1. Introduction
Therapeutic drug monitoring (TDM) aims to offer assistance to clinicians to improve drug dosing and clinical outcomes, while minimizing the probability of serious adverse effects in routine clinical practice. Disease conditions, drug-drug and drug-herbal interactions, noncompliance, and genetic features affect the relationship between drug concentrations in the body and clinical effects. They contribute to the pharmacokinetic inter-patient variability which ultimately impactsdrug response, resulting in some patients showing lack of therapeutic effects while others experience toxicity. Thus, the identification of the sources of pharmacokinetic variability contributes to the adequacy of the dosing regimen [Citation1]. However, performing TDM in Developing Nations as South American Countries is particularly challenging, mainly due to the lack of structure and economic aspects.
2. State of affairs in South America
South America consists of 12 countries and 3 dependent territories with a population estimated at the moment of the present report in 426 million, with average annual population growth between 1.1% and 1.5% [Citation2]. The increasing population presents an important challenge for the developing healthcare structure. In this context, it is vital to understand the demographic composition of the South American population as well as the economic context to define the priorities of the healthcare system and how each country may face its own challenges. Most countries in South America are middle-income, with total expenditure on health, as a percentage of gross domestic product, between 5% and 12% in all but one country of the region, Venezuela, with a value of 3.2 [Citation3].
Unfortunately, quantitative data about the number of TDM services provided in the region are not available. Recently, the Brazilian Federal Board of Pharmacy planned to start a nationwide survey of pharmacy practice in TDM services, but this project is currently on hold. It is important to note that no particular licensing or registration is required for TDM services, and a quantitative evaluation would require an active search through all hospitals of the region. In addition, a search for studies published by local scientists revealed a limited scientific interest in the topic. A Pubmed® search for publications in the last decade using the key words: ‘Therapeutic drug monitoring; pharmacogenetic; pharmacogenomic; pharmacoeconomic; dose individualization; personalized medicine; dried blood spot and drug’ revealed 658 articles, representing only 2.2% of the worldwide publications (29,755 articles) ().
Figure 1. Distribution of the number of articles returned from Pubmed® search on TDM topics in the last decade
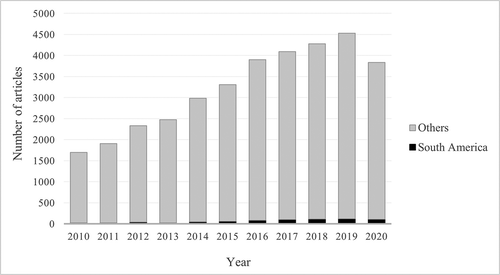
Brazil, the most populous country of South America, accounts for more than half of the publications, followed by Argentina, Colombia, and Chile (). TDM of immunosuppressants was subject of relevant studies, including a prospective randomized trial on tacrolimus therapy in kidney transplant [Citation4], from the group from the ‘Hospital do Rim’, a world reference center in kidney transplants located in São Paulo, Brazil, and a 2-year follow-up study from the Argentinean ‘J.P. Garrahan Hospital’ on pharmacogenetic and tacrolimus in pediatric liver patients [Citation5]. Pharmacogenetic was also explored in several Brazilian studies. Recently, Rodrigues-Soares and Suarez-Kurtz reviewed the status of pharmacogenetic/genomic (PGx) studies in the Brazilian population, focusing on the drugs and genes included in the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines [Citation6]. Dr. Suarez-Kurtz also coordinates the Brazilian Pharmacogenetics Network, an initiative of Brazilian researchers, which aims to promote and coordinate integrated research projects in pharmacogenetics/pharmacogenomics in the Brazilian population (REFARGEN). Still, interesting studies on TDM in children were performed in Argentina, a population evaluation of pharmacokinetics of amikacin in patients with cystic fibrosis [Citation7], and in Chile, an evaluation of drug interaction of voriconazole and cyclosporine in hematopoietic stem cell transplantation [Citation8].
Table 1. Number of articles published between 2010 and 2020 indexed in Pubmed®
In addition, performing TDM in dried capillary blood samples have been explored to promote further the TDM in Developing Countries. Dried blood spots (DBS) are an easy to collect sampling that overcomes the logistic problems related to venous blood transportation and biohazard risks. In developing countries, a central level laboratory equipped with advanced techniques such as LC-MS/MS could analyze DBS samples collected from peripheral health centers/laboratories. Our research group developed several bioanalytical methods and evaluated the clinical applicability of DBS in several TDM fields, including chemotherapies [Citation9,Citation10], immunossupressants [Citation11], antiretroviral, antiepileptic [Citation12], and psychiatric drugs [Citation13,Citation14].
To the best of our knowledge, Brazil, Argentina, Colombia, Chile, and Uruguay are the only South American countries with clinical units of TDM, where mostly pharmacists and biochemists provide the service, which in many cases are limited to measuring drug concentrations, without adequate clinical interpretation. An Argentinean nationwide survey of clinical centers with bioanalytical facilities and private laboratories that provide bioanalysis showed interesting results about the practice of bioanalysis for TDM of immunosuppressant drugs to identify areas of work and improvement. The survey indicated that only 17% of the assays reported the therapeutic range for the drug along with the drug concentration, immunoassays were the most frequent methodologies for drug quantitation as they require minimum training and instruments are usually available, while only 3 private laboratories had access to liquid chromatography-tandem mass spectrometry (LC-MS/MS) [Citation15]. These limitations attempt against the widespread implementation of state-of-the-art assays. Of concerned, only half of the analytical facilities participated in external quality assurance programs. A positive aspect was that almost all facilities calibrated their instruments and showed interest in training and establishing consensus documents for the practice of TDM and specifically bioanalysis.
Besides the challenges, some successful TDM experiences in South America are noteworthy. In Argentina, the Unit of Clinical Pharmacokinetics was established in 2001 at Hospital de Pediatría JP Garrahan and offers TDM services to pediatric in- and outpatients and provides pharmacotherapeutic recommendations on demand to other hospitals of the country. Also, the professionals intensively participate in educational activities of postgraduate students from Argentina and the Latin American region as well as in close collaboration with universities and research institutes. Preclinical and clinical research has played an important role in the advancement of innovative treatments with translation to international health-care centers specialized in pediatric oncology.
In Chile, professionals at Hospital Calvo Mackenna have been notoriously active in TDM despite no formal unit structure is available. Also, studies on the pharmacokinetics of HIV, antimicrobials, and anesthetics have been carried out in different universities and research centers of this country. The Drug and Poison Research and Information Center (CIEMTO) was established in 2014 at the University of Antioquia, Medellin, Colombia and has been offering extremely active services for professionals all over the country in the prevention and treatment of poisonings and drug treatment optimization. Of note, this is the only center in our region that includes physicians and bioengineers among the staff. As part of CIEMTO, the Integrated Laboratory of Specialized Medicine focuses on pharmacogenomic testing and precision medicine.
Lastly, since 1991, Uruguay has a highly active unity in TDM services and education of national and international graduate and postgraduate students on pharmacokinetics, clinical pharmacy, and pharmacotherapeutic monitoring. The unit at Hospital de Clinicas Dr. Manuel Quintela has a double dependency on the hospital and the University of La Republica and is closely related to the center of evaluation of bioavailability and bioequivalence (CEBIOBE). Interestingly, researchers of this unit have vast experience in TDM on saliva apart from conventional biological fluids (blood, plasma). Also, they actively participate in pharmacovigilance and interchangeability of commercial drug products.
3. Expert opinion
Large regional disparities exist in access to health-care services and availability of medicines among South American Countries and between different areas of the same Country. Together with economic aspects, the diversity of genetic background, the use of herbal medicines, and the existence of poisons pose additional challenges to the clinical treatment of local patients. In this sense, we face a spectrum of health-care systems that coexists with endemic diseases that need improvement in clinical outcome and could benefit from TDM in many settings.
The process of TDM requires clinical capabilities, analytical facilities, as well as health-care personnel trained in bioanalysis, pharmacokinetics, and informatics skills to interpret drug concentrations in the context of patient characteristics and provide pharmacotherapeutic recommendations. Rapid, accurate, and precise analytical assays to measure drug concentrations in biological specimens along with proficiency testing programs are key components for reliable TDM service. Nonetheless, TDM must be cost-effective to be implemented.
Setting up TDM services in Developing Countries that attend these requirements is particularly challenging due to: limited economic resources with minimal financial support from health-care centers for TDM services; high costs in acquisition and maintenance of high-end instruments, like LC-MS/MS systems; lack of commercially available test kits for commonly measured drugs; lack of highly trained personnel, there is a need for education programs; a limited number of proficiency testing programs; availability of appropriate reference materials; and patients need to travel long distances to be treated at centers where TDM is available.
In addition to structural issues, quality control, and economic aspects, some clinical characteristics are additional challenges shared by Developing Countries and should be taken into account while performing and interpreting TDM data: scarce availability of data on the genomic background of the local population, extrapolating data from other populations is not always reliable; higher prevalence of infectious diseases and nutritional deficiencies; variable quality of pharmaceuticals and generic formulations; and lack of appropriate drug concentrations reference range.
Considering this scenario, promoting studies to better understand the particularities and genetic background of the population, investing in education and structure, and following good laboratory practices are of great importance to expand TDM in Developing Nations. In addition, the use of immunoassays and intermediate instrumentations as liquid chromatography with UV or fluorescence detection could be an interesting compromise to have good data at lower costs. Also, due to its intrinsic stability and handling safety, DBS sampling could be a cost-effective strategy to promote further the TDM in South America, where sample logistics are a major difficulty, and only few laboratories are equipped with high-end instruments.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Touw DJ, Neef C, Thomson AH, et al. Cost-effectiveness of therapeutic drug monitoring: a systematic review. Ther Drug Monit. 2005 Feb;27(1):10–17.
- The World Bank. 2020. Available for: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=ZJ
- World Health Organization. Total expenditure on health as a percentage of gross domestic product. Available from: https://apps.who.int/nha/database
- Bessa AB, Felipe CR, Hannun P, et al. Prospective randomized trial investigating the influence of pharmaceutical care on the intra-individual variability of tacrolimus concentrations early after kidney transplant. Ther Drug Monit. 2016 Aug;38(4):447–455.
- Buendía JA, Halac E, Bosaleh A, et al. Frequency of CYP3A5 genetic polymorphisms and tacrolimus pharmacokinetics in pediatric liver transplantation. Pharmaceutics. 2020 Sep 22;12(9):E898.
- Rodrigues-Soares F, Suarez-Kurtz G. Pharmacogenomics research and clinical implementation in Brazil. Basic Clin Pharmacol Toxicol. 2019 May;124(5):538–549.
- Caceres Guido P, Perez M, Halac A, et al. Population pharmacokinetics of amikacin in patients with pediatric cystic fibrosis. Pediatr Pulmonol. 2019 Nov;54(11):1801–1810.
- Valenzuela R, Torres JP, Salas C, et al. Evaluación de interacción medicamentosa de voriconazol-ciclosporina en pacientes pediátricos que reciben trasplante de precursores hematopoyéticos (2013–2014) [Drug interaction of voriconazole-cyclosporine in children undergoing hematopoietic stem cell transplantation (2013–2014)]. Rev Chilena Infectol. 2017 Feb;34(1):14–18. Spanish. PMID: 28394976.
- Hahn RZ, Arnhold PC, Andriguetti NB, et al. Determination of irinotecan and its metabolite SN-38 in dried blood spots using high-performance liquid-chromatography with fluorescence detection. J Pharm Biomed Anal. 2018 Feb 20;150:51–58. Epub 2017 Dec 5. PMID: 29216585. DOI:10.1016/j.jpba.2017.11.079
- Andriguetti NB, Hahn RZ, Lizot LF, et al. Analytical and clinical validation of a dried blood spot assay for the determination of paclitaxel using high-performance liquid chromatography-tandem mass spectrometry. Clin Biochem. 2018 Apr;54:123–130. Epub 2018 Mar 4. PMID: 29514052. DOI:10.1016/j.clinbiochem.2018.02.020
- Arpini J, Antunes MV, Pacheco LS, et al. Clinical evaluation of a dried blood spot method for determination of mycophenolic acid in renal transplant patients. Clin Biochem. 2013 Dec;46(18):1905–1908. Epub 2013 Oct 22. PMID: 24161478.
- Hahn RZ, Antunes MV, Costa Arnhold P, et al. Determination of topiramate in dried blood spots using single-quadrupole gas chromatography-mass spectrometry after flash methylation with trimethylanilinium hydroxide. J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Mar 1;1046:131–137. Epub 2017 Feb 1. PMID: 28178597. DOI:10.1016/j.jchromb.2017.01.047
- Manfro ID, Tegner M, Krutzmann ME, et al. Determination of lithium in dried blood spots and dried plasma spots by graphite furnace atomic absorption spectrometry: method development, validation and clinical application. Talanta. 2020 Aug 15;216:120907. Epub 2020 Mar 19. PMID: 32456895. DOI:10.1016/j.talanta.2020.120907
- da Silva ACC, Raasch JR, Vargas TG, et al. Simultaneous determination of fluoxetine and norfluoxetine in dried blood spots using high-performance liquid chromatography-tandem mass spectrometry. Clin Biochem. 2018 Feb;52:85–93. Epub 2017 Oct 5. PMID: 28987790. DOI:10.1016/j.clinbiochem.2017.10.002
- Schaiquevich P, Taich P, Riva N, et al. Estado de situación y regulación de laboratorios bioanalíticos que cuantifican inmunosupresores para trasplantes en Argentina [Current status and regulation of bioanalytical laboratories engaged in quantifying immunosuppressants for transplant patients in Argentina]. Rev Panam Salud Publica. 2016 Mar;39(3):142–148. Spanish. Erratum in: Rev Panam Salud Publica. 2017 Apr 20;41:1.
