ABSTRACT
Introduction
The growing interest in subcutaneous delivery of larger single-dose volumes using handheld autoinjectors raises questions about the feasible upper limits for injection volume and rate. This review critically evaluates the literature on subcutaneous administration with dose volumes greater than 1.0 mL. In so doing, it examines how previous work has addressed limitations and considerations for designing and developing large-volume autoinjectors.
Areas covered
This article synthesizes 31 studies on large-volume subcutaneous delivery through a systematic review process and structures their findings based on three themes critical to developing large-volume autoinjectors: injection tolerability, suitability for self-administration, and pharmacokinetic equivalence with existing dosing options. This review highlights the answers provided by previous studies and identifies promising avenues for future research.
Expert opinion
This review finds that the literature supports the feasibility of delivering single large-dose subcutaneous volumes, providing a foundation for large-volume autoinjectors. Moreover, the review guides future research to address questions within and across themes critical to large-volume autoinjector development, helping to provide health-care professionals and patients with more effective and convenient dosing options.
1. Introduction
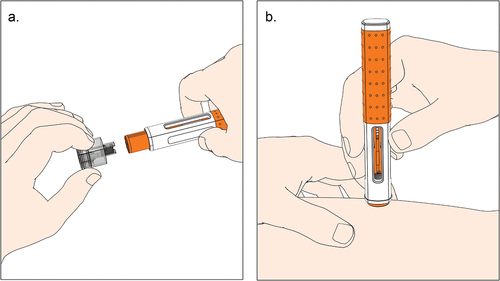
Subcutaneous drug delivery has emerged as a viable and often preferred alternative to intravenous infusion of biologics, offering patients and health-care providers new home-based treatment options that improve treatment adherence, reduce the cost of therapy, and decrease health-care resource utilization [Citation1–6]. Although subcutaneous delivery options advance patient-centered care, their development faces numerous challenges ranging from pharmacokinetics and efficacy to bioavailability, viscosity, stability, and the development of delivery devices for safe and effective home use [Citation7,Citation8]. It is, therefore, hardly surprising that the industry has shown great interest in handheld autoinjectors that allow patients and caregivers to administer medications safely and effectively [Citation9–11]. illustrates the user steps of a ready-to-use prefilled handheld autoinjector with visual and audible feedback at the beginning and end of the injection. These spring-actuated mechanical devices, activated by pressing a button or pushing against the injection site, provide a needle safety function and deliver a predetermined fixed volume from a prefilled syringe within 10 to 20 s [Citation12,Citation13]. Following delivery into the subcutaneous tissue, liquid-formulated biologics accumulate locally as a fluid depot and traffic through the interstitial matrix into the lymphatic system, then into the bloodstream [Citation14–16].
Figure 1. Illustration of user steps with a prefilled handheld autoinjector activated by push-on-skin. a. The user pulls the needle cap straight off and holds the autoinjector against the skin. b. The user holds the autoinjector down until the injection is complete (i.e. until the second click is heard and the plunger rod filled the viewing window).
The availability of user-tested device platforms has broadened access to handheld autoinjectors for biologics in chronic diseases [Citation10,Citation17–20]. Tumor necrosis factors to mediate rheumatoid arthritis and other chronic debilitating diseases have pioneered home treatment with autoinjectors [Citation20,Citation21]. Today, subcutaneous dosing options exist for different drug modalities, such as bispecific antibodies [Citation22] and small-interfering RNAs [Citation23,Citation24], and therapy areas, such as cardiovascular diseases and obesity [Citation18,Citation25–27], respiratory diseases [Citation27,Citation28], migraine [Citation19,Citation29–32], psoriasis [Citation31,Citation32], and rare diseases [Citation10,Citation19–22,Citation33]. Handheld autoinjectors have become a preferred option for safe and effective self-administration of single doses and have been widely adopted as the standard for subcutaneous drug delivery [Citation34,Citation35].
The industry for a long time assumed the delivery of 1.0 mL in less than 10 to 15 s to be the feasible upper limit for handheld autoinjectors [Citation31,Citation36]. However, recent approvals of products with single-dose volumes up to 2.0 mL have demonstrated the successful delivery of larger volumes. provides an overview of drugs marketed in the 2.25 mL single-dose prefilled syringe format. This has prompted pharmaceutical manufacturers to replace two sequential smaller dose-volumes with a single large-volume injection [Citation47,Citation48]. Moreover, research has shown that patients can safely and effectively complete large-volume injections lasting up to 30 s [Citation36].
Table 1. Approved products from the U.S. Food and Drug Administration and European Medicines Agency using 2.25 mL prefilled syringes, prefilled needle safety devices, and prefilled handheld autoinjectors×.
These recent advances have energized attempts to expand the feasible volume limit for handheld autoinjectors. Higher injection volumes not only reduce the frequency of injections and enhance patient preferences and therapy adherence [Citation49–51] but also help establish subcutaneous dosing for new therapeutic areas and drug modalities that require larger single-dose volumes [Citation2,Citation52,Citation53]. As such, the advent of handheld autoinjectors exceeding 2.0 mL capacity has garnered significant attention. provides an overview of investigational large-volume autoinjectors that enable the delivery of single-dose volumes up to 10.0 mL. These devices could provide an alternative to more complex electromechanical large-volume wearable injectors that remain patched on the skin and slowly inject a single-dose volume over minutes if not hours [Citation59–62]. However, there is uncertainty about the feasibility of the injection volume and the rate that can be achieved with high-volume handheld autoinjectors. Although previous literature reviews have summarized past work on large-volume subcutaneous injections [Citation63–65], they have provided limited and somewhat fragmented insights into the feasibility of handheld autoinjectors for high-rate large-volume subcutaneous dosing. To address this gap, this article analyzes prior literature based on three themes to gain a more coherent understanding of large-volume autoinjectors: injection tolerability, suitability for self-administration, and pharmacokinetic equivalence with existing delivery options.
Table 2. An overview of investigational large-volume handheld autoinjectors with pre-filled syringes and cartridges exceeding 2.25 mL capacity.
These three themes are essential for the successful development and approval of novel large-volume autoinjectors. First, increasing the subcutaneous injection volume and rate may intensify injection-site reactions, change the subcutaneous depot location, and increase pain [Citation15,Citation16,Citation64,Citation66]. Therefore, research on large-volume high-rate injections must examine injection tolerability as a necessary condition for regulatory approval and successful market uptake [Citation67–69]. Second, manufacturers must ensure safe and effective drug self-administration, as autoinjectors help shift the point of care from the hospital to the home [Citation29,Citation70,Citation71]. Regulators will scrutinize human factor validation to ensure users can perform self-injection at home [Citation72,Citation73]. In addition, patients must accept the administration of larger volumes, potentially resulting in longer injection time [Citation36,Citation74]. Third, the study of large-volume high-rate subcutaneous dosing with autoinjectors may lag behind the clinical evaluation of conventional subcutaneous injection methods [Citation75,Citation76]. Researchers may conduct large multicenter pivotal studies with safety and efficacy end points using conventional drug delivery methods and establish pharmacokinetic equivalence with emerging large-volume dosing options in subsequent bridging studies [Citation77].
In conclusion, these three themes must be thoroughly understood to assess the feasibility of handheld autoinjectors for the subcutaneous administration of larger volumes. With that aim, this review article critically assesses the existing literature on large-volume subcutaneous drug delivery and structures their findings along injection tolerability, suitability for self-administration, and pharmacokinetic equivalence with existing dosing options. It then summarizes those questions prior work has answered, examines potential barriers to developing large-volume handheld autoinjectors, and concludes by highlighting promising areas for future research.
2. Review methods
The objective of this systematic literature review is to structure and synthesize previous work on subcutaneous administration of single large-volume doses (>1.0 mL) of a therapeutic agent. To identify relevant articles, the authors performed a multi-step review process. First, a Boolean search of the National Library of Medicine (pubmed.ncbi.nlm.nih.gov) was conducted using the keywords subcutaneous’, ‘injection’, ‘volume’, ‘pain’, and ‘tolera*’ (to account for different terms, such as tolerability or tolerance) in the title, abstract, or keywords. The authors then repeated the Boolean search on Google Scholar. Due to the large number of results and automatic sorting by article relevance, the review on Google Search was limited to the first 200 search results. These search results identified 602 articles, which the authors manually reviewed to determine whether the article focused on the study of large-volume subcutaneous injections. Of the studies considered relevant, the authors examined the references, engaged in repeated discussions, and collected relevant articles known to the authors before the systematic review to ensure that the study included all relevant articles. This multi-step process identified 31 articles that provided relevant insights into large-volume subcutaneous injections. The authors then organized the 31 articles based on their contributions to injection tolerability, suitability for self-administration, and pharmacokinetic equivalence with existing dosing options.
3. Review of current research
3.1. Overview of studied injection volume-rate ranges and themes
highlights the increasing interest in studying large-volume subcutaneous injections. While the first article on the area was published in 1996, 48% of the sample (15 articles) have appeared since 2020. shows that previous work has explored various combinations of injection volumes and rates across different device technologies, including manual prefilled syringes and autoinjectors (1.0–2.0 mL; 0.1–0.9 mL/s), large-volume wearable injectors (5.0–10.0 mL; 0.02 mL/s), and manual large-volume syringes and syringe-type infusion pumps (15.0–60.0 mL; 0.01–0.1 mL/s). indicates that previous studies have mainly focused on the large-volume/low-rate and the small-volume/high-rate ranges. Although several studies have addressed injection rates up to 0.9 mL/s, these have only studied injection volumes up to 2.0 mL. Conversely, injection rates were limited to around 0.1 mL/s for injection volumes significantly greater than 2.0 mL. Researchers have seldom examined large-volume/high-rate ranges, which would be most relevant to the new device category of large-volume handheld autoinjectors. These devices require injection rates of up to 0.5 −1.0 mL/s to achieve injection times of less than 15 s. In conclusion, only few findings directly apply to large-volume autoinjectors, and available data must be carefully evaluated before being extrapolated to the new device category.
Figure 2. Evolution of previous research on large-volume subcutaneous drug delivery (N = 31), organized by three themes: injection tolerability, suitability for self-administration, and pharmacokinetic equivalence.
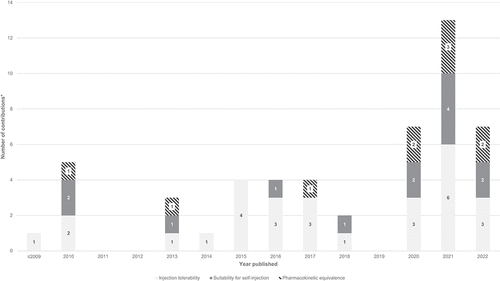
Figure 3. Overview of injection volume-rate ranges addressed by previous work and volume-rate ranges applicable to different drug delivery device categories.
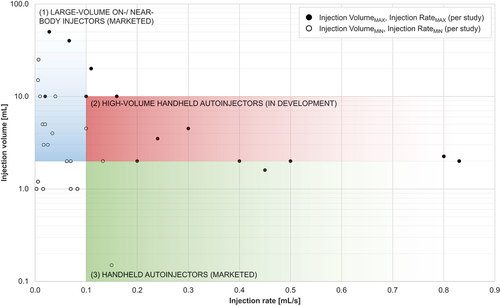
Table 3. Summary of literature review.
shows that prior work has covered all three themes critical to developing large-volume handheld autoinjectors. Although previous work has predominantly focused on advancing the injection tolerability theme (28 articles, 90% of the sample), 42% (13 articles) have contributed to the literature on suitability for self-administration. Thirty-two percent (10 articles) have advanced the pharmacokinetic equivalence theme. Five articles (16%) have covered all three themes, while 9 (29%) articles have addressed a combination of two of the three themes. The majority (17 articles, 55%) have provided insights into a single theme, leaving the others unaddressed.
3.2. Injection tolerability
3.2.1. Pain
Eighteen articles (58% of the reviewed articles) provide insights into the effects of injection volume and rate on injection-related pain. shows that 16 studies evaluated pain using a 100 mm Visual Analog Scale (VAS). Fifteen of the 16 studies reported mild injection-related pain according to the VAS-based pain categories defined by Jensen and colleagues [Citation100], while one study observed moderate-to-severe pain [Citation95]. The authors attributed these higher pain levels to irritations caused by the citrate co-formulation, possibly masking the effects of the injection volume and rate on pain. Prior reviews concurred with these findings, highlighting that besides injection system design, dosing parameters, injection technique, and patient-related attributes, the drug formulation is a critical factor in determining pain level [Citation64,Citation101].
provides an overview of the findings on the influence of injection rate and volume on pain. Nine studies examined the effect of increasing injection rates on pain. Results from eight studies showed no or no clinically relevant changes in pain. One study reported a significant decrease in pain with 3.5 mL injections administered in 10 min compared to 3.0 mL injected in 1 min [Citation68]. However, the authors considered this difference clinically irrelevant due to the slight absolute increase in pain [Citation68].
Table 4. A summary of review results for injection tolerance themes, including injection-related pain, injection-site reactions, and adverse events.
shows that four studies found a significant positive relationship between injection volume and pain. For instance, a 3.5 mL injection in 1 min was more painful than a 1.2 mL injection over 5 s – even though the results do not show clinical significance [Citation68]. Other studies also reported higher pain levels with larger volumes of 1.2 and 1.6 mL compared to lower volumes of 0.4 and 0.8 mL [Citation84] or with volumes of 1.0 and 1.5 mL than for 0.2 and 0.5 mL [Citation85]. Similarly, Zijlstra and colleagues [Citation74] concluded that a single 2.25 mL injection caused significantly more pain than injections of 0.1, 0.4, and 0.8 mL. Conversely, five articles found no significant or clinically relevant effects of injection volume on pain. However, the literature review does not provide conclusive evidence on the design space for large-volume autoinjectors, thus inviting further investigations to validate these aspects for injection volumes between 2.0 and 10.0 mL administered within less than 1 min.
Two studies analyzed the impact of fluid viscosity on perceived pain, with one study finding a statistically significant negative relationship [Citation15] and the other no significant effect [Citation61]. These conflicting results may be due to differences in experimental design, such as the device type used (syringe pump versus wearable injector), the injection volume (2.0–3.0 mL versus 5.0 mL), and the injection rate (0.02–0.30 mL/s versus 0.02 mL/s). Two studies found higher pain with injections into the thigh compared to the abdomen [Citation74,Citation84].
3.2.2. Adverse events and injection-site reactions
Twenty-two studies (71.0%) examined the incidence of adverse events (AEs) and injection-site reactions. provides a summary of the effect of injection volume and rate on AEs.
No study reported any injection-related AEs except for injection-site reactions. In two cases, drug-related AEs were reported [Citation31,Citation88]. The occurrence of injection-site reactions varied greatly, ranging from 2.5% up to 100.0% of injections, highlighting how drug product-specific attributes may mask the effects of injection parameters [Citation53,Citation74,Citation81,Citation95]. Reactions typically observed at the injection site were redness, itching, and swelling; in some cases, hematomas or bleeding occurred. Bruising, which has been reported in other work on the tolerability of subcutaneous drug delivery using autoinjectors [Citation102–104], was mentioned in only few of the studies [Citation31,Citation61,Citation84,Citation86,Citation95]. While most injection-site reactions were mild, one study reported moderate injection-site reactions [Citation78] and another study reported severe injection-site reactions [Citation79]. The authors of the latter study attributed the severe injection-site reactions to the use of a novel injection patch rather than injection volume or rate. This study therefore highlights that the injection device technology can have a significant impact on AEs.
Out of the 14 studies that evaluated the relationship between injection volume and injection-site reactions or injection-related AEs, four studies found a positive correlation between the two variables (two on the severity of AEs and two on the frequency of AEs), while 10 studies did not observe any correlation ().
Eleven studies examined the influence of injection rate on the frequency of injection-site reactions and injection-related AEs. One study found a positive correlation between the two variables [Citation66], while 10 observed no effects. None of these studies performed a statistical analysis determine significance.
3.2.3. Injection-site leakage
Ten articles (32.2%) investigated the occurrence of injection-site leakage (). The review showed that when injection-site leakage did occur, it was only in small amounts, and the injection parameters had little to no impact on its occurrence. Therefore, this review suggests that injection-site leakage can be considered negligible from both a practical and clinical perspective. Out of the 10 studies, eight studies observed no or minimal leakage (less than 1.0% of the injection volume), while one study reported ‘some’ leakage [Citation68]. Another study observed ‘limited’ leakage of around 2.0% of the injection volume [Citation66]. Zijlstra and colleagues [Citation74] found that leakage increased with increasing volume but remained below 1.0% of the injected volume. They also confirmed that the amount of leakage met the acceptable accuracy requirements in 99.6% of the cases as per the relevant standard. Similarly, Doughty and colleagues [Citation82] observed increased leakage for high-viscosity solutions, but it was still below 1.0% of the injection volume. Another study showed that injection-site leakage decreased with increasing injection depth but remained below 1.0% across scenarios studied [Citation95]. The remaining studies found no significant effects of injection volume, rate, or fluid viscosity on leakage.
3.3. Suitability for self-administration
Of the 31 articles reviewed, four (12.9%) focused on the safe and effective use of large-volume injection devices, and 10 (32.3%) studied user preferences, such as treatment acceptance and satisfaction. A single article studied both usability and user preference (). The review found broad agreement in support of the safe and effective administration of large-volume doses [Citation78,Citation81]. However, previous research has been inconclusive on how injection parameters, such as volume and rate, impact user preference.
Studies have confirmed the safe and effective use of 2.0 mL prefilled syringes [Citation31,Citation90] and handheld autoinjectors [Citation36]. A phase 3 clinical trial on patient ease of use and satisfaction concluded that 88% of patients completed all required user steps and 90% reported being very satisfied or satisfied with large-volume self-injection [Citation31]. Pager and colleagues [Citation90] found that a new 8.0 mm needle improved user experience for 2.0 mL injections with high-viscosity fluids compared to existing 12.7 mm needle syringes. Although large-volume doses translate into longer injection duration, Schneider and colleagues [Citation36] showed that participants in a simulated use study successfully completed injections up to 30 s with an autoinjector, regardless of disease state, age, or visual and dexterity limitations. Researchers consistently reported high patient satisfaction with high-volume injections across device categories, including large-volume wearable injectors. For instance, Lange and colleagues [Citation62] found high user acceptance for a large-volume wearable injector. While these insights have important implications for future device design and development, the simulated study design limits their findings to user-related aspects, thus remaining silent on others, such as pain or injection-site leakage. Woodley, Morel et al. [Citation99] fill these gaps with their in-human study and found that patients favorably perceived self-injection of 5.0 mL with a wearable large-volume injector and would use the device if prescribed.
This review uncovers conflicting results regarding user preferences for different large-volume dosing options. Kokolakis, Kreis et al. [Citation86] found patients prefer a single large-volume injection (2.0 mL) over two separate small-volume injections (1.0 mL each), while Müller-Ladner and colleagues [Citation88] found a strong user preference for smaller single-dose volumes. Preferences also varied depending on the injection rate. As such, Shapiro [Citation93,Citation94] found patient preferences for high-rate subcutaneous injection over slow infusion-pump-enabled administration. In contrast, Tangen et al. [Citation96] concluded that patient preferences for subcutaneous injection of 4.5 mL lidocaine were robust to changes in injection rate (0.1–0.3 mL/s) and duration (15 s − 45 s).
3.4. Pharmacokinetic equivalence with existing dosing options
summarizes the results of the 10 articles that studied pharmacokinetic (PK) equivalence of different drug delivery methods. The review found that changing from multiple low-volume to a single large-volume injection does not affect PK profiles and bioavailability [Citation31,Citation66,Citation79,Citation86]. For instance, the serum exposure of 300 mg secukinumab from a single 2.0 mL dose was similar to two sequential 1.0 mL injections [Citation31,Citation79]. Another study found no differences in the PK profile of tralokinumab when comparing different injection rates of a single 2.0 mL injection with two sequentially administered 1.0 mL injections [Citation66]. Comparing two 1.0 mL injections with a single 2.0 mL subcutaneous injection, a clinical study on tildrakizumab found supportive evidence [Citation86].
Table 5. A summary of review results for pharmacokinetic equivalence theme.
Changing the injection rates while keeping the injection volume constant did not change the PK profiles [Citation66,Citation79,Citation91]. For example, Portron et al. [Citation91] found no statistically significant differences in the PK profiles of gantenerumab patient groups with an injection time of 5 and 15 s. Similarly, varying the time required to inject 2.0 mL secukinumab between 5 min and 10 s did not affect the PK profile [Citation79].
Studies examining large-volume injections of monoclonal antibodies and immunoglobulins concluded that increasing the injection volume and rate did not affect serum concentrations [Citation53,Citation78,Citation81,Citation93,Citation94]. Larger injection volumes (<50.0 mL) and rates (<0.028 mL/s) did not impact safety and tolerability of immunoglobulins, simplified overall administration, and reduced the number of injection sites and injection duration [Citation78]. Cowan and colleagues [Citation81] also concluded that increasing injection rates up to 0.033 mL/s did not change immunoglobulin serum levels. These findings were consistent for patient cohorts with manual push-type infusion and pump-assisted delivery [Citation78,Citation93,Citation94]. Subcutaneous injection of crenezumab doses up to 40.0 mL (7200 mg) neither affected pharmacokinetics nor bioavailability parameters [Citation53]. Adding recombinant hyaluronidase improved PK profiles compared with intravenous infusion and subcutaneous bioavailability [Citation53]. The latter results have important implications for the development of large-volume injectors and show that adapting a formulation to meet the requirements of large-volume high-rate injections may affect PK equivalence.
4. Future research directions
4.1. Toward an agenda for future research
This review analyzes 31 articles on large-volume subcutaneous delivery and organizes their findings into three themes critical to the development and approval of large-volume autoinjectors: injection tolerance, suitability for self-administration, and pharmacokinetic equivalence. These themes are not only helpful in organizing the existing literature but also provide the basis for categorizing future research, as outlined in . First, the review results call for future studies within the three themes that address questions related to high-rate injections with high-volume autoinjectors. Second, they highlight the need for integrative work that spans the three themes. Advancing topics at the intersection of these themes will promote comprehensive views of the feasibility of large-volume autoinjectors, and help address critical trade-offs in their design and development.
Table 6. Proposed future research topics classified by theme.
4.2. Future research within themes
The review analyzed prior research on large-volume subcutaneous injections across therapeutic contexts and device categories, such as manual syringes [Citation31], syringe pumps [Citation61,Citation66,Citation84], wearable large-volume injectors [Citation61,Citation97], and handheld autoinjectors [Citation36,Citation95]. While these studies advance understanding of large-volume subcutaneous injections in general, they do not address certain issues specific to high-rate and large-volume injections with handheld devices (). Therefore, this review suggests avenues for future work on large-volume autoinjectors within the three themes ().
This review advances the key insight that although higher injection volume and rate may increase pain, the impact was low on the pain scale (), and drug formulation may mask these effects [Citation95]. These findings inform future work on handheld autoinjectors for high-volume dosing. Effective subcutaneous dosing with handheld autoinjectors will likely hinge on new formulations that allow rapid absorption of highly concentrated biologics in subcutaneous tissue [Citation65,Citation105]. For instance, the co-formulation of the dispersion enhancer hyaluronidase has effectively improved subcutaneous delivery [Citation95]. Still, new formulations may also affect pain perceptions. Pain is particularly significant in the case of large-volume autoinjectors, as users may remove the device prematurely from the injection site during prolonged injection times if it is too painful [Citation36]. Future work should therefore study the impact of such formulation advances on pain-related clinical outcomes.
This review invites future work to study the effects of high-volume autoinjectors on injection-related pain, adverse events, and injection-site leakage (). Bruin and colleagues [Citation79] have reported a new injection patch to cause severe injection-site reactions. Therefore, future research must ensure that the design of new high-volume autoinjectors does not cause such AEs. Various technical attributes of handheld devices, such as the user forces required to trigger the device or the type or design of the needle shield pressed against the injection site, should be considered in optimizing device design to prevent excessive tissue pressure, back-flow and leakage of injected solution, and tissue damage. In fact, previous work has shown that tissue resistance can cause problems with autoinjectors for subcutaneous injection [Citation106]. These studies will provide a more complete understanding of the challenges specific to high-volume injectors.
This review presents strong evidence of the feasibility of self-administrating single large-volume doses. Researchers have demonstrated safe and effective use for handheld autoinjectors up to 2.25 mL [Citation16,Citation36], wearable large-volume injectors [Citation62,Citation97,Citation99], and large-volume manual syringe and infusion pumps [Citation93,Citation94]. In particular, studies show that increasing injection volume and time was feasible for handheld autoinjectors [Citation36]. However, the review shows conflicting views on user preferences for different dosing options [Citation86,Citation88,Citation93,Citation94,Citation96]. Future work should therefore examine how user preferences for large-volume autoinjector-based dosing options change with changes in dosing regimens. Previous studies have shown that injection duration and frequency play a significant role in treatment choices and adherence [Citation1,Citation51,Citation107–109]. Moreover, the review calls for future work on user preferences across device categories, such as manual syringes, handheld autoinjectors, wearable large-volume injectors, and syringe pumps. As the variety of devices continues to increase, research must provide health-care professionals and patients with the necessary evidence to make treatment decisions for optimal adherence and therapy outcomes.
The review found the pharmacokinetic profiles to be stable in response to changes in injection parameters (). These findings are in line with the slow absorption rate of therapeutic proteins from the subcutaneous extracellular matrix [Citation8]. However, previous studies have mainly focused on switching from multiple small-volume injections to a single large-volume dose without adjusting the time intervals between injections [Citation31,Citation66,Citation79]. Future research should examine the effects of reducing injection frequency using large-volume autoinjectors on pharmacokinetic equivalence. Such a shift in dosing could potentially lead to improved treatment adherence and patient preference [Citation51,Citation107].
This review also highlights the potential for future work to evaluate novel approaches to assess the pharmacokinetic equivalence of high-volume autoinjectors compared to low-dose injections given more frequently. Currently, new subcutaneous dosing options are established through dedicated drug-by-drug bridging studies [Citation76]. However, future work could consider molecule-independent approaches to clinical bridging [Citation110] to facilitate access to and accelerate the time-to-market of new large-volume handheld autoinjectors.
4.3. Future research across themes
This review highlights that 55% of the articles advanced understanding of a single theme while not addressing others (). Although 29% of the articles combined two themes, only a limited number (16%) provided an integrated perspective on all three themes. Limited resources and complexities in research design limit the number of themes one can address in a single study. However, researchers need to acknowledge the risk of isolation of each of the three themes. Studies that advance insights within a single theme are problematic because they can lead to lop-sided empirical work that selectively emphasizes some aspects and ignores others. Blind spots at the intersection of themes can result in incomplete knowledge, which is an impediment to effective decision-making during the development of large-volume autoinjectors.
Therefore, this review underscores the importance of considering all three themes and developing an encompassing view of large-volume high-rate injections with handheld devices. Consider, for example, an innovative clinical trial design to bridge the current small-volume to new large-volume autoinjectors. Patients would administer multiple small-volume doses followed by a single large-volume autoinjector dose. In addition to reporting pharmacokinetics-related end points, the study may also examine pain perceptions, monitor use errors, and assess perceived ease of use and overall treatment satisfaction. Such a study could provide insights into injection tolerability, device usability, and pharmacokinetic equivalence, enabling broad adoption of handheld autoinjectors in clinical practice.
Another example of a research question that arises only at the intersection of the three themes is addressing the inherent trade-offs between injection tolerance, suitability for self-administration, and injection duration (). While lower-rate injections may result in reduced pain [Citation95], longer injection durations may increase the risk of use errors [Citation36]. Addressing this challenge through innovative research designs could lead to better informed specification of the injection time of large-volume handheld autoinjectors. Thus, the illustrative example re-emphasizes the importance of intertwining themes and calls for innovative research designs that provide integrated insights spanning injection tolerance, suitability for self-administration, and pharmacokinetic equivalence.
5. Conclusion
This review critically evaluates the existing literature on large-volume subcutaneous injections to assess the feasibility of large-volume handheld autoinjectors. The study structures the findings of previous work along three themes critical to large-volume autoinjector development. Despite the lack of prior work specifically studying autoinjectors exceeding 2.0 mL volume capacity, the review demonstrates that the insights provided by existing literature are relevant to and support using handheld autoinjectors for large-volume single-dose delivery. The review also identifies two areas for future research. First, it encourages scholars to address the specific challenges related to injection tolerability, suitability for self-administration, and pharmacokinetic equivalence in the context of handheld autoinjectors. Second, the review suggests integrating the three themes to develop a comprehensive view of large-volume high-rate injections and avoid lop-sided empirical work that selectively emphasizes some aspects while neglecting others.
6. Expert opinion
The study of large-volume subcutaneous drug delivery has developed into a vibrant field of research, where scholars have made significant headway in exploring the upper limits of subcutaneous injection. While researchers have yet to convince the industry, health-care providers, patients, and regulatory authorities of the feasibility of large-volume handheld autoinjectors for volumes between 2.0 and 5.0 mL or even beyond, the review provides a valuable basis for developing these new dosing options. With the increasing demand for safe and effective self-administration options, large-volume autoinjectors could soon gain a foothold in the market.
Autoinjectors have effectively emerged as a viable option for safe and effective subcutaneous self-injection of up to 2.0 mL (). Handheld devices for single doses exceeding 2.0 mL would be the next step in this incremental development. These devices under development (see ) leverage the well-accepted and proven push-on-skin handling principle [Citation29,Citation111] and may allow more seamless health-care provider and patient onboarding with lower training requirements. This is in contrast to large-volume wearable devices, which deviate more fundamentally from the standard handling principles of handheld devices [Citation51,Citation59,Citation62,Citation99].
Furthermore, large-volume autoinjectors leverage the potential of established technologies, manufacturing processes, and regulatory pathways, while other emerging device categories, such as capsules for the oral delivery of biologics [Citation112] or needle-free injectors [Citation113], pose additional challenges and require different kinds of expertise, development processes, and innovation routines. These advantages enable pharmaceutical companies to reinforce existing capabilities, mitigate risks in device development, and accelerate time-to-market. For example, some large-volume autoinjector device platforms are largely compatible with existing infrastructures and manufacturing processes, such as fill-finish, final assembly, and packaging (cf. ).
Finally, large-volume handheld autoinjectors reduce injection duration (), which has been shown to increase treatment preference and contribute to the widespread market acceptance of subcutaneous drug delivery [Citation1,Citation51,Citation93]. By allowing for faster injection of large single-volume doses, large-volume autoinjectors may further boost the acceptance of subcutaneous injections.
While large-volume autoinjectors offer significant potential benefits, these devices also face barriers to adoption. Pharmaceutical manufacturers must establish new primary packaging suitable for high-volume drug delivery and address questions around high-concentration drug formulation, process development, analytical methods, and drug stability [Citation114,Citation115]. For investigational new drugs where time-to-market is critical, pharmaceutical manufacturers are more likely to adopt well-characterized syringes or cartridges for low-volume dosing systems and turn toward innovative large-volume dosing options only later in the life cycle management process.
In conclusion, large-volume handheld autoinjectors have the potential to offer new dosing regimens for drugs already injected subcutaneously and to expand subcutaneous injections to new fields, such as cancer care [Citation1,Citation52]. Hence, these devices may become instrumental in broadening access to innovative medicine as they help shift the point of care from the hospital to the home. In oncology, for example, efforts are underway to establish more flexible care concepts where nurses can perform at-home injections [Citation3,Citation116] and patients self-report symptoms during therapy [Citation117,Citation118]. The high-rate delivery of biologics with large-volume autoinjectors may further improve patient satisfaction, reduce healthcare resource utilization, and increase advantageous effects on total health care and societal costs of subcutaneous drug administration. However, patient preferences for devices and dosing regimens are complex and subject to change [Citation51,Citation107,Citation108]. Thus, we anticipate multiple dosing options to co-exist in the future, providing more flexibility to personalize treatment decisions to patients’ diverse needs. The potential benefits to patients and health-care providers make handheld autoinjectors for the subcutaneous delivery of large-volumes a field worth exploring.
Article highlights
The systematic review assesses the feasibility of handheld autoinjectors with dose volumes greater than 2.0 mL by critically evaluating previous work on large-volume subcutaneous injections.
The analysis identifies 31 empirical studies on large-volume subcutaneous drug delivery and synthesizes their findings on injection tolerability, suitability for self-administration, and pharmacokinetic equivalence with existing dosing options.
Although there are few studies on autoinjectors with a capacity exceeding 2.0 mL, the review suggests that handheld autoinjectors are suitable for delivering a single large-volume dose.
The article highlights promising avenues for future research and presents a more comprehensive approach to answering critical questions in the development of large-volume autoinjectors.
Large-volume autoinjectors may emerge as a promising new device category that builds on established technologies and expertise, manufacturing processes, and regulatory pathways, while expanding subcutaneous drug delivery to new therapy areas.
Declaration of interest
The authors are employed by Ypsomed AG. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors are grateful to Mathias Romacker and Ian Thompson for their critical review of and insightful contributions to the competitive and market analysis of high-volume subcutaneous drug delivery.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Pivot X, Gligorov J, Müller V, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013 sep 01;14(10):962–970. DOI:10.1016/S1470-2045(13)70383-8.
- Pivot X, Verma S, Fallowfield L, et al. Efficacy and safety of subcutaneous trastuzumab and intravenous trastuzumab as part of adjuvant therapy for HER2-positive early breast cancer: final analysis of the randomised, two-cohort PrefHer study. Eur J Cancer. [ Oxford, England]. 1990 [2017 Nov];86:82–90. DOI:10.1016/j.ejca.2017.08.019.
- Franken M, Kanters T, Coenen J, et al. Hospital-based or home-based administration of oncology drugs? A micro-costing study comparing healthcare and societal costs of hospital-based and home-based subcutaneous administration of trastuzumab. Breast. 2020;52:71–77.
- Stoner KL, Harder H, Fallowfield LJ, et al. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient-Patient-Centered Outcomes Res. 2015;8(2):145–153. DOI:10.1007/s40271-014-0075-y
- Walsh C, Minnock P, Slattery C, et al. Quality of life and economic impact of switching from established infliximab therapy to adalimumab in patients with rheumatoid arthritis. Rheumatology. 2007;46(7):1148–1152. DOI:10.1093/rheumatology/kem074
- Martin A, Lavoie L, Goetghebeur M, et al. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin infusion therapy in adult patients with primary immune deficiency. Transfus Med. 2013;23(1):55–60. DOI:10.1111/j.1365-3148.2012.01201.x
- Jiskoot W, Hawe A, Menzen T, et al. Ongoing challenges to develop high concentration monoclonal antibody-based formulations for subcutaneous administration: quo Vadis? J Pharmaceut sci. 2022;111(4):861–867. DOI:10.1016/j.xphs.2021.11.008
- Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018 Oct;32(5):425–440.
- Tischer B, Mehl A. Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Preference Adherence. 2018;12:1413.
- García-Moguel I, Rosado A, Gómez-Cardeñosa A, et al. Reliability, satisfaction and effectiveness of benralizumab home self-administration in patients with severe eosinophilic asthma in real-world practice: the auto-benra study. Journal Asthma Allergy. 2022;15:623–632.
- Alpizar S, Megally A, Chen C, et al. Functionality And performance of an accessorized pre-filled syringe and an autoinjector for at-home administration of tezepelumab in patients with severe, uncontrolled asthma. J Asthma Allergy. 2021;14:381.
- Lange J, Thompson I. Self-injection devices. In: Swarbrick J. editor Encyclopedia of Pharmaceutical Science and Technology 4 NY, Taylor and Francis; 2013 p. 3132–3143. 10.1081/E-EPT4-120050350
- Dou Z, Eshraghi J, Guo T, et al. Performance characterization of spring actuated autoinjector devices for emgality and aimovig. Curr Med Res Opin. 2020;36(8):1–12. DOI:10.1080/03007995.2020.1783219
- Jones GB, Collins DS, Harrison MW, et al. Subcutaneous drug delivery: an evolving enterprise. Sci Transl Med. 2017 Aug 30;9(405). DOI:10.1126/scitranslmed.aaf9166
- Berteau C, Filipe-Santos O, Wang T, et al. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Devices (Auckl). 2015;8:473–484.
- Berteau C, Schwarzenbach F, Donazzolo Y, et al. Evaluation of performance, safety, subject acceptance, and compliance of a disposable autoinjector for subcutaneous injections in healthy volunteers. Patient Prefer Adherence. 2010 Oct 5;4:379–388. DOI:10.2147/PPA.S13132
- Klonoff DC, Bassock S, Dwyer A, et al. Evaluating the usability and safety of the semaglutide single-dose pen-injectors through summative (human factors) usability testing. J Diabetes Investig:N/A(n/A). 2021;12(6):978–987. DOI:10.1111/jdi.13429
- Frias JP, Koren MJ, Loizeau V, et al. The SYDNEY device study: a multicenter, randomized, open-label usability study of a 2-mL alirocumab autoinjector device. Clin Ther. 2020;42(1):94–107. e5. DOI:10.1016/j.clinthera.2019.11.008
- Mead J, Dammerman R, Rasmussen S. Patient reported ease-of-use with a disposable autoinjector in individuals with migraine. Patient Prefer Adherence. 2020;14:1137–1144.
- Kivitz A, Segurado OG. HUMIRA pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab. Expert Rev Med Devices. 2007 Mar;4(2):109–116.
- Paul C, Stalder JF, Thaci D, et al. Patient satisfaction with injection devices: a randomized controlled study comparing two different etanercept delivery systems in moderate to severe psoriasis. J Eur Acad Dermatol Venereol. 2012 Apr;26(4):448–455.
- Lewandowska M, Nasr S, Shapiro AD. Therapeutic and technological advancements in haemophilia care: quantum leaps forward. Haemophilia. 2022;28:77–92.
- Lamb YN. Inclisiran: first Approval. Drugs. 2021 jan 01;81(3):389–395.
- Keam SJ. Inotersen: first global approval. Drugs. 2018;78(13):1371–1376.
- Snitker S, Egebjerg C, Frederiksen M, et al. Ease-of-use and acceptability of the novel semaglutide 2.4 mg single-dose pen-injector in people with overweight or obesity in the STEP 8 phase III trial. Diabetes Obesity Metab. 2022 Jul 6;24(11):2273–2276. DOI:10.1111/dom.14809
- Roth EM, Bujas-Bobanovic M, Louie MJ, et al. Patient and Physician Perspectives on Mode of Administration of the PCSK9 Monoclonal Antibody Alirocumab, an Injectable Medication to Lower LDL-C Levels. Clin Ther. 2015 Sep 1;37(9):1945–1954 e6. DOI:10.1016/j.clinthera.2015.07.008
- Bernstein D, Pavord ID, Chapman KR, et al. Usability of mepolizumab single-use prefilled autoinjector for patient self-administration. J Asthma. 2019;57(9):1–12. DOI:10.1080/02770903.2019.1630641
- Barker P, Ferguson GT, Cole J, et al. Single-Use Autoinjector Functionality and Reliability for At-Home Benralizumab Administration: gRECO Trial Results. J Allergy Clin Immunol. 2019;143(2):AB96. DOI:10.1016/j.jaci.2018.12.292
- Brand-Schieber E, Munjal S, Kumar R, et al. Human factors validation study of 3 mg sumatriptan autoinjector, for migraine patients. Med Devices (Auckl). 2016 may 30;9:131–137.
- Andre AD, Brand-Schieber E, Ramirez M, et al. Subcutaneous sumatriptan delivery devices: comparative ease of use and preference among migraineurs. Patient Prefer Adherence. 2017 jan 19;11:121–129.
- Sigurgeirsson B, Schäkel K, Hong C-H, et al. Efficacy, tolerability, patient usability, and satisfaction with a 2 mL pre-filled syringe containing secukinumab 300 mg in patients with moderate to severe plaque psoriasis: results from the phase 3 randomized, double-blind, placebo-controlled ALLURE study. J DermatolTreat. 2022 apr 03;33(3):1718–1726.
- Blauvelt A, Gordon KB, Lee P, et al. Efficacy, safety, usability, and acceptability of risankizumab 150 mg formulation administered by prefilled syringe or by an autoinjector for moderate to severe plaque psoriasis. J Dermatological Treat. 2021;33(4):1–9. DOI:10.1080/09546634.2021.1914812
- Banerji A, Riedl MA, Bernstein JA, et al. Effect of Lanadelumab Compared with Placebo on Prevention of Hereditary Angioedema Attacks: a Randomized Clinical Trial. JAMA. 2018;320(20):2108–2121. DOI:10.1001/jama.2018.16773
- Fenwick S, Thakur K, Munro D. Nurse and Patient Perceptions and Preferences for Subcutaneous Autoinjectors for Inflammatory Joint or Bowel Disease: findings from a European Survey. J Rheumatology Therapy. 2019;6(2):1–12.
- Thakur K, Biberger A, Handrich A, et al. Perceptions and Preferences of Two Etanercept Autoinjectors for Rheumatoid Arthritis: a New European Union-Approved Etanercept Biosimilar (Benepali((r))) versus Etanercept (Enbrel((r))) - Findings from a Nurse Survey in Europe. Rheumatol Ther. 2016 Jun;3(1):77–89.
- Schneider A, Mueller P, Jordi C, et al. Hold the device against the skin: the impact of injection duration on user’s force for handheld autoinjectors. Expert Opin Drug Deliv. 2020 Feb;17(2):225–236. DOI:10.1080/17425247.2020.1704730
- Pharmaceutical manufacturer. Teva Pharmaceuticals USA I. FULL PRESCRIBING INFORMATION AJOVY (fremanezumab-vfrm) injection, for subcutaneous use. Teva Pharmaceuticals USA, Inc. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.PDF
- Novartis. Cosentyx: EPAR - Product Information: 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cosentyx
- Regeneron. Full Prescribing Information: DUPIXENT® (dupilumab) injection, for subcutaneous use. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761055s046lbl.pdf
- Novartis. Full Prescribing Information: LEQVIO® (inclisiran) injection, for subcutaneous use 2021. Located at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214012lbl.pdf
- Sanofi. Praluent: ePAR - Medicine Overview. 2023. https://www.ema.europa.eu/en/documents/product-information/praluent-epar-product-information_en.pdf
- Valeant. Full Prescribing Information: SILIQ™ (brodalumab) injection, for subcutaneous use. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Akcea. FULL PRESCRIBING INFORMATION: TEGSEDI (inotersen) injection, for subcutaneous use. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211172s004lbl.pdf
- Amgen. Full Prescribing Information: TEZSPIRE® (tezepelumab-ekko) injection, for subcutaneous use. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761224s000lbl.pdf
- Takeda. Full Prescribing Information: TAKHZYRO (lanadelumab-flyo) injection, for subcutaneous use. Lexington MA: Takeda; 2022.
- Aktiv. ARAI is the next generation rescue auto-injector for life threatening conditions 2023 [cited 2023 16 feb]. Available from: https://aktivpharmagroup.com/rescue-auto-injector
- Sigurgeirsson B, Browning J, Tyring S, et al. Secukinumab demonstrates efficacy, safety, and tolerability upon administration by 2 ml autoinjector in adult patients with plaque psoriasis: 52-week results from MATURE, a randomized, placebo-controlled trial. Dermatologic Therapy. 2022;35(3):e15285. DOI:10.1111/dth.15285
- Cohen YZ, Zhang X, Xia B, et al. Pharmacokinetics of Subcutaneous Dupilumab Injection with an Autoinjector Device or Prefilled Syringe. Clin Pharmacol Drug Dev. 2022;11(5):675–681. DOI:10.1002/cpdd.1073
- Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399.
- Blackwood C, Sanga P, Nuamah I, et al. Patients’ Preference for Long-Acting Injectable versus Oral Antipsychotics in Schizophrenia: results from the Patient-Reported Medication Preference Questionnaire. Patient Prefer Adherence. 2020;14:1093–1102.
- Schneider A, Kolrep H, Horn H-P, et al. Understanding patient preferences for handheld autoinjectors versus wearable large-volume injectors. Expert Opin Drug Delivery. 2022;20(2):273–283. just-accepted. DOI:10.1080/17425247.2022.2162037.
- Pivot X, Gligorov J, Müller V, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25(10):1979–1987. DOI:10.1093/annonc/mdu364
- Dolton MJ, Chesterman A, Moein A, et al. Safety, Tolerability, and Pharmacokinetics of High‐Volume Subcutaneous Crenezumab, with and without Recombinant Human Hyaluronidase in Healthy Volunteers. Clin Pharmacol Ther. 2021;110(5):1337–1348. doi:10.1002/cpt.2385.
- kaleo. Aerio™ Auto-Injection Platform 2023 [cited 2023 8 feb]. Available from: https://kaleo.com/what-we-do/auto-injection-platform/
- Oval. ArQ® Subcutaneous Platform 2023 [cited 2023 8 feb]. Available from: https://www.ovalmedical.com/platforms/arq/
- Gerresheimer. Gx InbeneoTM: our next generation Autoinjector 2023 [cited 2023 16 feb]. Available from: https://www.gerresheimer.com/en/drug-delivery-systems/platform-solutions/autoinjector
- Maggie® SHL 50.2023 [cited 2023 8 feb]. Available from: https://www.shl-medical.com/products-and-services/maggie-5-0-auto-injector/
- Jost R. The New YpsoMate™ 5.5–Taking Handheld Self-Injection Beyond Volumes of 2 mL. Lewes, United Kingdom: ONdrugDelivery; 2022.
- Andre AD, Mohr J, Cornelius B, et al. Successful Validation of a Wearable, On-body Infusor for Subcutaneous Administration of Furoscix® in Heart Failure Patients, Caregivers, And Health Care Practitioners. J Cardiac Failure. 2020 oct 01;26(10, Supplement):S68.
- Joshi RS, Egbuna OI, Cairns AS, et al. Performance of the pegfilgrastim on-body injector as studied with placebo buffer in healthy volunteers. Curr Med Res Opin. 2016;33(2):1–6. DOI:10.1080/03007995.2016.1257980
- Woodley WD, Morel DR, Sutter DE, et al. Clinical evaluation of large volume subcutaneous injection tissue effects, pain, and acceptability in healthy adults. Clin Transl Sci. 2022 Jan;15(1):92–104.
- Lange J, Schneider A, Jordi C, et al. Formative Study on the Wearability and Usability of a Large-Volume Patch Injector. Med Devices (Auckl). 2021;14:363–377.
- Mathaes R, Koulov A, Joerg S, et al. Subcutaneous Injection Volume of Biopharmaceuticals—Pushing the Boundaries. J Pharmaceut sci. 2016 aug 01;105(8):2255–2259.
- Clair-Jones A S, Prignano F, Goncalves J, et al. Understanding and Minimising Injection-Site Pain Following Subcutaneous Administration of Biologics: a Narrative Review. Rheumatol Ther. 2020;7(4):1–17. DOI:10.1007/s40744-020-00245-0
- Badkar AV, Gandhi RB, Davis SP, et al. Subcutaneous delivery of high-dose/volume biologics: current status and prospect for future advancements. Drug Design Develop Therapy. 2021;15:159.
- Jain M, Doughty D, Clawson C, et al. Tralokinumab pharmacokinetics and tolerability when administered by different subcutaneous injection methods and rates. Int J Clin Pharmacol Ther. 2017 Jul;55(7):606–620. DOI:10.5414/CP203023
- Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006 Oct;28(10):1619–1629.
- Dias C, Abosaleem B, Crispino C, et al. Tolerability of High-Volume Subcutaneous Injections of a Viscous Placebo Buffer: a Randomized, Crossover Study in Healthy Subjects. AAPS Pharm Sci Tech. 2015 Oct;16(5):1101–1107.
- Denys H, Martinez-Mena CL, Martens MT, et al. Safety and tolerability of subcutaneous trastuzumab at home administration, results of the phase IIIb open-label BELIS study in HER2-positive early breast cancer. Breast Cancer Res Treat. 2020 may 01;181(1):97–105.
- Andre A, Squittieri N, Patil S. Evaluating Use of the Octreotide Acetate Pen Injector in a Summative Human Factors Validation Study. Endocr Pract. 2022;28(4):414–419.
- Mahony MC, Patterson P, Hayward B, et al. Human factors engineering and design validation for the redesigned follitropin alfa pen injection device. Expert Opin Drug Deliv. 2015 May;12(5):715–725.
- van den Bemt BJ, Gettings L, Domańska B, et al. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Delivery. 2019;26(1):384–392. DOI:10.1080/10717544.2019.1587043
- Anderson D, Liu R, Subramony JA, et al. Design control considerations for biologic-device combination products. Adv Drug Delivery Rev. 2017;112:101–105.
- Zijlstra E, Jahnke J, Fischer A, et al. Impact of Injection Speed, Volume, and Site on Pain Sensation. J Diabetes Sci Technol. 2018 Jan;12(1):163–168.
- Xu Z, Marciniak SJ Jr., Frederick B, et al. Pharmacokinetic bridging approach for developing biologics-delivery devices: a case study with a golimumab autoinjector. Clin Ther. 2015 Feb 1;37(2):427–438. DOI:10.1016/j.clinthera.2014.09.012
- Hu P, Wang J, Florian J, et al. Systematic Review of Device Parameters and Design of Studies Bridging Biologic-Device Combination Products Using Prefilled Syringes and Autoinjectors. Aaps J. 2020 feb 27;22(2):52.
- Li Z, Easton R. Practical considerations in clinical strategy to support the development of injectable drug-device combination products for biologics. MAbs. 2018 Jan;10(1):18–33.
- Anderson JT, Bonagura VR, Cowan J, et al. Safety and Tolerability of Subcutaneous IgPro20 at High Infusion Parameters in Patients with Primary Immunodeficiency: findings from the Pump-Assisted Administration Cohorts of the HILO Study. J Clin Immunol. 2021 Feb;41(2):458–469.
- Bruin G, Hockey HP, La Stella P, et al. Comparison of pharmacokinetics, safety and tolerability of secukinumab administered subcutaneously using different delivery systems in healthy volunteers and in psoriasis patients. Br J Clin Pharmacol. 2020 Feb;86(2):338–351.
- Connor RJ, Taverna DM, Thrall K, et al. Use of computed tomography to assess subcutaneous drug dispersion with recombinant human hyaluronidase PH20 in a swine model. J Pharmacol Toxicol Methods. 2020;106:106936.
- Cowan J, Bonagura VR, Lugar PL, et al. Safety and Tolerability of Manual Push Administration of Subcutaneous IgPro20 at High Infusion Rates in Patients with Primary Immunodeficiency: findings from the Manual Push Administration Cohort of the HILO Study. J Clin Immunol. 2021 jan 01;41(1):66–75.
- Doughty DV, Clawson CZ, Lambert W, et al. Understanding Subcutaneous Tissue Pressure for Engineering Injection Devices for Large-Volume Protein Delivery. J Pharm Sci. 2016 Jul;105(7):2105–2113.
- Ferruccio LF, Murray C, Yee KW, et al. Tolerability of Vidaza (azacitidine) subcutaneous administration using a maximum volume of 3 ml per injection. J Oncol Pharm Pract. 2016 Aug;22(4):605–610.
- Heise T, Nosek L, Dellweg S, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: a single-centre, randomized controlled trial. Diab Obes Metab. 2014 Oct;16(10):971–976.
- Jørgensen JT, Rømsing J, Rasmussen M, et al. Pain assessment of subcutaneous injections. Ann Pharmacother. 1996 Jul;30(7–8):729–732.
- Kokolakis G, Kreis G, Falqués M, et al. High Tolerability, Favorable Safety, and Subjects’ Preference for a Single 200 mg/2 mL Tildrakizumab Injection: a Phase I, Open-Label, Randomized Crossover Trial in Healthy Volunteers. Dermatol Ther (Heidelb). 2022 Aug;19(9):2135–2144.
- Lundbom JS, Tangen LF, Wågø KJ, et al. The influence of Lidocaine temperature on pain during subcutaneous injection. J Plast Surg Hand Surg. 2017;51(2):118–121. DOI:10.1080/2000656X.2016.1194281
- Müller-Ladner U, Rockwitz K, Brandt-Jürgens J, et al. Tolerability and patient/physician satisfaction with subcutaneously administered methotrexate provided in two formulations of different drug concentrations in patients with rheumatoid arthritis. Open Rheumatol J. 2010 Mar 18;4(1):15–22. DOI:10.2174/1874312901004010015
- Ng P, Incekol D, Lee R, et al. Tolerability of Velcade (Bortezomib) subcutaneous administration using a maximum volume of 3 mL per injection site. J Oncol Pharm Pract. 2015 Aug;21(4):285–292.
- Pager A, Combedazou A, Guerrero K, et al. User experience for manual injection of 2 mL viscous solutions is enhanced by a new prefillable syringe with a staked 8 mm ultra-thin wall needle. Expert Opin Drug Delivery. 2020;17(10):1485–1498. DOI:10.1080/17425247.2020.1796630
- Portron A, Jordan P, Draper K, et al. A Phase I Study to Assess the Effect of Speed of Injection on Pain, Tolerability, and Pharmacokinetics After High-volume Subcutaneous Administration of Gantenerumab in Healthy Volunteers. Clin Ther. 2020 Jan;42(1):108–120.e1.
- Rini CJ, Roberts BC, Vaidyanathan A, et al. Enabling faster subcutaneous delivery of larger volume, high viscosity fluids. Expert Opin Drug Delivery. 2022;19(9):1–12. DOI:10.1080/17425247.2022.2116425
- Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol. 2010 Mar;30(2):301–307.
- Shapiro RS. Subcutaneous immunoglobulin therapy given by subcutaneous rapid push vs infusion pump: a retrospective analysis. Ann Allergy Asthma Immunol. 2013 Jul;111(1):51–55. doi:10.1016/j.anai.2013.04.015.
- Shi GH, Connor RJ, Collins DS, et al. Subcutaneous injection performance in Yucatan miniature pigs with and without human hyaluronidase and auto-injector tolerability in humans. AAPS Pharm Sci Tech. 2021;22(1):1–13. DOI:10.1208/s12249-020-01880-0
- Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50(1):7–9. doi:10.3109/2000656X.2015.1058269.
- Torjman MC, Machnicki R, Lessin J, et al. Evaluation of an investigational wearable injector in healthy human volunteers. Expert Opin Drug Deliv. 2017 Jan;14(1):7–13.
- Vultaggio A, Azzari C, Milito C, et al. Subcutaneous immunoglobulin replacement therapy in patients with primary immunodeficiency in routine clinical practice: the VISPO prospective multicenter study. Clin Drug Investig. 2015 Mar;35(3):179–185.
- Woodley WD, Yue W, Morel DR, et al. Clinical Evaluation of an Investigational 5 mL Wearable Injector in Healthy Human Subjects. Clin Transl Sci. 2021 May;14(3):859–869.
- Jensen MP, Engel JM, McKearnan KA, et al. Validity of pain intensity assessment in persons with cerebral palsy: a comparison of six scales. J Pain. 2003;4(2):56–63. DOI:10.1054/jpai.2003.9
- Usach I, Martinez R, Festini T, et al. Subcutaneous injection of drugs: literature review of factors influencing pain sensation at the injection site. Adv Ther. 2019 nov 01;36(11):2986–2996.
- Gittelman M, Jaffe JS, Kaminetsky JC. Safety of a new subcutaneous testosterone enanthate auto-injector: results of a 26-week study. J Sex Med. 2019;16(11):1741–1748.
- Landy S, Munjal S, Brand-Schieber E, et al. Efficacy and safety of DFN-11 (sumatriptan injection, 3 mg) in adults with episodic migraine: an 8-week open-label extension study. J Headache Pain. 2018;19(1):1–8. DOI:10.1186/s10194-018-0882-y
- Rogin J, Wheless J, Abou‐Khalil B, et al. Safety and effectiveness of long‐term treatment with diazepam auto‐injector administered by caregivers in an outpatient setting for the treatment of acute repetitive seizures. Epilepsia. 2014;55(9):1444–1451. DOI:10.1111/epi.12685
- Collins DS, Sánchez-Félix M, Badkar AV, et al. Accelerating the development of novel technologies and tools for the subcutaneous delivery of biotherapeutics. J Control Release. 2020 May 10;321:475–482. DOI:10.1016/j.jconrel.2020.02.036
- Allmendinger A, Fischer S. Tissue Resistance during Large-Volume Injections in Subcutaneous Tissue of Minipigs. Pharm Res. 2020 sep 04;37(10):184.
- McNamara M, Turner-Bowker DM, Westhead H, et al. Factors driving patient preferences for growth hormone deficiency (GHD) injection regimen and injection device features: a discrete choice experiment. Patient Prefer Adherence. 2020;14:781.
- Cowan R, Cohen JM, Rosenman E, et al. Physician and patient preferences for dosing options in migraine prevention. J Headache Pain. 2019 may 09;20(1):50.
- Rummel M, Kim T, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017;28(4):836–842. DOI:10.1093/annonc/mdw685
- Bittner B, Schmidt J. Chapter 3 - Clinical development of automated subcutaneous injection devices—established pathways and novel concepts. In: Bittner B Schmidt J. editors Formulation and Device Lifecycle Management of Biotherapeutics. Academic Press; 2022. p. 85–105. 10.1016/B978-0-12-823741-0.00009-9
- Schneider A, Kolrep H, Jordi C, et al. How to prevent medication errors: a multidimensional scaling study to investigate the distinguishability between self-injection platform device variants. Expert Opinion on Drug Delivery. 2019 aug 03;16(8):p. 883–894.
- Abramson A, Frederiksen MR, Vegge A, et al. Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nature Biotechnol. 2022 jan 01;40(1):103–109.
- Kelley EL, Smith RH, Corcoran G, et al. Advances in subcutaneous injections: pRECISE II: a study of safety and subject preference for an innovative needle-free injection system. Drug Deliv. 2021 Dec;28(1):1915–1922.
- Wang SS, Yan Y, Ho K. US FDA-approved therapeutic antibodies with high-concentration formulation: summaries and perspectives. Antibody Therapeutics. 2021;4(4):262–272.
- Sahin E, Deshmukh S. Challenges and considerations in development and manufacturing of high concentration biologics drug products. J Pharm Innov. 2020;15(2):255–267.
- Lassalle A, Thomaré P, Fronteau C, et al. Home administration of bortezomib in multiple myeloma is cost-effective and is preferred by patients compared with hospital administration: results of a prospective single-center study. Ann Oncol. 2016 feb 01;27(2):314–318.
- Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017 Jul 11;318(2):197–198. DOI:10.1001/jama.2017.7156
- Penedo FJ, Oswald LB, Kronenfeld JP, et al. The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet Oncol. 2020 may 01;21(5):e240–e251.
