre-filled multi-use pens were provided in dose sizes of 450IU and 300IU. Ovaleap® multi-use pen was provided with cartridges of 450IU and 300IU to be placed inside the pen. Bemfola® single-use pens were provided in doses of 150IU and 225IU, corresponding to the doses to be prepared in the user testing.
Each pen was provided together with their corresponding needles for injection, alcohol swabs, an injection sponge, and IFU as per the standard packing.
2.4. Usability testing
In routine clinical practice, most patients will initially be trained in the preparation and administration of pen injections by a nurse in the fertility center before continuing with the treatment course and self-administering the daily injections at home. The nurse should teach the patient how to use the pen in accordance with the respective IFU, a set of pre-defined steps that must be undertaken to ensure correct administration of the dose prescribed by the physician. Some steps in this process are more critical than others, and if these critical steps are not carried out correctly, it could result in a safety issue or administration error resulting in an incorrect dose being administered by the patient. For each pen, the IFU was assessed and the critical steps were identified ().
Table 1. Critical steps identified from the Instructions for Use (IFU) for each product
For the usability test, different scenarios were adopted for the nurses and patients. The first set of testing was carried out with the nurses only. Each nurse was provided with materials sufficient to prepare two 150IU doses of each of the three products (). Each nurse performed a simulated injection of 150IU – one of the most commonly used initial dosing regimens during ART stimulation [Citation19,Citation25,Citation26] – with each of the three pens. To eliminate any bias, the order of assessment of pens followed a rotational plan. During each simulation, the research interviewer recorded the handling of each step of the injection process according to the IFU, to assess whether or not each step had been carried out, whether it had been done correctly, and with particular focus on the critical steps/errors and potential actions that could have resulted in subsequent dosing errors.
Figure 1. Injection pens provided for nurse testing: (a). Gonal-f® pen, 300IU; (b). Ovaleap® pen and 300IU cartridge; (c). Bemfola® single-use pens, 2 × 150IU. Relative product sizes are shown to scale
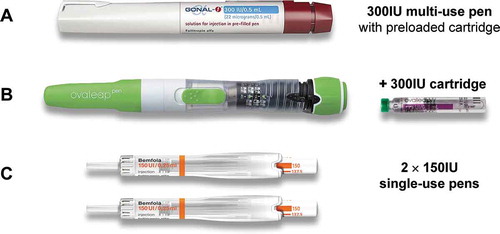
During this process, the research interviewer played the role of a non-participant/non-interventional observer in order not to influence the simulation being carried out by the nurse.
The second set of testing was carried out with the patients, who were provided with materials sufficient to prepare three doses – two 150IU injections and one 225IU injection – of each product (). Each patient was trained by an independent external moderator who was experienced in the use of all three pens. The decision to use an independent moderator, rather than an experienced fertility nurse, was taken further to some trial testing, which was carried out prior to the main study testing. During the trial testing, it became apparent that even experienced fertility nurses were making critical errors with each of the pens, which could result in the same errors and associated risks being passed on to the patients. The authors wanted to ensure that the study tested the correct usage according to the IFU for each product in a fair and comparable manner, and that patients’ performance would not be compromised by suboptimal training. Therefore, the independent moderator’s role was to ensure that patients understood the correct steps for use of each product according to the respective manufacturers’ IFUs.
Figure 2. Dosing scenarios and pen products tested by patients in the usability study: (a). Gonal-f®; (b). Ovaleap®; (c). Bemfola®. Relative product sizes are shown to scale
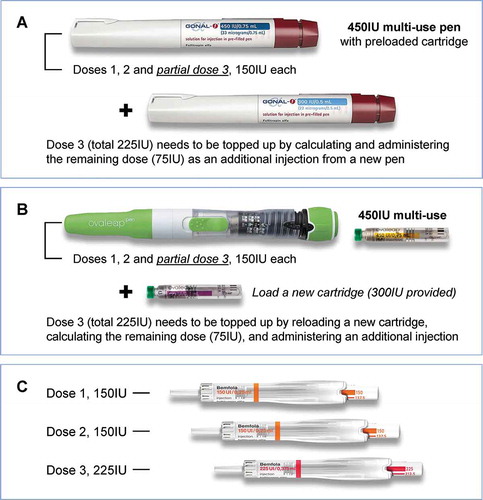
As with the nurses, to eliminate any bias in the patient tests, the order of pen assessments followed a rotational plan. For each pen, the external moderator first trained the patient in how to carry out an injection according to the IFU, using a simulation of a 150IU injection. The patient then simulates a further injection of 150IU, with the external moderator in the room. This scenario was to create a situation as close as possible to a ‘real-life’ training setting, where normally a nurse will train and assist the patient until she is comfortable with the use of the device. At this point, the external moderator left the room, and the patient was required to simulate two further injections: the first, another 150IU injection and the second a 225IU injection. The 225IU simulation was included to test the patient’s confidence and ability to change her dose during the course of treatment, as this situation has been previously described as having a potential risk of error and possible mis-dosing with multi-use pens [Citation24]. To help the patients to anticipate and manage this scenario as they would in a real-life situation, the scenario where a change in dose and pen may be required was explained to them during the training and the relevant section of the IFU was provided to give guidance on the practical steps to take should this situation arise. During each simulation, the research interviewer recorded the handling of each step of the injection process, according to the IFU, to assess whether or not the step had been carried out, and whether it had been completed correctly. The testing scenario was repeated, in turn, for each pen, according to the rotational plan.
In the test scenarios, for the Gonal-f® and Ovaleap® multi-use pens, the patients were first given a 450IU pen or cartridge, which allowed them to simulate three 150IU injections (one for the training with the external moderator, one with the moderator present, and the final as part of the 225IU injection scenario; ). For the final injection, the patient should only have 150IU left in the original pen, and therefore she had to recognize that she needed a second pen or cartridge to complete the 225IU injection. This requirement for a new pen/cartridge was used to simulate the scenario in a normal clinical setting where a doctor may change the daily dose during the treatment course and there may be insufficient drug left in the multi-use pen to administer the next full dose. For the Bemfola® pen, as this is a single-use pen, the patient was given a new pen for each simulated injection ()).
During each injection, the total number of handling errors, defined as any steps missed or carried out incorrectly, versus the total number of steps was recorded. The time taken to prepare each injection by the patients was also recorded.
2.5. Handling testing
After completion of the usability tests, the nurses and patients were asked a series of questions to assess their experiences of the handling of each of the pens, during which they were asked to rank the pens in order of preference. Nurses were asked to assess the following aspects: ease of correct dose selection for the patients (particularly when a dose change is required), confidence that the patient will correctly inject a full dose at home, and the most convenient pen for a patient’s daily life. Patients were asked to assess the following aspects of the pens: overall ease of learning how to use the pen correctly, ease of selecting the correct dose, confidence that she could dose herself correctly at home, and her overall level of stress associated with the use of each of the pens.
2.6. Data analysis
The sample size for this testing was based on Health Authority requirements, which stipulate that a minimum of 15 individuals from each distinct user group (patients and nurses) should participate in a usability test [Citation27,Citation28]. Each step of the injection process was assigned a scoring value where 0 = Operation missed or operation performed incorrectly and 1 = Operation performed correctly. For the steps in the process classified as major/critical, the number of users and percentage assigned a value of 0 was calculated.
As the multi-use pens require more steps overall in comparison to the single-use pens, the analysis of critical errors was calculated as a percentage of the total number of critical steps, rather than as an absolute number of critical errors. Thus, the critical error rate was calculated for each participant in each test according to the formula: critical error rate (%) = [(total number of critical steps omitted or carried out incorrectly)/(total number of critical steps)] × 100.
3. Results
3.1. Participant characteristics
The user testing took place in four countries, Germany, UK, Spain, and France. A total of 43 participants took part, 19 nurses and 24 patients (). The nurses had an average of 8 years of experience in a fertility clinic setting and usually provided training about fertility treatment – including the use of FSH pens – to between 10 and 20 patients per week. The patients included in the testing had a mean age (± SD) of 33 (± 4.9) years.
Table 2. Study participants and summary of inclusion criteria
3.2. Usability testing
summarizes the critical steps identified from the IFUs for each of the three pen products. The number of critical steps required for the whole process of preparing and administering the injections was fewest for the single-use device, whilst the multi-use pens both included additional critical steps, mostly required for preparing the injection and for calculating subsequent doses.
3.2.1. Nurse testing
During simulations of a 150IU injection by the nurses there was a mean overall critical error rate of 4% with the Bemfola® pen, 49% with the Gonal-f® pen, and 40% with the Ovaleap® pen ().
There were two main critical errors made by nurses with the Bemfola® pen. The first critical error was not fixing the needle in place by clicking it on (11% of nurses); instead, most nurses who made this error were trying to screw the needle into place. Nevertheless, in all cases the needle was attached in a successful manner to allow a valid injection to be undertaken. The second critical error made by some nurses (21%) was not holding the needle in the skin for the full 5 seconds after the injection had been administered, as stated in the IFU.
With regards to the Gonal-f® pen, most of the critical errors made by the nurses related to the priming of the pen. According to the IFU for Gonal-f®, once the needle has been attached, the user should check to make sure that a droplet of liquid is seen at the tip of the needle, and if this is not seen then the priming steps recommended for the pen should be carried out. However, nearly 70% of the nurses omitted this step completely and commented that despite the instructions in the IFU, they would not carry out this step, and subsequently would not train patients to carry out this step. Another critical error made by the nurses (75%) was failure to check that the dose window was at 0 prior to setting the dose for injection, which in turn could result in the wrong dose being dialed and subsequently administered. Finally, many nurses (47%) did not hold the needle in the skin for the IFU-recommended 5 seconds after the injection had been administered.
There were several areas of error reported during the nurses’ use of the Ovaleap® pen. The first of these was failing to put the cartridge into the pen correctly and ensuring the plunger was correctly aligned (22% of nurses). The other main areas of error were similar to those observed for the Gonal-f® pen, i.e. failure to check if the dose window was at 0 prior to setting the dose for injection (76% of nurses), omitting the priming step (58%), which according to the IFU for this pen is required prior to each injection, and finally the pen not being held in the skin for 10 seconds, as per IFU instructions (53%).
3.2.2. Patient testing
For the patients, the overall critical error rate was 7% with the Bemfola® pen, 38% with the Gonal-f® pen, and 16% with the Ovaleap® pen ().
For the Bemfola® pen, as with the nurses, there were two main critical errors made by patients: fixing the needle in place by trying to screw it into the device rather than clicking it on (11%); and not holding the needle in the skin for the recommended 5 seconds after the injection had been administered (17%). However, in all cases the needle was attached in a successful manner to allow a valid injection to be undertaken.
Patients made similar errors to the nurses during Gonal-f® pen manipulations: failure to check whether priming was required (52%), and failure to prime the injection when required (63%). Over 75% of patients omitted to check if the dose window was back at ‘0ʹ prior to an injection and nearly 40% did not hold the injection in the skin for the recommended 5 seconds after the injection.
For the Ovaleap® pen, patients also made similar errors to the nurses regarding not checking if the dose window was at ‘0ʹ prior to setting a dose (70%). However, with this pen, the patients were more diligent in the priming of the device than the nurses and only 18% made an error at this step. The one step during which the patients made more errors than the nurses with Ovaleap® was the actual administration of the injection, where many patients tried to use the button on the top of the pen (dial-back button), rather than the side button that delivers the injection. In a real-world situation, this could potentially have resulted in either no dose being administered (if the patient did not check that the dose window had returned to 0 to confirm that the dose had been injected); or a decrease in dose being administered (if she pushed the top button and then realized her mistake, but went on to push the correct administration button without re-adjusting to the correct dose).
The last scenario during the patient usability testing was the simulation of a 225IU injection. With the Bemfola® pen, this did not result in any difficulties as the single-use design allowed patients to use a new 225IU pen and administer the full dose in one injection as described in the IFU. However, for both the Gonal-f® and Ovaleap® pens, the need to use a second pen or cartridge to complete the dose was generally found to be complicated and led to confusion for the patients. The majority of patients were unaware that they did not have sufficient drug in the original device to administer the 225IU dose and tried to administer the dose, without checking the dosing window after the injection had been simulated, or ignoring the dose remaining in the window. Several other patients tried to calculate manually the amount of drug left in the pen, trying to account for any priming they had carried out, which in turn led to additional stress and erroneous calculations.
Regarding the time taken for the patients to carry out the injections, the Bemfola® pen was observed to take the shortest average time (2.12 minutes), compared with 3.28 minutes for Gonal-f® and 5.07 minutes for Ovaleap®. For four patients, the time taken to prepare and carry out the simulated injection with the Ovaleap® pen was over 10 minutes.
3.3. Handling testing
In relation to the ease of selecting the correct dose, 68% of the nurses chose the Bemfola® pen as their preferred option, whilst 32% of nurses chose the Gonal-f® pen. In a similar trend, 63% of nurses chose the Bemfola® pen as the one with which they would have the most confidence that a patient would dose herself correctly at home, compared with 37% for the Gonal-f® pen. Overall, 63% of the nurses also chose the Bemfola® pen as the one they felt to be the most convenient for patients, with 37% choosing the Gonal-f® pen. None of the nurses chose the Ovaleap® pen in any of these preference assessments: they all reported that it was the most difficult with which to select the correct dose – and therefore gave them the least confidence that the patient would dose themselves correctly at home – and the pen that would be the most inconvenient for a patient’s daily life ()).
Figure 3. Critical error rates recorded during the pen usability study. Critical error rate (%) = [(total number of critical steps omitted or carried out incorrectly) / (total number of critical steps)] × 100.
![Figure 3. Critical error rates recorded during the pen usability study. Critical error rate (%) = [(total number of critical steps omitted or carried out incorrectly) / (total number of critical steps)] × 100.Figure 4](/cms/asset/131a3724-cf7b-4f22-b23e-6a7b92c22a7d/iedd_a_1863944_f0003_oc.jpg)
Figure 4. Results of preference rankings in the handling tests. Data show the product preferred in each aspect of handling characteristics by (a) Nurses (N = 19) and (b) Patients (N = 24)
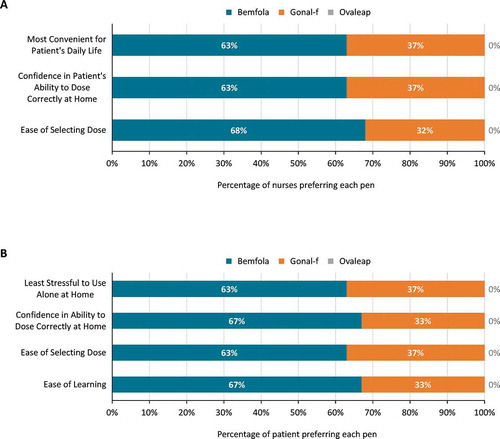
In the patients’ preference assessments, 67% chose Bemfola® as the pen that was the easiest to learn, compared with 29% who chose Gonal-f®. Bemfola® was considered the easiest with which to select the correct dose by 63% of patients compared with 37% who chose Gonal-f®, and 67% chose the Bemfola® pen as the one with which they would have most confidence in dosing themselves correctly at home, compared with 29% who chose Gonal-f®. Overall, 63% of the patients chose Bemfola® as the pen they would find least stressful to use alone at home, compared with 37% who chose the Gonal-f® pen. As in the nurse assessments, the Ovaleap® pen was ranked last for all patient preference assessments ()).
4. Discussion
A number of different pen injector devices have been developed to enable women undergoing ART to self-administer the daily injections of r-hFSH required in controlled ovarian stimulation protocols. This study aimed to compare the features and usability of three of these products and their safety implications, with a particular focus on comparing single-use versus multi-use devices.
For single-use pens there are, overall, fewer steps involved in the process of preparing and administering injections in comparison to the multi-use pens, and this in turn means that there are fewer steps at which a critical error leading to a dosing error can occur. During the testing, it was clearly observed that there was a higher risk of critical errors with the multi-use pens than with the single-use pen. This was particularly apparent when a dose change was required and there was insufficient product left in a multi-use pen to administer the required dose. The majority of patients either did not realize they had insufficient product and did not check the dosing windows to ensure a full dose had been administered, or tried to calculate the dosing manually, adjusting for any priming that had been required. This reflects a scenario that is commonplace in the real-world setting and raises the possibility that dosing errors may occur even with devices intended to facilitate self-dosing by patients. The most worrying aspect of this was that in most cases the patients did not realize that they had made an error and could have unknowingly given themselves a smaller dose than that prescribed by the physician. For the multi-use pens, in particular Gonal-f®, a diary card is provided to the patients to record their daily dose, and to assist with calculating the amount of drug left in the pen after each injection, and the potential need to complete a dose with a second pen. However, the task of having to complete a diary card further adds to the overall burden of treatment and does not necessarily ensure that part-doses are correctly calculated and combined to deliver the correct full dose. This was especially apparent during the current testing, where for example there was confusion about the amount of drug used in a priming step, when required, and whether this should be subtracted from the total dose; this in turn added to the complexity in understanding exactly how much dose was available in the pen. Given that the burden of treatment is one of the main reasons a patient may discontinue or make a mistake during treatment, this added stress of dose calculation only adds to the overall complexity of the use of multi-use pens for the patient.
Minimizing drug administration errors is essential to optimize the chance of a successful outcome of an ART treatment. The failure of an ART cycle has important consequences in terms of economics, time, emotional, and even organizational costs for both patients and healthcare providers. The fact that many drug administration errors may go undetected is of special concern as in many situations, particularly a failed cycle, the next cycle dose adjustment will be based on previous cycle response. This could have the result of a doctor increasing the patient’s dose, thinking that the failed cycle was due to an insufficient dose, whereas it was actually the result of the patient unknowingly injecting the incorrect dose. Unnecessary increases in dose have the potential to increase the risk of overstimulation, which can lead to ovarian hyperstimulation syndrome, a potentially life-threatening exaggerated response to ovarian stimulation regimens. For those reasons, a pen that is easy to use in terms of both correct dose selection and self-injection is highly likely to minimize any drug administration mistakes. The results of the current study showed that the single-use Bemfola® pen was the clearly preferred option among the devices tested for both patients and nurses with respect to ease of selecting the dose and having the confidence to inject a correct dose at home.
An important finding during the usability testing was that approximately three-quarters of nurses did not follow the IFU with regards to the priming steps, when required, for the Gonal-f® and Ovaleap® pens and, consequently, said that they would not teach these steps to the patients. For Gonal-f®, this might be explained in part by the fact that the pen has been on the market for the longest period of time and the nurses might consider that they were already familiar with its use. However, a similar level of incorrect use was also observed with Ovaleap®. This finding has important consequences on patient education, since the skills that the nurse passes on will obviously influence how a patient uses the pens and could again result in potential dosing errors in the real world. In addition, given that in a normal environment the more experienced nurses are responsible for teaching any new/less experienced nurses, if these errors in procedure are passed on to the next generation of trainers, they will in turn pass these errors to any patients they subsequently train. For the Bemfola® pen this was not an issue as the priming of the pen is an integral part of the pen functioning, and as such cannot be omitted.
Another common critical error by both nurses and patients – observed in approximately 20% of single-use pen test injections and around half of the multi-use pen tests – was failure to hold the needle in the skin for the IFU-recommended 5–10 seconds after injection. Since the single- versus multi-use functionalities of the pens are not expected to impact on the actual injection process, the reason for the apparent difference between single-and multi-use pens in compliance to this step is unclear. The authors propose two underlying factors that might have contributed to these findings. Firstly, as there are fewer steps involved in preparing the single-use pen injections, reflected in the shorter average time to complete the whole procedure, users are more likely to remember the correct injection instructions, whereas multi-use pen users might be more focused on the larger number of steps required to prepare the injection and may have forgotten the instructions for injection by the time they reach the end of the procedure. A second possible factor might be the difference in size between the single- and multi-use pen devices: the Bemfola® pen device is lighter and smaller to hold than the other pens, and this may allow the user to hold the pen more stably for the required duration without feeling the need to remove it more quickly. On the other hand, differences in the IFU-recommended duration of injection – 5 seconds for Bemfola® and Gonal-F® and 10 seconds for Ovaleap® – did not appear to influence these findings, since there was no noticeable difference between Gonal-F® and Ovaleap® in the observed critical error rates for this step.
An interesting observation made during the user testing were comments from several nurses and many of the patients that they felt the single-use pens were more hygienic than the multi-use pens. They commented that they would feel much more secure from a hygiene perspective with the single-use pens, as they are only removed from their original packaging when they are needed for use, then disposed of after injection, whereas the multi-use pens could be left on surfaces, touched/used on multiple occasions, and this in turn could increase the risk of microbial contamination. For patients, this consideration was particularly important in the current circumstances of the COVID-19 pandemic, where at the time of the study, strict infection control measures were affecting every aspect of daily living in countries throughout the world.
The current testing, however, did have some limitations. Firstly, this was a simulated scenario in which none of the prepared injections was actually administered to the patient, a factor that might have influenced the attitude of participants toward the injection process. Secondly, during the patient user testing, the external moderator only remained with the patient for the first injection simulation for each device, leaving her alone to prepare the injections for all subsequent scenarios. In real life, the patient would always have the ability to call a nurse or experienced person to ask any questions she had about the correct steps required to prepare and carry out the injection. Another study limitation was the fact that the Gonal-f® pen has been on the market longer than the other devices, and the nurse participants were generally more familiar with its use, which might have biased their performance or opinions across the range of pens tested.
5. Conclusion
Among the different follitropin alfa pen injection devices available for use in controlled ovarian stimulation regimens self-administered by patients during ART, there are several critical errors that can occur during the process of preparing and injecting prescribed doses, which can lead to dosing errors that potentially compromise patient safety and the success of ART. The results of this user experience study demonstrate that single-use pens, requiring fewer critical steps in their preparation and administration, are associated with fewer critical errors and higher user preference scores than the multi-use pens. Furthermore, the single-use pens are felt to be more convenient and more hygienic to use than the multi-use options. Overall, both nurses and patients have more confidence in the use of the single-use pens to deliver correct doses, which could in turn be reflected in both treatment outcomes and patient safety. The study also highlighted the importance of following the IFUs in accordance with the approved product labeling, especially when experienced nurses are training patients or less experienced nurses. Incorrect instruction, particularly with the multi-dose pens, can lead to patient self-administration errors and in turn could compromise patient safety and lead to a less favorable ART cycle outcome.
Declaration of interest
H Saunders and H Donat are employed by PregLem S.A.; M Messner and M Reder are employed by Point-Blank International; L Bjärgestad Lamp is employed by Stockholm IVF, Stockholm, Sweden and has received honoraria to participate in advisory board meetings for Preglem/Gedeon Richter; H Kendrew is employed by the CARE Fertility Group, England, UK and has received honoraria to participate in advisory board meetings for Preglem/Gedeon Richter and Theramex. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial support and assistance in the preparation of figures were provided by Joanne Fitz-Gerald and Jonathan A C Lee at FourWave Medical Communications, the funding for which was provided by PregLem S.A./Gedeon Richter.
Additional information
Funding
References
- Papanikolaou EG, Kolibianakis E, Devroey P. Emerging drugs in assisted reproduction. Expert Opin Emerg Drugs. 2005;10(2):425–440.
- Rettenbacher M, Andersen AN, Garcia-Velasco JA, et al. A multi-centre phase 3 study comparing efficacy and safety of Bemfola® versus Gonal-f® in women undergoing ovarian stimulation for IVF. Reprod Biomed Online. 2015;30(5):504–513.
- Ferrando M, Coroleu B, Rodríguez-Tabernero L, et al. The continuum of ovarian response leading to BIRTH, a real world study of ART in Spain. Fertil Res Pract. 2020;6:13.
- Allahbadia GN. The ideal stimulation protocol: is there one? J Obstet Gynaecol India. 2015;65(6):357–361.
- Howles CM, Saunders H, Alam V, et al. Predictive factors and a corresponding treatment algorithm for controlled ovarian stimulation in patients treated with recombinant human follicle stimulating hormone (follitropin alfa) during assisted reproduction technology (ART) procedures. An analysis of 1378 patients. Curr Med Res Opin. 2006;22(5):907–918.
- Weiss N. Gonadotrophin products: empowering patients to choose the product that meets their needs. Reprod Biomed Online. 2007;15(1):31–37.
- Schertz JC, Saunders H, Hecker C, et al. The redesigned follitropin alfa pen injector: results of the patient and nurse human factors usability testing. Expert Opin Drug Deliv. 2011;8(9):1111–1120.
- Steinke DT, Zarroug OH, Mathur R, et al. Qualitative risk assessment of follicle stimulating hormone injectable products. Expert Opin Drug Deliv. 2020;17(11):1647–1654.
- Domar AD, Rooney K, Hacker MR, et al. Burden of care is the primary reason why insured women terminate in vitro fertilization treatment. Fertil Steril. 2018;109(6):1121–1126.
- Lunenfeld B. Historical perspectives in gonadotrophin therapy. Hum Reprod Update. 2004;10(6):453–467.
- de Mora F, Fauser B. Biosimilars to recombinant human FSH medicines: comparable efficacy and safety to the original biologic. Reprod Biomed Online. 2017;35(1):81–86.
- US Department of Health and Human Services Food and Drug Administration (FDA). Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring, MD: FDA; 2009. cited 2020 Oct 19. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims
- Rubin RR, Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care. 2004;27(10):2495–2497.
- Asakura T, Jensen KH. Comparison of intuitiveness, ease of use, and preference in two insulin pens. J Diabetes Sci Technol. 2009;3(2):312–319.
- Gameiro S, Boivin J, Peronace L, et al. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update. 2012;18(6):652–669.
- Longobardi S, Seidler A, Martins J, et al. An evaluation of the use and handling errors of currently available recombinant human follicle-stimulating hormone pen injectors by women with infertility and fertility nurses. Expert Opin Drug Deliv. 2019;16(9):1003–1014.
- Imthurn B, McVeigh E, Stiller R, et al. Evaluation of the use and handling of three different pen systems considered for in vitro fertilization treatment. Expert Opin Drug Deliv. 2014;11(12):1859–1864.
- Mahony MC, Patterson P, Hayward B, et al. Human factors engineering and design validation for the redesigned follitropin alfa pen injection device. Expert Opin Drug Deliv. 2015;12(5):715–725.
- Foxon G, Mitchell P, Turner N, et al. Bemfola(R) fixed dose pens potentially reduce drug wastage and associated costs of infertility treatment. Hum Fertil (Camb). 2018;21(4):275–280.
- Strowitzki T, Kuczynski W, Mueller A, et al. Randomized, active-controlled, comparative phase 3 efficacy and safety equivalence trial of Ovaleap® (recombinant human follicle-stimulating hormone) in infertile women using assisted reproduction technology (ART). Reprod Biol Endocrinol. 2016;14:1.
- Strowitzki T, Kuczynski W, Mueller A, et al. Safety and efficacy of Ovaleap® (recombinant human follicle-stimulating hormone) for up to 3 cycles in infertile women using assisted reproductive technology: a phase 3 open-label follow-up to main study. Reprod Biol Endocrinol. 2016;14(1):31.
- International Electrotechnical Commission (IEC). IEC 62366-1. Medical devices - Part 1: Application of usability engineering to medical devices. Geneva: International Electrotechnical Commission; 2015.
- Weinhold T, Del Zotto M, Rochat J, et al. Improving the safety of disposable auto-injection devices: a systematic review of use errors. AAPS Open. 2018;4(1):7.
- Mahony M, Dwyer A, Barkume R, et al. US human factors engineering evaluation of an updated follitropin alfa pen injector (GONAL-f®) RFF Redi-ject®) and instructions for use. Expert Opin Drug Deliv. 2018;15(1):5–15.
- Rombauts L. Is there a recommended maximum starting dose of FSH in IVF? J Assist Reprod Genet. 2007;24(8):343–349.
- Popovic-Todorovic B, Loft A, Bredkjaeer HE, et al. A prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a ‘standard’ dose of 150 IU/day in ‘standard’ patients undergoing IVF/ICSI treatment. Hum Reprod. 2003;18(11):2275–2282.
- Association for the Advancement of Medical Instrumentation (AAMI). Human factors design process for medical devices. Report No.: ANSI/AAMI HE 74:2001. Arlington, VA: Association for the Advancement of Medical Instrumentation; 2001.
- Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Methods Instrum Comput. 2003;35(3):379–383.
