1. Introduction
Almost all current orally inhaled drug products (OIDPs) are for the treatment of respiratory diseases, and many have been designed using molecular or formulation strategies to retain drug in the lungs. After deposition in the lungs, the rate at which aerosol particles release drug into the lung lining fluid is a determinant of local bioavailability for drugs with poor solubility. Slow dissolution provides an absorption rate-limiting mechanism for prolonging lung exposure to an inhaled drug and extending the duration of pharmacodynamic effects. Historically, the Inhalation Ad Hoc Advisory Panel of the United States (US) Pharmacopoeia (2008) considered that there was an absence of ‘compelling evidence that dissolution testing is kinetically important for currently approved inhaled drug products’; a conclusion supported in 2012 by an IPAC-RS Dissolution Working Group [Citation1]. Paradoxically, there has since been an intensification of interest in dissolution testing for OIDPs, driven at least in part by the US Food and Drug Administration sponsorship of research in this area [Citation2,Citation3].
More recently, solubility has been proposed as a key attribute of the drug substance and dissolution as a key attribute of the drug product under the foundational principles of an inhaled biopharmaceuticals classification system (iBCS) [Citation4]. This brings closer the prospect of dissolution being recognized formally as a critical quality attribute for certain classes of OIDP and that in vitro dissolution methods will be required to characterize OIDPs in a way that is biorelevant, predictive, and can be used with confidence by medicine developers and regulators. Developing a biorelevant dissolution assay for OIDPs is challenging in practice, requiring collection of a relevant aerosol fraction, a dissolution apparatus, and dissolution medium that replicate the key determinants of dissolution in the lungs and methods for data analysis and interpretation. This article summarizes briefly recent developments in dissolution testing with a focus on pharmaceutical, regulatory, and clinical relevance ().
Figure 1. The pharmaceutical, regulatory, and clinical relevance of dissolution testing for orally inhaled drug products. Noyes Whitney equation for dissolution: dC/dt = dissolution rate, D = diffusion coefficient, S = surface area of dissolution, (Cs-Ct) = concentration gradient of diffusion. Created with BioRender.com.
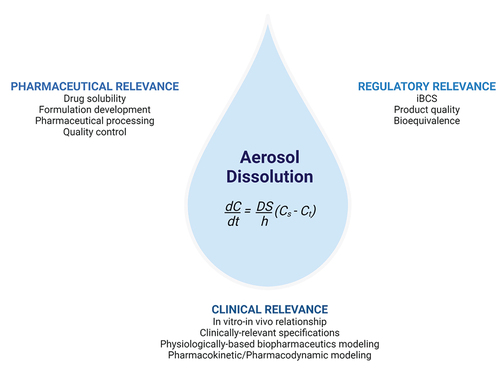
2. Development of dissolution test methods
Dissolution testing has long been recognized as an important part of drug product development and assessment of product quality for medicines. For example, there is a multiplicity of standardized compendial in vitro dissolution methods for orally ingested drug products. In contrast, there is no compendial method for OIDPs. Recent development of disease-specific dissolution methods for orally delivered medicines includes simulated intestinal fluids to represent patients with different gastrointestinal diseases, ulcerative colitis, Crohn’s disease,s and celiac disease [Citation5]. This further illustrates how far inhalation of biopharmaceutics lags as little attention has been given to dissolution medium compositions that represent lung epithelial lining fluids in either health or disease. An often-neglected consideration is that lung lining fluid is different in the conducting airways (mucus-sol layers) compared to the peripheral lung (surfactant layer).
To date, methods utilized for collecting respirable aerosols and studying the dissolution of aerosol particles have largely involved adjustments to existing pharmacopoeial apparatuses [Citation6], together with a few innovative, bespoke technical developments, such as Unidose [Citation2], DissolvIt [Citation7], and RespiCell [Citation8]. The dissolution methods can be broadly categorized as bulk fluids (e.g. USP apparatuses II, IV, and VII) or membrane techniques (e.g. Transwells, Franz cells). The former may not represent the fluid restriction in the lungs and overestimate dissolution rates, whereas the latter may introduce drug transfer effects and sink condition limitations. Inter-laboratory differences in the approaches adopted for the development and attempted optimization of both bulk and membrane dissolution methods for OIDPs make comparisons between studies difficult [Citation9]. At some point, it will be beneficial for medicines developers and regulators to define and standardize readily available, robust, reproducible, methods for inhaled product dissolution that can provide clinically relevant discriminatory product profiling. The analysis and interpretation of aerosol dissolution data have employed similar models and statistics to those used for non-inhaled products. When included in physiologically based pharmacokinetic (PBPK) modeling for inhaled medicines, the dissolution rate can be calculated according to particle size based on dissolution theory [Citation10] or derived from experimental data [Citation11].
3. Pharmaceutical, regulatory, and clinical relevance
The dissolution methods which are currently being used for OIDPs have limited validation for their predictive power and sensitivity. Here, we illustrate the need for greater efforts in this area by considering the pharmaceutical, regulatory, and clinical relevance of OIDP dissolution.
3.1. Pharmaceutical
Dissolution has obvious applications as an in vitro performance test during formulation development, manufacture, and for quality control of OIDPs. Dissolution of inhaled dosage forms is increasingly being investigated as medicines developers seek to balance the needs of dose, formulation, device, and relationship between these factors [Citation12]. During product development, dissolution testing has an important role for some inhaled products in supporting the selection of particle size distributions and excipients, e.g. excipient ratio, excipient type, wetting agents, hydrophobic barriers, and viscosity modifiers. Examples include investigations into the dissolution of inhaled micronized drug powders showing differentiation in release profiles for both a poorly soluble drug candidate with two different particle size distributions and drugs of different solubility possessing similar particle size distributions [Citation9] and slowing of dissolution by the presence of magnesium stearate in tobramycin powders [Citation8]. Dissolution can also discern the effects of pharmaceutical processing, evaluate the performance of modified-release formulations, determine the effect of storage conditions, and be stability-indicating [Citation13]. For example, different blending techniques for lactose dry powder mixtures have been shown to influence the dissolution profile of a poorly soluble inhaled drug candidate [Citation8].
3.2. Regulatory
To license a new drug product, regulatory agencies require evidence of product quality, safety, and efficacy. The development of an iBCS [Citation4] is anticipated to be helpful during product development by classifying when dissolution is a critical quality attribute that should be controlled and is relevant to include in the product quality profile. The iBCS links the physicochemical properties of inhaled drugs and essential material attributes to in vivo pharmacokinetics, i.e. area under the plasma concentration–time curve (AUC), peak plasma concentration (Cmax), and time to reach Cmax (Tmax) and provides a framework under which dissolution is governed by dose and solubility. Dissolution profiles are already being included in regulatory submissions for OIDPs, which creates a need for justified assay conditions or standardized dissolution procedures for such work. Given the current interest, it appears likely that dissolution may become a requirement in regulatory submissions for certain OIPDs. As part of a product quality profile, dissolution testing can also be used to confirm consistent product performance through lifecycle management.
Dissolution assays may also be useful when developing generic products, for which achieving equivalent bioavailability at the site of action depends on an interplay between the processes of aerosol deposition in the lungs, dissolution, and pharmacokinetic processes [Citation14]. A representation of how dissolution parameters might supplement more established in vitro and in vivo data for comparing test (T) and reference (R) products is proposed in .
Table 1. Possible interpretation of the available outcomes when dissolution is considered alongside aerosol and pharmacokinetic properties when evaluating orally inhaled drug product bioequivalence *.
3.3. Clinical
For inhaled medicines, it is difficult to demonstrate experimentally the influence of dissolution on bioavailability at the site of action. It is difficult to know exactly how much drug deposits in the lungs and where it deposits and to make measurements of drug concentration in lung lining fluid. A therapeutically relevant dissolution assay for orally ingested drug products requires drug release profiles to distinguish between attributes in a product that impact in vivo performance [Citation5]. This concept applies to all drug products regardless of route of administration; thus, the challenge for inhaled medicines is to link the dissolution of OIDPs to in vivo performance.
Efficacy for respiratory medicines is associated with drug availability in the lungs, with systemic exposure generally regarded as a measure of safety. However, it is drug concentration in blood vs time profiles that are most readily available to compare inhaled product performance in humans. Studies have been conducted to assess whether pharmacokinetic analysis can provide insights into the pulmonary fate of inhaled drug formulations, with preliminary analysis concluding that systemic PK can discriminate doses available to the lungs and residence time and may also be sensitive to differences in regional lung deposition [Citation3].
There are relatively few examples where the clinical relevance of dissolution has been demonstrated definitively. An interesting investigation into dose proportionality for an inhaled fixed dose combination of fluticasone furoate and vilanterol trifenatate in healthy subjects and found a less than dose-proportionate increase in partial AUC and Cmax of the poorly soluble fluticasone furoate in plasma due to absorption limiting pharmacokinetics, which were attributed to slower dissolution at higher doses [Citation15]. A study designed to probe the effect of two different primary particle sizes of AZD5423, a poorly soluble drug in clinical development, found a 2-fold difference in Cmax in healthy subjects that was attributed to faster dissolution of the smaller particle size [Citation16].
4. Expert opinion
The data-driven developments and limitations in the field are summarized in . The imminent publication of a proposal for an iBCS, heralded by Hastedt et al. [Citation4], promises to provide a framework to classify inhaled products according to the properties of the API and identify those for which dissolution is pharmaceutically, regulatorily, and clinically important. If widely adopted, this will compel the development of dissolution methodology based on sound scientific principles to provide technical platforms to guide and enable advancements in rational inhaled product design and regulation.
Table 2. Developments affecting the dissolution testing for orally inhaled drug products and unmet needs requiring further attention.
The future for pulmonary drug delivery is anticipated to include the development of new treatment modalities and improvements/adaptations to current respiratory medicines as part of moves toward more affordable inhaled medicines and devices that are environmentally sustainable. The development of new and improved medicines is increasingly guided by in silico models to quantitatively link in vitro data on product and drug attributes that govern dose deposition, dissolution rate, permeability, and solubility to systemic exposure, which can be validated and give confidence in estimates of local exposure [Citation17]. In terms of dissolution, it will be necessary to differentiate between (i) discriminatory quality control tools, and (ii) biorelevant methods that predict drug dissolution in vivo.
The challenge is to understand the importance of dissolution and how it interplays with other facets of inhaled product performance, e.g. aerosol deposition, tissue binding, and permeation. With greater availability and refinement of in silico tools, prediction of local and systemic exposure will increasingly be integrated into product development. It remains to be seen where and how the iBCS might serve as a guideline for when dissolution becomes important across areas of pharmaceutical, regulatory, and clinical relevance. The role of epithelial lining fluids as the dissolution media in the lungs is an important area that has received relatively little attention to date. Eagerly anticipated advances in biorelevant in vitro and in silico tools are expected to enable more predicative IVIVC and tease out the role of dissolution in the performance of the current and next generations of inhaled products.
Declaration of interest
Karin Somby, Martin Hingle, and Ivana Tomic are affiliated with Novartis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Riley T, Christopher D, Arp J, et al. Challenges with developing in vitro dissolution tests for orally inhaled products (OIPs). AAPS Pharm Sci Tech. 2012 Sep;13(3):978–989.
- Price R, Shur J, Ganley W, et al. Development of an aerosol dose collection apparatus for in vitro dissolution measurements of orally inhaled drug products. AAPS J. 2020 Mar;22(2). DOI:10.1208/s12248-020-0422-y.
- Hochhaus G, Chen M-J, Kurumaddali A, et al. Can pharmacokinetic studies assess the pulmonary fate of dry powder inhaler formulations of fluticasone propionate? AAPS J. 2021 May;23(3). DOI:10.1208/s12248-021-00569-x.
- Hastedt JE, Bäckman P, Cabal A, et al. iBCS: 1. principles and framework of an inhalation-based biopharmaceutics classification system. Mol Pharm. 2022 May;19(7):2032–2039.
- McAllister M, Flanagan T, Cole S, et al. Developing Clinically Relevant Dissolution Specifications (CRDSs) for oral drug products: virtual webinar series. Pharmaceutics. 2022 May;14(5):1010.
- Mercuri A, Fotaki N. In Vitro Dissolution for Inhalation Products. In In Vitro drug release testing of special dosage forms. Wiley; 2019. DOI:10.1002/9781118675748.ch5.
- Gerde P, Malmlöf M, Havsborn L, et al. DissolvIt: an in vitro method for simulating the dissolution and absorption of inhaled dry powder drugs in the lungs. Assay Drug Dev Technol. 2017 Feb;15(2):77–88.
- Sonvico F, Chierici V, Varacca G, et al. RespicellTM: an innovative dissolution apparatus for inhaled products. Pharmaceutics. 2021 Oct;13(10):1541.
- Franek F, Fransson R, Thörn H, P. Bäckman P, Andersson U, and Tehler U. Ranking in vitro dissolution of inhaled micronized drug powders including a candidate drug with two different particle sizes. Mol Pharm. 2018 Nov;15(11):5319–5326. DOI:10.1021/acs.molpharmaceut.8b00796.
- Ruzycki CA, Murphy B, Nathoo H, et al. Combined in vitro-in silico approach to predict deposition and pharmacokinetics of budesonide dry powder inhalers. Pharm Res. 2020;37(10):209.
- Hassoun M, Malmlöf M, Scheibelhoferd O, et al. Use of PBPK modeling to evaluate the performance of DissolvIt, a biorelevant dissolution assay for orally inhaled drug products. Mol Pharmaceut. 2019;16(3):1245–1254.
- Floroiu A, Klein M, Krämer J, et al. Towards standardized dissolution techniques for in vitro performance testing of dry powder inhalers. In: Dissolution Technologies. Aug 1, 2018. Vol. 253. Dissolution Technologies Inc. DOI:10.14227/DT250318P6.
- Forbes B, Richer NH, Buttini F. Dissolution: a critical performance characteristic of inhaled products? Pulm Drug Deliv. 2015;223–240. DOI:10.1002/9781118799536.ch10.
- Forbes B, Bäckman P, Christopher D, et al. In Vitro testing for orally inhaled products: developments in science-based regulatory approaches. Aaps J. 2015 Jul;17(4):837–852. DOI:10.1208/s12248-015-9763-3.
- Allen A, Bal J, Moore A, et al. Bioequivalence and dose proportionality of inhaled fluticasone furoate. J Bioequivalence Bioavailab. 2014;6(1):24–32.
- Bäckman P, Tehler U, Olsson B. Predicting exposure after oral inhalation of the selective glucocorticoid receptor modulator, AZD5423, based on dose, deposition pattern, and mechanistic modeling of pulmonary disposition. J Aerosol Med Pulm Drug Deliv. 2017;30(2):108–117.
- Bäckman P, Cabal A, Clark A, et al. iBCS. 2: mechanistic modeling of pulmonary availability of inhaled drugs versus critical product attributes. Mol Pharm. 2022 May;19(7):2040–2047.
