ABSTRACT
Introduction
Poor or inconsistent adherence to daily oral pre-exposure prophylaxis (PrEP) has emerged as a key barrier to effective HIV prevention. The advent of potent long-acting (LA) antiretrovirals (ARVs) in conjunction with advances in controlled release technologies has enabled LA ARV drug delivery systems (DDS) capable of providing extended dosing intervals and overcome the challenge of suboptimal drug adherence with daily oral dosing.
Areas covered
This review discusses the current state of the LA PrEP field, recent advances, and emerging technologies, including ARV prodrug modifications and new DDS. Technological challenges, knowledge gaps, preclinical testing considerations, and future directions important in the context of clinical translation and implementation of LA HIV PrEP are discussed.
Expert opinion
The HIV prevention field is evolving faster than ever and the bar for developing next-generation LA HIV prevention options continues to rise. The requirements for viable LA PrEP products to be implemented in resource-limited settings are challenging, necessitating proactive consideration and product modifications during the design and testing of promising new candidates. If successfully translated, next-generation LA PrEP that are safe, affordable, highly effective, and accepted by both end-users and key stakeholders will offer significant potential to curb the HIV pandemic.
1. Introduction
The HIV/AIDS epidemic remains a major global health crisis, with approximately 37.7 million people currently living with HIV, 1.5 million people newly infected, and 28.2 million accessing antiretroviral (ARV) therapy (ART) as of June 2021 [Citation1]. While we are still far from achieving UNAIDS (The Joint United Nations Programme on HIV/AIDS) global targets for reducing annual HIV infections and AIDS-related deaths, one of the top priorities for successfully reducing HIV sexual transmission and infection is to develop safe, effective, and highly acceptable products that can fit the needs and desires of end-users and health-care providers (HCP) globally, minimize burden on the health-care system, and readily promote uptake and adherence [Citation2,Citation3].
Currently, several ARVs targeting different stages of the HIV replication process are clinically available for treatment or pre-exposure prophylaxis (PrEP) [Citation4,Citation5] to prevent infection in at-risk uninfected individuals. The primary route of administration of marketed ARVs is via daily oral pills in single-tablet regimens, in large part due to a simplified dosage regimen, patient convenience, and low cost of manufacturing. However, many ARVs have a short plasma half-life and first-pass metabolism, which necessitates high daily oral doses in order to maintain therapeutic and/or prophylactic concentrations, thus presenting significant adherence challenges. There remain additional challenges for daily oral ARV application: (a) pill burden and fatigue, which can negatively affect adherence; (b) degradation of sensitive molecules by an acidic/enzymatic gastrointestinal (GI) environment; (c) prevention of drug absorption by the GI mucus barrier that leads to low oral bioavailability and therapeutic concentration in plasma [Citation6]; and (d) need for strict adherence to the ARV regimen to prevent emergence of resistant viral strains in infected individuals [Citation7]. Due to these issues, frequent high oral doses of ARVs must be taken throughout an individual’s sexually active life, also raising concerns about drug-related toxicities. Thus, there is considerable interest in the development of long-acting (LA) ARVs and drug delivery systems (DDS) for HIV prevention or treatment.
Several excellent reviews have been published in recent years that discuss LA formulations for HIV prevention (and treatment) from various perspectives, including those focused on multipurpose prevention technologies (MPTs) (e.g. HIV prevention combined with contraception) [Citation8,Citation9], clinical pharmacokinetics (PK) [Citation10], and health inequity viewpoints, as well as recent formulation-, ARV-, and antibody-based LA PrEP strategies [Citation10–12]. Yet, because the HIV prevention R&D landscape is evolving faster than ever, this review aims to provide a current update on the state of the field, inclusive of the many recent successes, setbacks, and challenges ahead. Further, this manuscript will review recent advances in next-generation LA PrEP active and controlled-release DDS strategies, including their advantages, disadvantages, and anticipated challenges for clinical translation. A roadmap of LA HIV prevention product development combined with the key technological challenges, knowledge gaps, preclinical testing considerations, and future directions for clinical advancements and the development of LA systems integrating end-user perspective is also provided.
1.1. State of the field and the need for next-generation LA HIV prophylaxis approaches
The prevention of new infections has arisen as a prominent approach for curtailing the HIV epidemic. Tenofovir disoproxil fumarate/emtricitabine (TDF/FTC; Truvada®), the first approved daily oral PrEP regimen, reduces the risk of getting HIV from sex by about 99% with perfect adherence [Citation13,Citation14] and by more than 75% with typical use [Citation15], although this is dependent on the population of users. Several large-scale clinical trials of oral PrEP, such as VOICE, FEM-PrEP, iPrEx, and TDF2, have had adherence estimates ranging from 29% to 79% that have greatly affected efficacy across these study populations [Citation16]. Thus, the development of a more potent, less user-dependent, longer-acting dosing regimen has become a necessary option to overcome challenges to consistent use and poor adherence.
With a positive opinion (Article 58) by the European Medicines Agency in July 2020 for use by women 18 years and older in developing countries, WHO recommendation and prequalification, and regulatory authority approvals since received in five African countries and counting [Citation17], the dapivirine vaginal ring (DVR) has recently become an additional long-acting preventative option for African women. Developed by the International Partnership for Microbicides (IPM) and very recently acquired by the Population Council [Citation18], the DVR is a flexible silicone ring that releases dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), locally in the vagina, providing protection for 1 month. Approvals are based on the statistically significant results from two double-blinded placebo-controlled Phase 3 clinical trials, the Ring Study (IPM-027) and ASPIRE (MTN-020), in which no severe treatment adverse events and an approximate 30% reduction in a woman’s risk of HIV infection were observed [Citation19,Citation20]. A secondary analysis revealed a reduction in the risk of HIV ranging from 75% to 91% for participants with consistent use of the ring [Citation21]. Follow-up open-label Phase 3b trials, DREAM (IPM-032) and HOPE (MTN-025), found an HIV incidence of 1.8 and 2.7 per 100 person years, respectively [Citation22,Citation23]. The REACH study (MTN-034), where women were randomized to DVR and daily oral TDF/FTC in a crossover design and then allowed to choose, has also shown that adolescent girls and young women can have high adherence to the ring [Citation24]. While the DVR is now a tangible PrEP option for many women at high risk and can be a desirable choice for HIV PrEP, it comes with limitations, such as use in women only, protection for 1 month, user-dependence, potential for urogenital side effects, and need for frequent refills [Citation20,Citation23].
Recently, the LA cabotegravir (CAB, an integrase inhibitor) intramuscular (IM) injectable nanosuspension [Citation25] from ViiV Healthcare became the first US FDA (the United States Food and Drug Administration) approved LA systemic product (called APRETUDE) for HIV-1 prevention [Citation26]. In two large Phase 3 trials (HIV Prevention Trials Network (HPTN) 083 and 084) [Citation27,Citation28], CAB LA administered once every 2 months was more effective than daily oral TDF/FTC for HIV prevention. In December 2021 and following a fast-track review process, the US FDA approved CAB LA for use in at-risk adults and adolescents (≥35 kg) for PrEP to reduce the risk of sexually acquired HIV. With impressive effectiveness rates (69% lower incidence of HIV compared to daily oral TDF/FTC in HPTN 083 and 90% lower incidence of HIV compared to TDF/FTC in HPTN 084) and excellent safety profile, CAB LA represents a great advance for the HIV prevention field. However, CAB LA has several suboptimal characteristics that may limit its implementation in developing countries and widespread acceptability. These include (a) high dosing volumes of IM injection (≥3 ml); (b) dosing frequency of every 2 months requiring frequent HCP visits; (c) non-removability of the dosage form in case of adverse reactions or a change in user preference; (d) current high cost of the final product; and (e) long-term sub-therapeutic PK tail after PrEP discontinuation. In the HPTN 077 trial, a Phase 2a study of safety, tolerability, and acceptability of oral and injectable CAB for PrEP in HIV uninfected men and women, the median time from the last injection to the time when CAB decreased below the detectable level was 43.7 and 67.3 weeks for male and female participants, respectively [Citation29]. Collectively, these limitations of CAB LA nanosuspension highlight the opportunities for further improvements such as reduced injection volume, alternate formulation type, different route of administration, prolonged dosing interval, and shortened PK tail. Numerous R&D efforts are ongoing to address these shortcomings. To help mitigate the high cost of CAB LA [Citation30] in the nearer term, ViiV Healthcare has signed a license agreement with Medicines Patent Pool to enable more affordable, at-scale access to CAB LA in low- and middle-income countries (LMICs) by allowing select generic manufacturers to supply generic CAB LA products in 90 different countries, concomitant with regulatory approval there [Citation31].
While these recent advances in the field have resulted in additional options for HIV prevention that have expanded method choice beyond daily oral pills, there are still shortcomings and gaps in coverage with the current product landscape. Therefore, the development of novel LA technologies (including those to further improve upon the first-generation DVR and CAB LA products) remains a priority for HIV research. To that end, investigation of novel mechanisms of action for HIV PrEP has proceeded across the LA prevention product development field and a number of promising compounds have risen to prominence.
Islatravir (EFdA, MK-8591, ISL) is a highly potent nucleoside reverse transcriptase translocation inhibitor (NRTTI) with substantial efficacy in HIV prevention with doses as low as 0.1 mg/kg/day [Citation32,Citation33]. In December 2021, however, ISL clinical trials were put on hold because of safety concerns related to dose dependent decreases in total lymphocyte and CD4 counts in participants [Citation34]. LA formulations such as subcutaneous (SC) implants are also being explored [Citation35] for EFdA (discussed later in this manuscript) but are also in clinical hold for similar safety concerns [Citation34].
Lenacapavir (LEN, formerly known as GS-6207 or GS-CA2), in development by Gilead Sciences, Inc., is the most advanced of a new class of ARVs called capsid inhibitors, which have shown high picomolar antiviral potency, metabolic stability, low aqueous solubility and systemic clearance, and long half-life, which allows for an ultra-long, extended-release SC administration [Citation36,Citation37]. LEN is currently in two Phase 3 trials (PURPOSE-1, NCT04994509; PURPOSE-2, NCT04925752) as a subcutaneous injectable given once every 6 months for HIV prevention [Citation38] and was just recently approved in the European Union for treatment in adults with multi-drug resistant HIV infection [Citation39]. PURPOSE 3 and 4, two acceptability studies, are currently in planning stages in collaboration with the NIH HIV Prevention Trials Network (HPTN) [Citation40]. A previous hold on trials of LEN issued by the FDA in December 2021 due to concerns over borosilicate vial compatibility with injectable formulations was lifted in May 2022 following review of an alternate plan to use aluminosilicate vials [Citation41].
The identification of broadly neutralizing antibodies (bnAbs) as an immunoprophylaxis approach to HIV prevention has generated substantial interest in recent years, demonstrating robust protection in animal models [Citation42–44] and favorable safety, tolerability, and PK profiles when tested alone or in combinations in both HIV-infected and uninfected individuals in early clinical trials [Citation45,Citation46]. Linker substitution mutated bnAbs (called ‘LS’ for substituted amino acids) have been designed for extended half-life and neutralizing activity by increased binding affinity to the neonatal Fc receptor [Citation47]. Phase 1 clinical trials of intravenously (IV) or SC delivered VRC01-LS and VRC07-523LS in healthy adults confirmed their enhanced safety, tolerability, and half-life while maintaining neutralizing activity [Citation48,Citation49]. Bi- and tri-specific bnAbs targeting different regions of Env-protein are also being explored [Citation50,Citation51], and other potent bnAbs (e.g. 3BNC117, PGT121, and 10–1074) are currently being tested in clinical trials [Citation45,Citation46,Citation52,Citation53]. The increased potency and half-life of newly developed bnAbs could potentially enable LA PrEP products for 6-month dosing by SC injection. However, there is evidence to suggest that bnAb monotherapies or even combinations of two are insufficient to prevent viral escape due to the diversity of HIV, necessitating a use of three or more bnAbs in a single formulation, which will increase the cost and complexity of therapeutic design and developing an appropriate DDS [Citation54,Citation55].
2. Approaches to improving existing ARVs for LA HIV prevention
The majority of ARVs are not well suited for developing as LA products using conventional approaches, due to relatively low potency and/or inadequate physicochemical properties. Approaches to enhance the LA potential of existing ARVs, thereby extending the dosing intervals for better adherence, include structural modification to the dug itself or encapsulation into novel LA DDS. These approaches are briefly discussed below.
2.1. Prodrug chemical modification for generating potent LA ARVs
A substantial improvement in potency of existing ARVs can be achieved by prodrug modification, which may also allow their efficient encapsulation into an LA DDS. Prodrugs in general do not possess intrinsic biological activity but are designed to be transformed in vivo into the active drug [Citation56,Citation57]. The approaches to developing LA ARV prodrugs (all under preclinical testing) are discussed below and illustrated in .
Figure 1. Schematic representation of prodrug technologies for generating long-acting (LA) antiretrovirals (ARVs). (a) ProTide prodrug concept; (b) Fatty acid ester prodrug conjugates; (c) Drugamer-based prodrug approach for TAF (Reproduced with permission from [Citation76]).
![Figure 1. Schematic representation of prodrug technologies for generating long-acting (LA) antiretrovirals (ARVs). (a) ProTide prodrug concept; (b) Fatty acid ester prodrug conjugates; (c) Drugamer-based prodrug approach for TAF (Reproduced with permission from [Citation76]).](/cms/asset/cf50871a-85e0-4eaf-8604-bf65c21ada60/iedd_a_2135699_f0001_oc.jpg)
2.1.1. ProTide prodrugs
The ProTide approach was designed to deliver nucleotide analogues intracellularly by masking the phosphate group with an amino acid ester and an aryl moiety [Citation58–60]. This approach has been used to increase the plasma stability and to reduce the toxicities of tenofovir (TFV) through the development of TAF prodrug [Citation61]. ProTide prodrugs of FTC [Citation62], lamivudine (3TC) [Citation63], and TFV [Citation64] were synthesized and subsequently encapsulated into poloxamer nanocrystals to further extend their half-life, intracellular delivery, retention, and antiretroviral activities. GS-9131 (rovafovir etalafenamide), a prodrug of nucleotide analog GS-9148, has also been designed to enhance the cellular permeation and delivery of the parent drug [Citation65–67].
2.1.2. Lipophilic fatty acid ester prodrugs
In this approach, the drugs are linked to fatty acids generally using an activating agent, resulting in an ester or amide-linked conjugates. Agarwal et al. showed that the fatty acid prodrugs of FTC [Citation68] and 3TC [Citation69] improved anti-HIV activity and cellular uptake. Other examples in this category include fatty acid conjugates of CAB [Citation70] and dolutegravir (DTG) [Citation71], later developed as LA injectable poloxamer nanocrystal formulations [Citation72,Citation73]. The palmitic acid prodrug of DTG has been formulated into biodegradable microparticles [Citation74], and PK evaluation in rabbits (SC injection, 30 mg/kg) showed that the IC90 of DTG was maintained for >3 months. LA polyunsaturated fatty-acid prodrugs of FTC and elvitegravir (EVG) exhibited superior antiviral activities in HIV replication tissue models and LA characteristics in mice [Citation75].
2.1.3. Drug-polymer conjugates
The recently developed ‘drugamer’ technology involves conjugating drug through a linker to produce a prodrug vinyl monomer, which is then polymerized into injectable depots [Citation76]. This platform was recently used for TAF and demonstrated maintaining drug stability and a controlled, sustained drug release from an SC hydrogel reservoir (through hydrolysis or enzyme-mediated cleavage at the linker site) for 2 months in mice [Citation76]. Conjugation of ARVs with polypeptides and polysaccharides has previously been shown to improve drug activity and cellular uptake [Citation77–79]. The drugamer technology offers several key advantages, including high drug loading capability, control over drug release rates and degradation kinetics for the depot, and increased drug substance stability due to the molecular architecture of the polymer formulation [Citation76]. The technology also offers scalable clinical manufacturing of the final product.
Overall, significant development has happened in prodrug modification for LA HIV prevention approaches. In general, the formulation of a clinically relevant and viable prodrug requires the balancing of a number of factors to achieve an efficient prodrug design. This includes a comprehensive understanding of the physicochemical/biological properties of the parent drug to produce the desired improvements while maintaining its safety, stability, and efficacy. Sophisticated analytical methods are also required to analyze the prodrug, parent drug, and the released by-products (chemical linker, pro-moiety) in the same sample.
2.2. Drug delivery technologies for LA HIV prophylaxis
The prodrug approach sustains the drug release by modulating physicochemical and PK properties, but further control in LA drug release can also be achieved by modifying the DDS. Improved innovative methods for controlled/extended delivery of potent ARVs are currently being investigated or are in advanced preclinical stages to deliver active drugs over months. The most promising developments in this area are discussed below and summarized in (for subdermal/SC reservoir or matrix-type implants) and (for DDS other than subdermal/SC implants).
Table 1. Long-acting (LA) HIV PrEP subdermal/subcutaneous reservoir or matrix-type implants in clinical and preclinical phases.
Table 2. Long-acting (LA) HIV PrEP formulations (other than reservoir or matrix-type implants) in clinical and preclinical phases.
2.2.1. Subdermal/subcutaneous (SC) implants
Implants have the potential to provide a controlled drug release for prolonged duration and can be removed if safety or acceptability concerns arise. Several LA biodegradable or non-biodegradable implants containing potent ARVs (e.g. EFdA, TAF, and CAB) with different designs are currently in preclinical and clinical development stages ().
Figure 2. Long-acting (LA) subdermal/subcutaneous implants in clinical development. (a) Merck EFdA implant (Reproduced with permission from [Citation35]); (b) RTI thin film TAF implant (Reproduced with permission from [Citation85] under the terms and conditions of the Creative Commons Attribution (CC BY) license http://creativecommons.org/licenses/by/4.0/); (c) Oak Crest TAF subdermal implant (Reproduced with permission from [Citation95]) (Three-dimensional model (A) and cross-sectional drawings (B and C) of TAF implant. The TAF core (black) inside the silicone scaffold with PVA membrane coating is shown (not to scale). Cross sections were sliced through the y-z (B) and x-y planes (C). (d) HMRI nanofluidic refillable CAB implant (Reproduced with permission from [Citation98]) ((A) Rendered image of cross-section of polyether ether ketone (PEEK) (left) and titanium (right) drug reservoirs. (B) Assembled PEEK βCAB (left) and titanium CAB (middle) drug reservoirs and 13 nm nanofluidic membrane (right). (C) SEM image of nanochannel membrane cross-section displaying drug release through perpendicular microchannels and horizontal nanochannels r, reservoir, e, epoxy, m, nanochannel membrane, s, implant shell); (e) HMRI nanofluidic refillable TAF/FTC implant (Reproduced with permission from [Citation93]) ((G) Cross-sectional rendering of nanochannel delivery implant depicting drug refill needles through the loading ports with resealable silicone plugs. (H) Nanochannel membranes of two different sizes, square (TAF) and rectangle (FTC); top and bottom view of nanochannel delivery implant in medical-grade titanium for TAF (with silicone plug) and FTC (before affixing silicone plug); (f) Northwestern CAB PU reservoir implant (Reproduced with permission from [Citation99]); (g) CONRAD subdermal biodegradable CAB pellet implant system (Reproduced with permission from [Citation100] under the terms and conditions of the Creative Commons Attribution (CC BY) license http://creativecommons.org/licenses/by/4.0/).
![Figure 2. Long-acting (LA) subdermal/subcutaneous implants in clinical development. (a) Merck EFdA implant (Reproduced with permission from [Citation35]); (b) RTI thin film TAF implant (Reproduced with permission from [Citation85] under the terms and conditions of the Creative Commons Attribution (CC BY) license http://creativecommons.org/licenses/by/4.0/); (c) Oak Crest TAF subdermal implant (Reproduced with permission from [Citation95]) (Three-dimensional model (A) and cross-sectional drawings (B and C) of TAF implant. The TAF core (black) inside the silicone scaffold with PVA membrane coating is shown (not to scale). Cross sections were sliced through the y-z (B) and x-y planes (C). (d) HMRI nanofluidic refillable CAB implant (Reproduced with permission from [Citation98]) ((A) Rendered image of cross-section of polyether ether ketone (PEEK) (left) and titanium (right) drug reservoirs. (B) Assembled PEEK βCAB (left) and titanium CAB (middle) drug reservoirs and 13 nm nanofluidic membrane (right). (C) SEM image of nanochannel membrane cross-section displaying drug release through perpendicular microchannels and horizontal nanochannels r, reservoir, e, epoxy, m, nanochannel membrane, s, implant shell); (e) HMRI nanofluidic refillable TAF/FTC implant (Reproduced with permission from [Citation93]) ((G) Cross-sectional rendering of nanochannel delivery implant depicting drug refill needles through the loading ports with resealable silicone plugs. (H) Nanochannel membranes of two different sizes, square (TAF) and rectangle (FTC); top and bottom view of nanochannel delivery implant in medical-grade titanium for TAF (with silicone plug) and FTC (before affixing silicone plug); (f) Northwestern CAB PU reservoir implant (Reproduced with permission from [Citation99]); (g) CONRAD subdermal biodegradable CAB pellet implant system (Reproduced with permission from [Citation100] under the terms and conditions of the Creative Commons Attribution (CC BY) license http://creativecommons.org/licenses/by/4.0/).](/cms/asset/6613416d-4351-4dd5-9f9f-34153ca5963a/iedd_a_2135699_f0002_oc.jpg)
Implants consisting of EFdA (ISL) dispersed within biodegradable (polylactic acid: PLA, and polycaprolactone: PCL) and non-biodegradable (ethylene co-vinyl acetate: EVA) polymers have been developed and achieved clinically relevant exposures of EFdA, with plasma levels maintained for >6 months corresponding to efficacious EFdA-TP (EFdA-triphosphate) levels in rodents and nonhuman primates (NHPs) [Citation35]. Notably, the reformulated, radiopaque EFdA implant evaluated in a Phase 1 study is projected to provide HIV prophylaxis for 1 year [Citation81,Citation82]. In recently published Phase 1 data in adults without HIV-1 infection, two doses (54 and 62 mg) of ISL in the implants provided mean concentrations above the PK threshold through 12 weeks [Citation83]. However, as previously noted, because of safety concerns related to dose-dependent decreases in total lymphocyte and CD4 counts in participants [Citation34], the FDA has put a clinical hold on all investigational new drug applications (INDs) for oral and implant ISL formulations for HIV-1 PrEP and the injectable formulation for treating and preventing HIV-1.
Several groups have formulated TAF into various implantable drug delivery technologies. Research Triangle Institute (RTI) developed biodegradable TAF reservoir implants, sheathed with rate controlling PCL membrane, which showed tunable in vitro TAF release (0.2–1.2 mg/day) through controlling formulation variables [Citation84–86]. In NHPs, the implant showed low (below limit of quantitation) sustained TFV exposure in plasma but high levels of TFV-diphosphate (TFV-DP) in PBMCs [Citation87]. Implants releasing 0.7 mg/day of TAF provided complete protection against vaginal SHIV infection; however, local inflammation and tissue necrosis were noted near the implantation sites [Citation88]. A Northwestern University-based consortium developed a non-biodegradable reservoir-based polyurethane (PU) implant containing compressed TAF [Citation89] and tested it in rabbit and rhesus macaque models, but findings also showed TAF-related local inflammation and severe necrosis around the implant even at the lowest TAF dose of ~10 µg/kg/day [Citation90]. Intarcia Therapeutics explored its proprietary osmotic pump system to develop non-biodegradable TAF implants [Citation91], however histopathology of tissue surrounding the SC infusion sites in rats and dogs receiving ≥300 and ≥25 µg/kg/day of TAF, respectively, for 28 days revealed signs of inflammation, edema, mass formation, and fibrosis that was more severe in animals that received TAF compared to vehicle control [Citation92].
Not all TAF implants have reported local safety issues, however. Houston Methodist Research Institute (HMRI) has developed a nanochannel delivery implant containing TAF/FTC [Citation93] and incorporated ports, which allowed for transcutaneous drug refilling, representing an innovative approach in addressing adherence issues. In rhesus macaques, the implant appeared safe, maintained the TFV-DP concentration above the clinically protective levels, and resulted in a 62.5% reduction in the risk of SHIV infection within a low-dose rectal challenge [Citation94]. Oak Crest Institute of Science has also developed a TAF (free base form) containing non-biodegradable silicone pod-type reservoir implant with delivery channels and coated with polyvinyl alcohol (PVA) polymer [Citation95]. The PK in dogs demonstrated intracellular TFV-DP above therapeutic levels for up to 40 days. In mice and sheep, the implant showed no concerning toxicity effects when observed macroscopically with TAF doses of ≤1 mg/day [Citation91,Citation96] though no histological evaluations were reported. In collaboration with CAPRISA (Centre for the AIDS Programme of Research in South Africa), this implant is currently being tested in humans (CAPRISA 018 Phase 1/2 trials) to evaluate the safety, acceptability, tolerability, and PK [Citation97].
Implants for the extended release of CAB are also in development. HMRI has explored the nanochannel delivery implant technology described above for a sustained delivery of CAB (modified with 2-hydroxypropyl-β-cyclodextrin for enhanced solubility) in rats for 3 months and showed that 2xPA-IC90 plasma concentration of CAB was maintained [Citation98]. Northwestern University also developed a PU reservoir implant for compressed CAB, and studies in rhesus macaques showed drug release of ~350 µg/day over 3 months [Citation99]. CONRAD is currently developing a subdermal resorbable multi-pellet implant system, capable of 6–12 months delivery of CAB [Citation100]. The biodegradable pellets are manufactured using a simple scalable process and are designed to be administered through a subdermal insertion via a low-cost, human-centered designed device also in development by CONRAD for delivery of ARVs and/or contraceptive implants.
Recent end-user acceptability surveys [Citation100–102] of biodegradable or non-biodegradable implants confirm that these implantable systems represent a meaningful and desirable option for HIV PrEP, particularly for people with adherence challenges or desire LA, highly effective systemic protection with reduced clinical visits. Reservoir-type implants can deliver drugs with sustained zero-order release kinetics and enable adding excipients in the core microenvironment to enhance drug solubility and stability [Citation86,Citation103]. The implants also support powdered or liquid drug transcutaneous refilling for enhanced patient acceptability with avoiding implantation and removal for the follow-up doses [Citation93,Citation104]. The drug loading in solid form in both degradable and non-degradable implants provides additional benefits of enhancing drug stability, thus the extended therapeutic duration [Citation104]. The biodegradable pellet-based modular implants offer very high drug loading (up to 90% w/w), long-term stability by way of a solid dosage form, and are tunable to adjust the release rate and duration of drug release through adjustment of size, number of pellets, and coating membrane composition [Citation100].
A key consideration in the clinical translation of implants into resource-limited clinics is whether surgical removal is required, which, while potentially burdensome to health-care systems with respect to added training and clinic visit time, enables immediate removal of the payload and reversal of effects. Biodegradable implants/pellets do not require removal; however, full characterization of the PK profile throughout the slow degradation process requires intensive characterization and long IND-enabling and early clinical studies, unless intentionally removed early by surgical incision for proof-of-concept in first-in-human trials. Other concerns that require further investigation for clinical advancements of implants include the risk of foreign body reactions around the implanted site or implant migration from the site of insertion, in vivo accumulation of the degraded polymers if not eliminated as predicted, variability of degradation and drug release rates of implants due to any biological changes in the patients, and need for specialized training and intervention by HCP for surgical insertion or drug refilling (in the case of the nanochannel device).
2.2.2. In-situ forming implants (ISFIs)
ISFIs are polymeric solutions that become semisolid or solidify when injected into the body (). An LA removable ISFI containing DTG, a biodegradable polymer (poly(lactic-co-glycolic acid): PLGA), and N-methyl-2-pyrrolidone (NMP) as a solvent delivered DTG for up to 9 months in mice and 140 days in NHPs and effectively protected against repeated high-dose vaginal HIV challenges [Citation105]. The concept was expanded for sustained delivery of multiple drugs in a single injection for HIV treatment or prophylaxis [Citation106]. A biodegradable ISFI containing CAB was also recently developed and tested in macaques [Citation107]. The formulation provided sustained CAB release above established PrEP benchmarks and durable protection against SHIV infection for up to 6 months and resulted in a short drug tail upon removal [Citation107]. Combining ISFI technology with micronization and compression, polymeric solid implants (PSI) were fabricated to provide sustained release of DTG [Citation108] or DTG/RPV in combination [Citation109] over several months. An injectable LA formulation of doravirine (NNRTI) that forms an implant after SC administration has also been developed and showed that drug plasma concentrations exceeded IC95 for >5 months in mice and efficiently prevented vaginal HIV transmission after multiple HIV challenges [Citation110]. Recently, laurate and myristate prodrugs of DTG were synthesized, formulated into a biodegradable ISI, and tested in vitro for LA delivery [Citation111]. The data demonstrate the flexibility of ISFI formulations to achieve long-term drug release with varying rates depending upon the composition of the system. However, for ISFIs, additional development efforts are still needed to address the reproducibility of the depot shape/size and drug distribution/encapsulation in vivo across patients and its subsequent impact on PK variability in vivo.
Figure 3. (a) Design and mechanism of release of an ISFIs (Reproduced with permission from [Citation9]); (b) The design of the gastric resident dosage forms consisting of an elastomeric core (grey) and six rigid arms loaded with a drug polymer matrix (multi-colored) (Reproduced with permission from [Citation122]); (c) Schematics of intradermal administration of TAF using dissolving and implantable microarray patch (MAP) (Reproduced with permission from [Citation131]). All the figures are reproduced under the terms and conditions of the Creative Commons Attribution (CC BY) license http://creativecommons.org/licenses/by/4.0/).
![Figure 3. (a) Design and mechanism of release of an ISFIs (Reproduced with permission from [Citation9]); (b) The design of the gastric resident dosage forms consisting of an elastomeric core (grey) and six rigid arms loaded with a drug polymer matrix (multi-colored) (Reproduced with permission from [Citation122]); (c) Schematics of intradermal administration of TAF using dissolving and implantable microarray patch (MAP) (Reproduced with permission from [Citation131]). All the figures are reproduced under the terms and conditions of the Creative Commons Attribution (CC BY) license http://creativecommons.org/licenses/by/4.0/).](/cms/asset/43f6fdba-2e82-4971-8f7f-c22dcadc12f3/iedd_a_2135699_f0003_oc.jpg)
2.2.3. Biodegradable shear-thinning hydrogel depot
Shear-thinning is the ability of a material to decrease in viscosity with increasing shear for improved injectability, an advantageous attribute for injectable DDS to better maintain drug suspension homogeneity and physical stability and support use of small gauge needles for low-pain SC or IM administration. The most advanced formulation in this category is a silica hydrogel matrix technology developed by DelSiTechTM, which allows drugs to be encapsulated either in silica microparticles or suspended directly in the hydrogel matrix to provide a controlled long-term release based on the silica matrix dissolution and PK properties of the drug itself. CONRAD in collaboration with DelSiTech initiated development of a multipurpose formulation containing DTG and levonorgestrel (LNG) in combination for the prevention of HIV acquisition and unintended pregnancy [Citation112]. Due to the changing landscape of FDA-approved drugs for PrEP, CONRAD has pivoted to CAB combined with LNG, and CAB only as next-generation formulations for 4–6 months of protection with a single low volume and less painful SC injection. Using their silica matrix technology, DelSiTech is also developing LA injectable biologics for HIV prevention[Citation113]. Although promising, further work is needed to ensure the manufacturability upon scale-up of this technology to ensure low cost of goods.
2.2.4. Injectable nanoformulations
While the most advanced injectable for HIV prevention is ViiV’s CAB LA nanosuspension (discussed earlier in this manuscript), additional compounds are being developed in nanoformulation DDS. In preclinical development are bictegravir (BIC: an integrase inhibitor) or BIC/TAF encapsulated PLGA nanoparticles (NPs), which have demonstrated sustained drug-release in vitro or in vivo [Citation114,Citation115]. The nanosuspension of VM1500A (deselsulfavirine; NNRTI), a parent drug of elsulfavirine [Citation116], has been tested in a Phase 1 study, which demonstrated that a monthly injection was well tolerated and had an acceptable PK profile [Citation117]. Gendelman et al. developed CAB [Citation70] and DTG [Citation71] fatty acid prodrug-based LA injectable poloxamer nanocrystals [Citation72,Citation73,Citation118]. CAB nanoformulations were tested in different animals and showed CAB plasma levels above the PA-IC90 (~167 ng/ml) for up to a year [Citation119,Citation120]. While these injectable formulations are quite promising with respect to achieving ultra-long durations, challenges still needing to be addressed prior to their clinical testing include controlling initial burst drug release, reducing the dosing volume and frequency while achieving high drug loading, feasibility of using drug combinations, and removability in case of adverse effects.
2.2.5. Oral LA formulations
Oral LA formulations can provide a simple alternative to both a daily oral pill regimen and an injectable/implant for HIV PrEP. A once-weekly oral dosing of EFdA (ISL) at 1.3 and 0.43 mg/kg protected rhesus macaques against SHIV infection [Citation121]; however, as mentioned previously, clinical development of ISL has been put on clinical hold due to safety concerns [Citation34]. Kirtane et al. demonstrated sustained delivery of DTG, RPV, and CAB via novel star-shaped matrices (gastric resident dosage form) with an elastomeric core attached to six PCL arms (), achieving therapeutic plasma drug levels with once weekly dosing in pigs [Citation122]. Once swallowed, acid in the stomach dissolves the outer layer, allowing the arms to unfold to resist the forces pushing the system down the digestive tract. After the drugs have been released, the device breaks up and passes into the intestines [Citation122]. This technology offers a highly versatile platform that can be formulated with multiple drugs and different polymers, allowing for a long-term release of drugs at different release rates.
LA oral HIV prevention approaches provide an attractive user-friendly option as they are devoid of any invasive dosing. However, the limitations for clinical application are restricted loading capacity requiring specific drugs with low dosage of which there are relatively few acceptable candidates, multifaceted manufacturing requirements, and non-suitability for drugs unstable in low pH aqueous environments of the stomach and at high temperature of the manufacturing process. Additionally, the impact of parameters such as the effects of diet and risk of GI ulceration on the safety, therapeutic efficacy and the tolerability of the device need to be assessed.
2.2.6. LA transdermal patches
Drug delivery using transdermal patches is beneficial to potential users, since these can be painlessly self-applied with no need for specialized disposal [Citation123–125]. CONRAD, in collaboration with Mercer University, developed a silicone-based suspension patch for sustained weekly delivery of TAF free base [Citation123]. The patch was tested in female hairless rats and achieved the target PK profile, duration, and safety for a once-weekly TAF delivery for HIV prevention or treatment [Citation126]. Eliminating the need for wearing a patch for a prolonged period of time and to provide a LA drug delivery, the recently developed microarray patches (MAP) are minimally invasive devices that are applied and removed minutes later, consisting of microscopic projections to penetrate the skin’s upper layers or mucosal tissues to administer the drug [Citation125,Citation127,Citation128]. Donnelly et al. in collaboration with PATH, developed a dissolving MAP containing LA nanosuspension of RPV [Citation129]. Most recently, they have also developed a bilayer MAP loaded with CAB [Citation130] and a dissolvable and implantable MAP loaded with TAF [Citation131] for long-term HIV PrEP (). CONRAD in partnership with the University of Connecticut is further developing this technology for the delivery of bnAbs (manuscript submitted).
Being non-invasive and convenient to apply (self-administer) without the intervention of HCPs, transdermal patches could expand access and provide more user adherence than injectable or implantable systems for HIV PrEP, especially in low-resource settings. To help translate this technology for clinical application, further studies are required to confirm scale-up development, impact of skin application sites, and variabilities in skin layers among individuals on insertion capability of MAPs. The limitations on patch sizes to achieve a higher drug loading and LA delivery duration require testing of clinical dose-relevant size patches in large animal models (e.g. primates) to understand the ease of application. In addition, the non-removability of inserted MAPs in case of adverse reactions may create patient compliance issues and require safety confirmation in animal models before clinical testing.
3. Roadmap of LA HIV prevention product development
LA formulations offer a promising new avenue for HIV prevention approaches, but since several formulations are in preclinical development, a thorough examination of each product is required since not all LA technologies are the same and have different development and dosing considerations in LMICs. While there have been tremendous advances in LA modalities and multiple research avenues are generating promising developments in LA HIV PrEP, there are still several important questions that need to be addressed for reliable clinical translation. Here, we briefly discuss the technical challenges, design factors to consider, and knowledge gaps (also summarized in ) for the development and evaluation of LA formulations in terms of their safety, efficacy, stability, and acceptability in diverse populations.
Figure 4. Technical challenges, design considerations, and knowledge gaps in the development of long-acting (LA) HIV products.
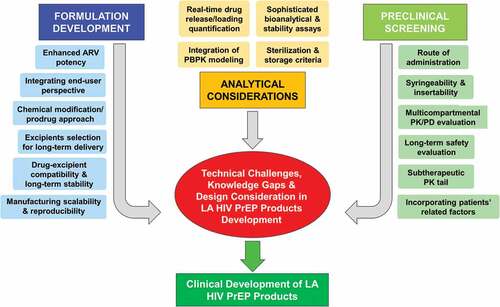
3.1. Formulation development
Enhancing ARV potency: Though significant advances have been reported in this area, there are still challenges that need to be addressed [Citation56,Citation132]. These include increased potency with IC90 values below 1 nM, cost-effective design and scalability of the chemical process, maintaining parent drug safety and stability throughout the modification approach, and the requirement of sophisticated methods to analyze the modified molecules and released chemical linker or by-products for safety considerations. Thus, it is advisable to select the drug or prodrug design based on estimated long-term safety, target duration, target site biology, and potency. This demands thorough understanding of the chemical interaction between the parent drug, linker, and biological targets.
Excipient selection and compatibility with the drug/formulation: The quality of excipients is critical to assuring the safety, stability, and efficacy of LA products. Although parenteral grade excipients listed by the FDA as ‘generally recognized as safe (GRAS)’ are preferred in LA formulations, the effect of these excipients on the long-term safety, tolerability, and PK profiles cannot always be extrapolated from the existing data, which is mostly on oral or immediate-release formulations. Therefore, long-term studies in preclinical models are required to confirm the safety, compatibility, stability, and efficacy of excipients and/or drugs in combination in LA formulations. With this critical information and the continuous improvement of functionalized excipients, the LA formulation development process will be accelerated. Feasibility of incorporation of multiple drugs into a single formulation also needs a detailed understanding of drug-drug or drug-excipient interactions to attain an ideal prolonged release profile for each drug.
Manufacturing scalability and reproducibility: The scalable development of LA formulations under current good manufacturing practice (cGMP) conditions presents typical challenges, since it involves multiple steps in the manufacturing process and subtle variations can significantly affect the therapeutic outcomes of the final product. The physicochemical properties and integrity of LA DDS need to be maintained throughout the process and during the product’s shelf life. Thus, a careful selection of manufacturing parameters meant to formulate a product with desired characteristics in a high-throughput manner is an important early consideration in the development process.
3.2. Analytical considerations
Real-time quantification of residual drug and drug release: To facilitate the product development process, whenever possible, the remaining drug from retrievable LA products (e.g. implants, depots) should be quantified to indirectly estimate in vivo release rate and duration. There are limitations to estimating in vivo release particularly when there is an initial burst of drug release prior to reaching steady state. Under such circumstances, estimated daily release may be less reliable and hence real-time drug quantification methods may be required. Addressing this, recently developed laser-induced breakdown spectroscopy (LIBS) [Citation133] and high-resolution X-ray microscopy (XRM) [Citation134] hold promise for rapid assessment of drugs in real-time, further accelerating the development of LA formulations.
Expedited development using modeling approaches: Preclinical evaluations (efficacy, safety, and stability) are desired to ensure the robust performance of LA products; however, evaluating the long-term profiles of these products in real time, in vivo, is time-consuming. In this regard, physiologically based pharmacokinetic (PBPK) modeling may be a helpful tool to predict PK using in vitro data to reduce the time required to gather information and accelerate the LA product development process [Citation135–137].
Sterilization method: Finding an appropriate sterilization method that can be used without compromising the stability and efficacy of final LA formulation is important. The common sterilization methods such as autoclaving and ionizing or nonionizing radiations may release toxic residues and/or affect the formulation and drug properties, which could in turn modify the drug release profile [Citation138,Citation139]. This demands in-depth understanding and precise controls of the process to meet the critical attributes of the product.
3.3. Preclinical and end-user testing
Syringeability, insertability, and route of administration: These are important parameters not only for patient compliance but also to deliver an accurate drug dose for a prolonged period. The effects of factors such as dosing volume, injection route (SC, IM, IV), and formulation parameters (e.g. drug physicochemical properties) on safety, injectability, and drug release/duration should be assessed appropriately and early.
Selection of an animal model for PK and safety: Selection of an appropriate animal model is critical for accurately defining the PK and safety profile of an LA product. For instance, differences in drug stability in plasma across species have been observed with the antiretroviral TAF [Citation140]. Specifically in rodents, an animal model commonly used for preclinical assessments, there was a complete loss of TAF and an overestimation of TFV in plasma [Citation140], suggesting the rodent may not be the optimal animal model for preclinical testing of certain drugs. Furthermore, establishing an in vitro-in vivo correlation (IVIVC) using dissolution and plasma profiling can assist with screening iterations of an LA product for a more refined and expedited approach during development [Citation141,Citation142]. Safety assessments are dependent on the type of DDS and may require following national or international standards and ISO (the International Organization for Standardization) 10,993 if classified as a device or combination drug/device. Test methods for evaluating biocompatibility are specified for implants, absorbable and non-absorbable, and include, but are not limited to, study interval recommendations to span degradation period, implant retrieval procedures, and microscopic evaluations.
Understanding the role of immune cells: There is not much information available regarding how the immune cells may influence LA depots and drug transport. Consideration should be given to the roles of macrophages in the uptake and transport of ARV nanoparticles from the injection site. For example, a higher concentration of RPV in lymph nodes after 1 month of IM administration compared to SC has been shown [Citation143]. Hence, it is worth exploring how immune cell populations may influence the IM/SC administered LA formulations.
Influence of individuals’ biological variability and integrating end-user perspective for better acceptability: Factors such as gender and physiological or anatomical differences may influence the PK, drug release kinetics, and therapeutic efficacy of LA products [Citation144,Citation145]. The HPTN 077 study showed that the LA CAB half-life was significantly longer in females compared to males and influenced by body mass index [Citation29]. Hence, evaluating the effect of biological differences is worth investigating to minimize variability in developing a safe, adaptable, and efficacious LA formulation. Integrating end-user preferences into early stages of product development may also improve the therapeutic outcome and acceptability. For example, the complexity of surgical procedures has significant effects on patient acceptability of implants and future designs should employ minimally invasive approaches to minimize or ideally avoid intensive insertion (and removal) procedures. Other key attributes that can determine acceptability of LA products include cost-benefits, removability, duration of effectiveness, insertion location, side effects, visibility/palpability, and health clinic versus pharmacy provided [Citation100,Citation146]. Product preferences as a whole vary among individual users leading to the need for different DDS that can be selected by individuals based on suitability [Citation147]. A single DDS for HIV PrEP may not meet the needs of all users and with more options available, the chances of making an impact on HIV incidence increase.
4. Conclusions
The HIV prevention field has entered a new era as first-generation LA PrEP options imminently rollout into the hands of end-users and next-generation LA PrEP products provide greater value-added to the method mix, including notably less frequent (3–12 months) dosing with improved patient adherence, minimal risk of resistance development, strategically accelerated clinical development and regulatory paths, and reduced costs to end-users in LMICs especially. While recent scientific advances have provided promising approaches for the long-term prevention of new HIV infections, there are still several important questions that need to be addressed regarding LA dosage forms and knowledge gaps, which will greatly influence successful clinical translation of LA products. In summary, the growing pipeline of LA HIV prevention options is promising, with CAB LA recently approved and marketed in the US, additional longer acting products in advanced clinical stages, and an array of innovative next-generation approaches being tested preclinically. However, development and translational challenges remain to be addressed for the successful clinical application of some of these novel modalities such as targeted consideration of unmet need and target populations, cost-effectiveness, efficacy, design, and scalability of the modification process and of the desired final product.
5. Expert opinion
There are several LA delivery options demonstrating promising clinical and preclinical results in the HIV PrEP field. In general, the LA potential of existing ARVs can be achieved either through their prodrug chemical modification or through delivering them using LA novel DDS approaches. One critical question in this respect is what approach should be implemented. While deciding how to proceed with a given ARV, multiple elements should be taken into consideration, such as whether chemically modifying the parent ARV or delivering the existing ARV from an improved DDS provides better value-added and applicability in target populations and settings. New product design must consider that an ARV should be chemically modified when the prodrug or derivative provides added benefits to the parent compound’s profile in terms of enhancing its potency, half-life, stability, cell permeability, and/or bioavailability. If this is not the case, formulating the existing ARVs into an improved LA DDS would be a superior approach. The LA potential of newly developed ARVs could be enhanced by generating molecules with novel mechanisms (or targeting multiple steps in viral replication) or through their encapsulation into a novel DDS.
Since several new potent ARVs are in the pipeline for HIV prevention, further exploration of new LA approaches is important for implementation in a feasible and inexpensive way. Moreover, high barriers to resistance and the transport/storage of LA products being compatible with use in resource-limited settings (i.e. avoiding the need for cold-chain storage) are ideal. New LA formulation technologies include oral star-shaped devices that allow sustained drug release for at least a week [Citation121,Citation122] and improved implant designs such as the refillable ARV implants [Citation93], which provide advantages of transcutaneous drug filling without the need to remove and re-implant a new device. These new LA technologies entail innovation but also raise challenges in their clinical feasibility and cost-effective and scalable manufacturing. For example, the major limitations of oral devices are the complex geometry and unsuitability for materials unstable in the acidic environment of the stomach such as TAF [Citation148], whereas for refillable implants, further studies are needed to confirm the clinical feasibility for better patient compliance. Future approaches comprise drug encapsulation in nanoformulations embedded in non-resorbable and biodegradable implants, injectable depots, and microarray patches, in order to maximize the intended therapeutic effects of LA products. Overall, with several highly potent drugs in preclinical/clinical testing and rapid progress in new delivery technologies, the prospects for LA HIV prevention are encouraging.
The requirements for LA HIV PrEP products for resource-limited settings where the HIV epidemic has the highest health burden can be even more challenging. This necessitates consideration, collaboration, and precise planning to design and prioritize the most promising ARVs and DDS that facilitate implementation in these settings. Furthermore, products in development must be tested in a timely fashion in local populations, while key stakeholders should be engaged early in the development process to provide feedback and better support ultimate introduction and implementation. This may help develop comprehensive approaches to ensure safe, scalable, cost-effective, and effective LA HIV products that end-users will want to use, providers will want to administer, and policy-makers will want to procure and distribute. The partnership of ViiV Healthcare and the Medicines Patent Pool (MPP) supporting an ongoing licensing agreement negotiation to help widen access to LA HIV prevention drugs [Citation149] with cost-effective and scalable manufacturing capacity represents a recent example of such collaborative approaches. There are also significant developments happening in LA formulations for other indications, such as ocular therapy, cancer, contraception, and tuberculosis, and leveraging LA formulation designs from other fields may support and expedite the HIV PrEP product development process. In summary, there is a growing pipeline of potent LA ARVs and bnAbs, as well as several novel LA formulation and drug delivery technologies for HIV prevention that are highly promising. Nevertheless, further innovation, technology advancements, and new partnerships are needed to expedite development of clinically translatable and implementable LA HIV PrEP products that are less invasive and more applicable, and acceptable, particularly in special populations of children, adolescents, and pregnant women.
Article highlights
State of the current HIV PrEP field and the need for the development of next-generation LA PrEP approaches
Review of approaches enhancing the LA potential of existing ARVs through chemical modification
Novel drug delivery strategies for LA HIV prophylaxis
Technical challenges and knowledge gaps in LA HIV prevention products development and clinical advancements
This box summarizes key points contained in the article.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The contents are the responsibility of the authors and do not necessarily reflect the views of USAID, NIH, NIAID, or the United States Government. The authors would also like to thank USAID for their support in organizing a one-day Technology Innovation Workshop on ‘Current Challenges & Emerging Approaches in Long Acting HIV Prevention,’ on 8 November 2018 (hosted by CONRAD in Arlington, VA, USA) and the workshop attendees. The meeting discussions inspired the field landscaping that culminated with this review.
Additional information
Funding
References
- UNAIDS. Global HIV & AIDS statistics - fact sheet [Internet]. UNAIDS. 2021 [cited June 28 2022]. cited: https://www.unaids.org/en/resources/fact-sheet.
- UNAIDS; Sabin K. The prevention gap report. 2016.
- Global AIDS Strategy 2021-2026 — end Inequalities. End AIDS. [Internet]. UNAIDS. 2021 [cited Sep 15, 2022]. Available from: https://aidstargets2025.unaids.org.
- Granich R, Crowley S, Vitoria M, et al. Highly active antiretroviral treatment as prevention of HIV transmission: review of scientific evidence and update. Curr Opin HIV AIDS. 2010;5(4):298–304.
- Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society-USA panel. JAMA. 2018;320(4):379–396.
- Sosnik A, Augustine R. Challenges in oral drug delivery of antiretrovirals and the innovative strategies to overcome them. Adv Drug Deliv Rev. 2016 ;103:105–120.
- Hobson JJ, Owen A, Rannard SP. The potential value of nanomedicine and novel oral dosage forms in the treatment of HIV. Nanomedicine (Lond). 2018 Aug;13(16):1963–1965.
- Krovi SA, Johnson LM, Luecke E, et al. Advances in long-acting injectables, implants, and vaginal rings for contraception and HIV prevention. Adv Drug Deliv Rev. 2021;176:113849.
- Young IC, Benhabbour SR. Multipurpose prevention technologies: oral, parenteral, and vaginal dosage forms for prevention of HIV/STIs and unplanned pregnancy. Polymers (Basel). 2021;13(15):2450.
- Thoueille P, Choong E, Cavassini M, et al. Long-acting antiretrovirals: a new era for the management and prevention of HIV infection. J Antimicrob Chemother. 2022;77(2):290–302.
- Flexner C, Owen A, Siccardi M, et al. Long-acting drugs and formulations for the treatment and prevention of HIV infection. Int J Antimicrob Agents. 2021 ;57(1):106220.
- Philbin MM, Perez-Brumer A. Promise, perils and cautious optimism: the next frontier in long-acting modalities for the treatment and prevention of HIV. Curr Opin HIV AIDS. 2022;17(2):72–88.
- Pre-Exposure Prophylaxis (PrEP) [Internet]. Centers for disease control and prevention 2019 [cited July 24, 2019]. cited: https://www.cdc.gov/hiv/risk/prep/.
- Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125–151ra125.
- Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410.
- Riddell J, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. Jama. 2018;319(12):1261–1268.
- Access to the dapivirine vaginal ring: a timeline on progress [Internet]. AVAC 2022 cited August 17, 2022]. Available from: https://www.avac.org/infographic/access-dapivirine-vaginal-ring-timeline-progress.
- Population Council Acquires the Monthly Dapivirine Ring and Other Woman-centered HIV Prevention Technologies from the International Partnership for Microbicides. [Internet]. Population Council; 2022; 21 July 2022. Available from: https://www.popcouncil.org/news/population-council-acquires-the-monthly-dapivirine-ring-and-other-woman-cen
- Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121–2132.
- Nel A, Bekker LG, Bukusi E, et al. Safety, acceptability and adherence of dapivirine vaginal ring in a microbicide clinical trial conducted in multiple countries in Sub-Saharan Africa. PLoS One. 2016;11(3):e0147743.
- Brown ER, Hendrix CW, van der Straten A, et al. Greater dapivirine release from the dapivirine vaginal ring is correlated with lower risk of HIV-1 acquisition: a secondary analysis from a randomized, placebo-controlled trial. J Int AIDS Soc. 2020;23(11):e25634.
- Baeten JM, Palanee-Phillips T, Mgodi NM, et al. Safety, uptake, and use of a dapivirine vaginal ring for HIV-1 prevention in African women (HOPE): an open-label, extension study. Lancet HIV. 2021 Feb;8(2):e87–e95.
- Nel A, van Niekerk N, Van Baelen B, et al. Safety, adherence, and HIV-1 seroconversion among women using the dapivirine vaginal ring (DREAM): an open-label, extension study. Lancet HIV. 2021 Feb;8(2):e77–e86.
- Nair G, Ngure K, Szydlo D, et al. Adherence to the dapivirine vaginal ring and oral PrEP among adolescent girls and young women in Africa: interim results from the REACH study. 11th IAS Conference on HIV Science2021, Berlin, Germany. Jul 18-21 2021; Virtual.
- Trezza C, Ford SL, Spreen W, et al. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS. 2015 Jul;10(4):239–245.
- Cabotegravir (Apretude) for HIV-1 pre-exposure prophylaxis. Med Lett Drugs Ther. 2022;64:29–31. Feb 21.
- Delany-Moretlwe S, Hughes JP, Bock P, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. 2022;399(10337):1779–1789.
- Marzinke MA, Grinsztejn B, Fogel JM, et al. Characterization of HIV infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J Infect Dis. 2021;224(9):1581–1592.
- Landovitz RJ, Li S, Eron JJ Jr., et al. Tail-phase safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in HIV-uninfected adults: a secondary analysis of the HPTN 077 trial. Lancet HIV. 2020;7(7):e472–e481.
- Neilan AM, Landovitz RJ, Le MH, et al. Cost-Effectiveness of Long-Acting Injectable HIV Preexposure Prophylaxis in the United States. Ann Intern Med. 2022 [2022 April 19];175(4):479–489.
- ViiV healthcare and the medicines patent pool sign new voluntary licensing agreement to expand access to innovative long-acting HIV prevention medicine [Internet]. Medicines Patent Pool; 2022; 28 July 2022. Available from: https://medicinespatentpool.org/news-publications-post/viiv-healthcare-and-the-medicines-patent-pool-sign-new-voluntary-licensing-agreement-to-expand-access-to-innovative-long-acting-hiv-prevention-medicine
- Markowitz M, Sarafianos SG. 4’-Ethynyl-2-fluoro-2’-deoxyadenosine, MK-8591: a novel HIV-1 reverse transcriptase translocation inhibitor. Curr Opin HIV AIDS. 2018 Jul;13(4):294–299.
- Stoddart CA, Galkina SA, Joshi P, et al. Oral administration of the nucleoside EFdA (4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine) provides rapid suppression of HIV viremia in humanized mice and favorable pharmacokinetic properties in mice and the rhesus macaque. Antimicrob Agents Chemother. 2015;59(7):4190–4198.
- Merck announces clinical holds on studies evaluating islatravir for the treatment and prevention of HIV-1 infection [Internet]. 2021; December 13, 2021. Available from: https://www.merck.com/news/merck-announces-clinical-holds-on-studies-evaluating-islatravir-for-the-treatment-and-prevention-of-hiv-1-infection/
- Barrett SE, Teller RS, Forster SP, et al. Extended-duration MK-8591-eluting implant as a candidate for HIV treatment and prevention. Antimicrob Agents Chemother. 2018 Oct;62(10).
- Tse WC, Link JO, Mulato A et al. Discovery of novel potent HIV capsid inhibitors with long-acting potential. Conference on Retroviruses and Opportunistic Infections (CROI), Feb 14-17, 2017. Seattle, WA.
- Singh K, Gallazzi F, Hill KJ, et al. GS-CA compounds: first-in-class HIV-1 capsid inhibitors covering multiple grounds [Original research]. Front Microbiol. 2019 10:1227 doi: 10.3389/fmicb.2019.01227.
- Dvory-Sobol H, Shaik N, Callebaut C, et al. Lenacapavir: a first-in-class HIV-1 capsid inhibitor. Curr Opin HIV AIDS. 2022;17(1):15–21.
- Gilead Announces First Global Regulatory Approval of Sunlenca® (Lenacapavir). The only twice-yearly HIV treatment option [Internet]. Gilead Sciences; 2022; 22 August 2022. Available from: https://www.gilead.com/news-and-press/press-room/press-releases/2022/8/gilead-announces-first-global-regulatory-approval-of-sunlenca-lenacapavir-the-only-twiceyearly-hiv-treatment-option
- HPTN Annual Meeting Presentations. The HIV prevention trials network (HPTN) annual meeting. 2022 June 5-8. Washington D.C: The HIV Prevention Trials Network; 2022.
- FDA Lifts Clinical Hold on Investigational Lenacapavir for the Treatment and Prevention of HIV [Internet]. Gilead; 2022; May 16, 2022. Available from: https://www.gilead.com/news-and-press/press-room/press-releases/2022/5/fda-lifts-clinical-hold-on-investigational-lenacapavir-for-the-treatment-and-prevention-of-hiv
- Grobben M, Stuart RAL, van Gils MJ. The potential of engineered antibodies for HIV-1 therapy and cure. Curr Opin Virol. 2019 ;38:70–80.
- Pegu A, Hessell AJ, Mascola JR, et al. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev. 2017;275(1): 296–312.
- Gautam R, Nishimura Y, Pegu A, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533(7601):105–109.
- Mahomed S, Garrett N, Baxter C, et al. Clinical trials of broadly neutralizing monoclonal antibodies for human immunodeficiency virus prevention: a review. J Infect Dis. 2021;223(3):370–380.
- Walsh SR, Seaman MS. Broadly neutralizing antibodies for HIV-1 prevention [Review]. Front Immunol. 2021;12(2903). doi: 10.3389/fimmu.2021.712122.
- Gautam R, Nishimura Y, Gaughan N, et al. A single injection of crystallizable fragment domain–modified antibodies elicits durable protection from SHIV infection. Nat Med. 2018;24(5):610–616.
- Gaudinski MR, Coates EE, Houser KV, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a Phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15(1):e1002493.
- Gaudinski MR, Houser KV, Doria-Rose NA, et al. Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07-523LS in healthy adults: a phase 1 dose-escalation clinical trial. Lancet HIV. 2019;6(10):e667–e679.
- Steinhardt JJ, Guenaga J, Turner HL, et al. Rational design of a trispecific antibody targeting the HIV-1 Env with elevated anti-viral activity. Nat Commun. 2018;9(1):877.
- Davis-Gardner ME, Alfant B, Weber JA, et al. A bispecific antibody that simultaneously recognizes the V2- and V3-glycan epitopes of the HIV-1 envelope glycoprotein is broader and more potent than its parental antibodies. mBio. 2020;11(1).
- Mahomed S, Garrett N, Karim QA, et al. Assessing the safety and pharmacokinetics of the anti-HIV monoclonal antibody CAP256V2LS alone and in combination with VRC07-523LS and PGT121 in South African women: study protocol for the first-in-human CAPRISA 012B phase I clinical trial. BMJ open. 2020;10(11):e042247.
- Cohen YZ, Butler AL, Millard K, et al. Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10-1074 in healthy adults: a randomized, phase 1 study. PloS one. 2019;14(8):e0219142–e0219142.
- Julg B, Stephenson KE, Wagh K, et al. Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial. Nat Med. 2022;28(6):1288–1296.
- Wagh K, Bhattacharya T, Williamson C, et al. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog. 2016;12(3):e1005520.
- Rautio J, Kumpulainen H, Heimbach T, et al. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008 Mar;7(3):255–270.
- Rautio J, Meanwell NA, Di L, et al. The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov. 2018;17(8):559–587.
- Mehellou Y, Rattan HS, Balzarini J. The ProTide prodrug technology: from the concept to the clinic. J Med Chem. 2018; 61(6):2211–2226.
- Mehellou Y. The ProTides Boom. ChemMedChem. 2016;11(11):1114–1116.
- Serpi M, Pertusati F. An overview of ProTide technology and its implications to drug discovery. Expert Opin Drug Discov. 2021 May;24:1–13.
- Ray AS, Fordyce MW, Hitchcock MJM. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res. 2016 ;125:63–70.
- Soni D, Bade AN, Gautam N, et al. Synthesis of a long acting nanoformulated emtricitabine ProTide. Biomaterials. 2019;222:119441.
- Smith N, Bade AN, Soni D, et al. A long acting nanoformulated lamivudine ProTide. Biomaterials. 2019;223:119476.
- Cobb DA, Smith N, Deodhar S, et al. Transformation of tenofovir into stable ProTide nanocrystals with long-acting pharmacokinetic profiles. Nat Commun. 2021 [2021 September 16];12(1):5458.
- Cihlar T, Ray AS, Boojamra CG, et al. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob Agents Chemother. 2008;52(2):655–665.
- Ray AS, Vela JE, Boojamra CG, et al. Intracellular metabolism of the nucleotide prodrug GS-9131, a potent anti-human immunodeficiency virus agent. Antimicrob Agents Chemother. 2008;52(2):648–654.
- Mackman RL, Ray AS, Hui HC, et al. Discovery of GS-9131: design, synthesis and optimization of amidate prodrugs of the novel nucleoside phosphonate HIV reverse transcriptase (RT) inhibitor GS-9148. Bioorg Med Chem. 2010;18(10):3606–3617.
- Agarwal HK, Chhikara BS, Bhavaraju S, et al. Emtricitabine prodrugs with improved anti-HIV activity and cellular uptake. Mol Pharm. 2013;10(2):467–476.
- Agarwal HK, Chhikara BS, Hanley MJ, et al. Synthesis and biological evaluation of fatty acyl ester derivatives of (-)-2’,3’-dideoxy-3’-thiacytidine. J Med Chem. 2012;55(10):4861–4871.
- Zhou T, Su H, Dash P, et al. Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles. Biomaterials. 2018;151:53–65. doi: 10.1016/j.biomaterials.2017.10.023
- Sillman B, Bade AN, Dash PK, et al. Creation of a long-acting nanoformulated dolutegravir. Nat Commun. 2018;9(1):443.
- McMillan J, Szlachetka A, Slack L, et al. Pharmacokinetics of a long-acting nanoformulated dolutegravir prodrug in rhesus macaques. Antimicrob Agents Chemother. 2018;62(1). doi:10.1128/AAC.01316-17.
- McMillan J, Szlachetka A, Zhou T, et al. Pharmacokinetic testing of a first-generation cabotegravir prodrug in rhesus macaques. AIDS. 2019;33(3):585–588.
- Khuroo T, Dharani S, Mohamed EM, et al. Ultra-long acting prodrug of dolutegravir and delivery system - physicochemical, pharmacokinetic and formulation characterizations. Int J Pharm. 2021;607:120889.
- Krovi SA, Gallovic MD, Keller AM, et al. Injectable long-acting human immunodeficiency virus antiretroviral prodrugs with improved pharmacokinetic profiles. Int J Pharm. 2018;552(1–2):371–377.
- D-K H, LeGuyader C, Srinivasan S, et al. Fully synthetic injectable depots with high drug content and tunable pharmacokinetics for long-acting drug delivery. J Control Release. 2021;329:257–269.
- Pemmaraju BP, Malekar S, Agarwal HK, et al. Design, synthesis, antiviral activity, and pre-formulation development of poly-L-arginine-fatty acyl derivatives of nucleoside reverse transcriptase inhibitors. Nucleosides Nucleotides Nucleic Acids. 2015;34(1):1–15.
- Agarwal HK, Chhikara BS, Quiterio M, et al. Synthesis and Anti-HIV activities of glutamate and peptide conjugates of nucleoside reverse transcriptase inhibitors. J Med Chem. 2012;55(6):2672–2687.
- Agarwal HK, Kumar A, Doncel GF, et al. Synthesis, antiviral and contraceptive activities of nucleoside–sodium cellulose sulfate acetate and succinate conjugates. Bioorg Med Chem Lett. 2010 ;20(23):6993–6997.
- New Phase 3 Data Support the Sustained, Long-acting efficacy of lenacapavir, gilead’s investigational HIV-1 capsid inhibitor. [Internet]; Foster City, CA: Gilead Sciences, Inc. 2021.
- Matthews RP, Barrett SE, Patel M, et al. First-in-human trial of MK-8591-eluting implants demonstrates concentrations suitable for HIV prophylaxis for at least one year. 10th IAS Conference on HIV Science; July 21-24 Mexico City, Mexico: The International AIDS Conference (IAS); 2019.
- Matthews RP, Zang X, Barrett S, et al. Next-generation islatravir implants projected to provide yearly HIV prophylaxis. Conference on Retroviruses and Opportunistic Infections (CROI); 2021 March 6-10.
- Matthews RP, Patel M, Barrett SE, et al. Safety and pharmacokinetics of islatravir subdermal implant for HIV-1 pre-exposure prophylaxis: a randomized, placebo-controlled phase 1 trial. Nat Med. 2021;27(10):1712–1717.
- Schlesinger E, Johengen D, Luecke E, et al. A tunable, biodegradable, thin-film polymer device as a long-acting implant delivering tenofovir alafenamide fumarate for HIV pre-exposure prophylaxis. Pharm Res. 2016 Jul;33(7):1649–1656.
- Johnson LM, Krovi SA, Li L, et al. Characterization of a reservoir-style implant for sustained release of tenofovir alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP). Pharmaceutics. 2019;11(7):315.
- Li L, Johnson LM, Krovi SA, et al. Performance and stability of tenofovir alafenamide formulations within subcutaneous biodegradable implants for HIV Pre-exposure prophylaxis (PrEP). Pharmaceutics. 2020;12(11):1057.
- Massud I, Krovi A, Ruone S, et al. Pharmacokinetics and safety of long-acting tenofovir alafenamide implants in macaques for HIV prevention. PEA0087. AIDS 2020.
- Massud I. High protection against vaginal SHIV infection in macaques by a biodegradable implant releasing tenofovir alafenamide. J Int AIDS Soc. 2021;24:11.
- Simpson SM, Widanapathirana L, Su JT, et al. Design of a drug-eluting subcutaneous implant of the antiretroviral tenofovir alafenamide fumarate. Pharm Res. 2020;37(4):83.
- Su JT, Simpson SM, Sung S, et al. A subcutaneous implant of tenofovir alafenamide fumarate causes local inflammation and tissue necrosis in rabbits and macaques. Antimicrob Agents Chemother. 2020;64(3): e01893–19.
- Romano JW, Baum MM, Demkovich ZR, et al. Tenofovir alafenamide for HIV prevention: review of the proceedings from the gates foundation long-acting TAF product development meeting. AIDS Res Hum Retroviruses. 2021;37(6):409–420.
- Zane D, Roller S, Shelton J, et al. A 28-day toxicity study of tenofovir alafenamide hemifumarate by subcutaneous infusion in rats and dogs. Microbiol Spectr. 2021;9(1):e0033921.
- Chua CYX, Jain P, Ballerini A, et al. Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis. J Control Release. 2018;286:315–325.
- Pons-Faudoa FP, Sizovs A, Shelton KA, et al. Preventive efficacy of a tenofovir alafenamide fumarate nanofluidic implant in SHIV-challenged nonhuman primates. Adv Ther. 2021;4(3). doi:10.1002/adtp.202000163.
- Gunawardana M, Remedios-Chan M, Miller CS, et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob Agents Chemother. 2015;59(7):3913–3919.
- Gunawardana M, Remedios-Chan M, Sanchez D, et al. Multispecies evaluation of a long-acting tenofovir alafenamide subdermal implant for HIV prophylaxis [Original Research]. Front Pharmacol. 2020;11(1866). Doi:10.3389/fphar.2020.569373.
- Gengiah TN, Abdool Karim Q, Harkoo I, et al. CAPRISA 018: a phase I/II clinical trial study protocol to assess the safety, acceptability, tolerability and pharmacokinetics of a sustained-release tenofovir alafenamide subdermal implant for HIV prevention in women. BMJ open. 2022;12(1):e052880–e052880.
- Pons-Faudoa FP, Sizovs A, Di Trani N, et al. 2-Hydroxypropyl-β-cyclodextrin-enhanced pharmacokinetics of cabotegravir from a nanofluidic implant for HIV pre-exposure prophylaxis. J Control Release. 2019;306:89–96.
- Karunakaran D, Simpson SM, Su JT, et al. Design and testing of a cabotegravir implant for HIV prevention. J Control Release. 2021;330:658–668. DOI:10.1016/j.jconrel.2020.12.024
- Little KM, Flomen L, Hanif H, et al. HIV pre-exposure prophylaxis implant stated preferences and priorities: results of a discrete choice experiment among women and adolescent girls in Gauteng Province, South Africa. AIDS Behav. 2022;26(9):3099–3109.
- Krogstad EA, Atujuna M, Montgomery ET, et al. Perspectives of South African youth in the development of an implant for HIV prevention. J Int AIDS Soc. 2018;21(8):e25170.
- Ngure K, Mugo NR, Bukusi EA, et al. Pills, injections, rings, or implants? PrEP formulation preferences of PrEP-experienced African women for HIV prevention. J Acquir Immune Defic Syndr. 2021;88(4):e30–e32.
- Sizovs A, Pons-Faudoa FP, Malgir G, et al. Trans-urocanic acid enhances tenofovir alafenamide stability for long-acting HIV applications. Int J Pharm. 2020;587:119623.
- Di Trani N, Pons-Faudoa FP, Sizovs A, et al. Extending drug release from implants via transcutaneous refilling with solid therapeutics. Adv Ther. 2022;5(2):2100214.
- Kovarova M, Benhabbour SR, Massud I, et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun. 2018;9(1):4156.
- Benhabbour SR, Kovarova M, Jones C, et al. Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nat Commun. 2019;10(1):4324.
- Ivana M, Kovarova, M, Wong-Sam A. In situ forming implants with cabotegravir for ultra-long-acting PrEP. Conference on Retroviruses and Opportunistic Infections (CROI), Feb 12-16, 2022.
- Maturavongsadit P, Paravyan G, Kovarova M, et al. A new engineering process of biodegradable polymeric solid implants for ultra-long-acting drug delivery. Int J Pharm X. 2021;3:100068.
- Maturavongsadit P, Shrivastava R, Sykes C, et al. Biodegradable polymeric solid implants for ultra-long-acting delivery of single or multiple antiretroviral drugs. Int J Pharm. 2021;605:120844.
- Kovarova M, Manse K, Wessel SE, et al. Long acting doravirine for treatment and prevention of vaginal HIV transmission. Conference on Retroviruses and Opportunistic Infections (CROI), Feb 12-16, 2022; 12–16.
- Khuroo T, Mohamed EM, Dharani S, et al. In-situ implant formulation of laurate and myristate prodrugs of dolutegravir for ultra-long delivery. J Pharm Sci. 2022;111(8):2312–2321.
- Project horizon: hydrogel injectable depot system for next-generation long-acting HIV prevention and contraception [Internet]. National Institute of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID). 2019-2022 [cited August 10 2022]. Available from: https://grantome.com/grant/NIH/R61-AI142685-03.
- DelSiTech Ltd announces the development of novel long-acting injectable biologics for HIV prevention [Internet]. Turku, Finland: DelSiTech. March 12, 2021.
- Mandal S, Prathipati PK, Belshan M, et al. A potential long-acting bictegravir loaded nano-drug delivery system for HIV-1 infection: a proof-of-concept study. Antiviral Res. 2019 Jul;167:83–88.
- Mandal S, Prathipati PK, Sunagawa SW, et al. A concept evaluation study of a new combination bictegravir plus tenofovir alafenamide nanoformulation with prolonged sustained-drug-release potency for HIV-1 preexposure prophylaxis. Antimicrob Agents Chemother. 2021;65(4). doi: 10.1128/AAC.02320-20.
- Al-Salama ZT. Elsulfavirine: first global approval. Drugs. 2017 Oct;77(16):1811–1816.
- Viriom announces initiation of phase 2 study investigating efficacy of intramuscular long-acting injectable nanoformulation of VM1500A in HIV-infected patients. [Internet]. San Diego (CA): Viriom, Inc.; May 4, 2020.
- Deodhar S, Sillman B, Bade AN, et al. Transformation of dolutegravir into an ultra-long-acting parenteral prodrug formulation. Nat Commun. 2022;13(1):3226.
- Kulkarni TA, Bade AN, Sillman B, et al. A year-long extended release nanoformulated cabotegravir prodrug. Nat Mater. 2020;19(8):910–920.
- Gautam N, McMillan JM, Kumar D, et al. Lipophilic nanocrystal prodrug-release defines the extended pharmacokinetic profiles of a year-long cabotegravir. Nat Commun. 2021;12(1):3453.
- Markowitz M, Gettie A, St. Bernard L, et al. Once-weekly oral dosing of MK-8591 protects male rhesus macaques from intrarectal challenge with SHIV109CP3. J Infect Dis. 2020;221(9):1398–1406.
- Kirtane AR, Abouzid O, Minahan D, et al. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat Commun. 2018;9(1):2.
- Puri A, Bhattaccharjee SA, Zhang W, et al. Development of a transdermal delivery system for tenofovir alafenamide, a prodrug of tenofovir with potent antiviral activity against HIV and HBV. Pharmaceutics. 2019;11(4):173.
- Puri A, Sivaraman A, Zhang W, et al. Expanding the domain of drug delivery for HIV prevention: exploration of the transdermal route. Crit Rev Ther Drug Carrier Syst. 2017;34(6):551–587.
- Waghule T, Singhvi G, Dubey SK, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258.
- Jiang Y, Gao X, Singh ON, et al. Pharmacokinetics of a weekly transdermal delivery system of tenofovir alafenamide in hairless rats. Int J Pharm. 2020;582:119342. doi: 10.1016/j.ijpharm.2020.119342.
- Tuan-Mahmood TM, McCrudden MT, Torrisi BM, et al. Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci. 2013;50(5):623–637.
- Larrañeta E, Lutton REM, Woolfson AD, et al. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1–32.
- Mc Crudden MTC, Larraneta E, Clark A, et al. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. J Control Release. 2018;292:119–129. doi: 10.1016/j.jconrel.2018.11.002.
- Tekko IA, Vora LK, Volpe-Zanutto F, et al. Novel bilayer microarray patch-assisted long-acting micro-depot cabotegravir intradermal delivery for HIV pre-exposure prophylaxis. Adv Funct Mater. 2022;32(9):2106999.
- Paredes AJ, Volpe-Zanutto F, Vora LK, et al. Systemic delivery of tenofovir alafenamide using dissolving and implantable microneedle patches. Mater Today Bio. 2022 ;13:100217.
- Palombo MS, Singh Y, Sinko PJ. Prodrug and conjugate drug delivery strategies for improving HIV/AIDS therapy. J Drug Deliv Sci Technol. 2009;19(1):3–14.
- Liang Z, Giles MB, Stenslik MJ, et al. Direct visualization of the drug release process of non-conductive polymeric implants via molecular imaging. Anal Chim Acta. 2022;1230:340395.
- Nagapudi K, Zhu A, Chang DP, et al. Microstructure, quality, and release performance characterization of long-acting polymer implant formulations with X-ray microscopy and quantitative AI analytics. J Pharm Sci. 2021;110(10):3418–3430.
- Rajoli RKR, Back DJ, Rannard S, et al. Physiologically based pharmacokinetic modelling to inform development of intramuscular long-acting nanoformulations for HIV. Clin Pharmacokinet. 2015;54(6):639–650.
- Rajoli RKR, Demkovich ZR, Flexner C, et al. Predicting pharmacokinetics of a tenofovir alafenamide subcutaneous implant using physiologically based pharmacokinetic modelling. Antimicrob Agents Chemother. 2020;64(8). doi: 10.1128/AAC.00155-20.
- Rajoli RKR, Flexner C, Chiong J, et al. Modelling the intradermal delivery of microneedle array patches for long-acting antiretrovirals using PBPK. Eur J Pharm Biopharm. 2019;144:101–109.
- Nkanga CI, Fisch A, Rad-Malekshahi M, et al. Clinically established biodegradable long acting injectables: an industry perspective. Adv Drug Deliv Rev. 2020;167:19–46.
- Faisant N, Siepmann J, Oury P, et al. The effect of gamma-irradiation on drug release from bioerodible microparticles: a quantitative treatment. Int J Pharm. 2002;242(1–2):281–284.
- Parsons TL, Gwenden KN, Marzinke MA. Interspecies differences in tenofovir alafenamide fumarate stability in plasma. Antimicrob Agents Chemother. 2020;64(9):e00930–20.
- Bao Q, Wang X, Zou Y, et al. In vitro release testing method development for long-acting injectable suspensions. Int J Pharm. 2022;622:121840.
- Li D, Chow PY, Lin TP, et al. Simulate SubQ: the methods and the media. J Pharm Sci. 2021. DOI: 10.1016/j.xphs.2021.10.031
- van ‘t Klooster G, Hoeben E, Borghys H, et al. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother. 2010;54(5):2042–2050.
- Das Neves J, Notario-Pérez F, Sarmento B. Women-specific routes of administration for drugs: a critical overview. Adv Drug Deliv Rev. 2021;16:113865.
- Sharifi S, Caracciolo G, Pozzi D, et al. The role of sex as a biological variable in the efficacy and toxicity of therapeutic nanomedicine. Adv Drug Deliv Rev. 2021;174:337–347.
- Minnis AM, Atujuna M, Browne EN, et al. Preferences for long-acting pre-exposure prophylaxis (PrEP) for HIV prevention among South African youth: results of a discrete choice experiment. J Int AIDS Soc. 2020;23(6):e25528.
- Ngure K, Mugo NR, Bukusi EA, et al. Pills, injections, rings, or implants? PrEP formulation preferences of PrEP-experienced African women for HIV prevention. J Acquir Immune Defic Syndr. 2021;88(4):e30–e32.
- Golla VM, Kurmi M, Shaik K, et al. Stability behaviour of antiretroviral drugs and their combinations. 4: characterization of degradation products of tenofovir alafenamide fumarate and comparison of its degradation and stability behaviour with tenofovir disoproxil fumarate. J Pharm Biomed Anal. 2016;131:146–155. doi: 10.1016/j.jpba.2016.08.022.
- ViiV Healthcare is working with medicines patent pool to progress voluntary licensing for cabotegravir long-acting for prep. [Internet]. Brentford, UK: ViiV Healthcare; 2022.
