ABSTRACT
Purpose
To compare the pharmacokinetics, pharmacodynamics and safety of the new prolonged-release leuprorelin acetate microspheres for injection (3.75 mg) with the reference product Enantone® (3.75 mg).
Method
48 healthy male volunteers were enrolled and randomly received a single 3.75 mg dose of the test drug or Enantone®.
Results
There were no significant differences in Cmax, AUC0-t and AUC0-48 between the test group and reference group (P > 0.05). The 90% confidence intervals of the two groups were 87.49%~112.74%, 97.15%~154.25%, and 80.85%~109.01%, respectively. Twenty-eight days after administration, both groups reached 100.0% castration level; there was no difference in the time from administration to reaching castration level between the two groups (P > 0.05); However, the difference between the two groups in the duration of castration level was statistically significant (P < 0.05). There were no major or serious adverse events, and the severity was mild to moderate.
Conclusion
The pharmacokinetic characteristics of leuprorelin in two groups were consistent. The two groups exhibited similar inhibitory effects on testosterone and more subjects in the test group maintained a longer castration time than those in the reference group.
1. Introduction
The American Cancer Society estimated in 2020 that the incidence of prostate cancer was highest among American men is based on the conclusion drawn from in [Citation1]. Bilateral orchiectomy and luteinizing hormone-releasing hormone (LHRH) analogs can be used as the main treatment for local or advanced prostate cancer. Although orchiectomy is a relatively simple and safe method, it is invasive and has negative psychological effects; thus, many patients choose to use LHRH analogs for treatment [Citation2–7].
LHRH is a decapeptide hormone synthesized in the hypothalamus. By binding to specific receptors of gonadotropins and activating them, LHRH regulates the expression of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The pulsed administration of low-dose LHRH agonists will cause receptor activation and stimulate the secretion of gonadotropin. However, long-term use of LHRH agonists can lead to desensitization of LHRH receptors, thereby inhibiting the function of the pituitary gonad [Citation8].
Leuprolide acetate is a synthetic nonapeptide LHRH analog that is more effective than the natural LHRH peptide, has a stronger receptor affinity and a longer half-life and is used to treat a variety of sex hormone-related disorders [Citation9–13]. A wide range of dosages and many administration forms of leuprolide acetate are available. To date, a variety of sustained-release formulations of leuprolide acetate have been developed, such as Lupron depot® and Eligard® [Citation6,Citation14–17].
Enantone® is a microspheres composed of the active substance leuprorelin acetate and a polymers of lactic acids [Citation18]. The prolonged-release leuprorelin acetate microspheres for injection (3.75 mg) in this study is a new type of sustained-release microencapsulated leuprolide, which has adopted a new formula. Its main components include biodegradable polymers and triethyl citrate. Compared with the marketed products such as Lupron depot® and Eligard® [Citation6], it only needs half the dose to obtain the same therapeutic effectiveness. In previous studies, this new type of drug treatment has been proven to achieve castration and inhibit the level of testosterone at least as effectively as the reference drugs [Citation19,Citation20]. Considering potential racial and ethnic differences, this trial aimed to evaluate the pharmacokinetics, pharmacodynamics and safety of the new prolonged-release leuprorelin acetate microspheres for injection (3.75 mg) compared with the reference product Enantone® (3.75 mg) in healthy Chinese male volunteers.
2. Materials and methods
2.1. Study design
This randomized, single-dose, single-blind, parallel-group study meets the requirements of relevant laws and regulations. This research was approved by the Clinical Medical Research Ethics Committee of the First Affiliated Hospital of Bengbu Medical University. Before beginning any research-related procedures, all subjects signed a written informed consent form. This study has been registered in www.chinadrugtrials.org.cn, and the registration number is CTR20180698. In this trial, the test group and the reference group each included 20 subjects, which should meet the statistical requirements. Considering the dropout rate of approximately 20%, the sample size per group was 24 cases (48 cases in total).
Inclusion criteria: male volunteers aged 18 to 40 years old, a group of subjects with an age gap of less than 10 years; weight not less than 50 kg; body mass index of 19.0 to 24.0 kg/m2. Participants were excluded if they had the following conditions: heart, liver, or kidney disease or acute or chronic gastrointestinal disease; blood, endocrine, respiratory, or nervous system diseases; depression or sexual dysfunction; allergies to leuprolide, synthetic LHRH/LHRH derivatives, or the excipients of the study drug; abnormal testosterone concentration during screening (fine-tuning to determine the testosterone level (168–781 ng/dL) was performed by the local laboratory of the clinical research center, with reference to the testosterone determination kit (chemiluminescence method) instructions); blood donation or blood loss ≥400 ml or participation in clinical trials of other drugs within 3 months before screening; use of other drugs within two weeks before screening; drug abuse, alcoholism, and smoking within one year before screening; or skin inflammation or skin abnormalities that interfere with the administration of the study drug at or around the administration site.
2.2. Study procedures
During screening, each subject was assigned a unique screening number by the research center. Subjects who passed the screening remained blinded during the research process and were randomly assigned to the test group or the reference group. After fasting for 10 h, the subjects received a single 3.75 mg injection of the new prolonged-release leuprorelin acetate microspheres (intramuscular injection in the buttocks) or a single 3.75 mg injection of the reference drug Enantone® (subcutaneous injection in the buttocks). The drug was administered by specialized researchers, and each drug provider gave only the test drug or the reference drug.
Leuprolide blood samples were collected on Day 1 prior to dose administration, and 1, 3, and 6 h post dosing; and 2, 3, 4, 8, 15, 22, 29, 36, 43, 50 and 57 days post dosing. Centrifuge conditions: 1600 × g, for 15 min at 4°C. The serum was stored at −70 ± 10°C, and was detected by LC-MS/MS. Testosterone, LH and FSH blood samples were collected on screening period; day 1 prior to dose administration, and 1, 3, and 6 h post dosing; and 2, 3, 4, 8, 15, 22, 29, 36, 43, 50 and 57 days post dosing. And the blood samples were evaluated in the local laboratory of the clinical research center. Testosterone was measured by chemiluminescence. The limitation of this detection method is that heterophilic antibodies in human serum can react with immunoglobulins in the kit components and interfere with in vitro immunoassays.
2.3. Analysis
Pharmacokinetics (PK) (leuprolide parameters): the area under the serum concentration/time curve from 0 h to 48 h after administration (AUC0-48); the area under the serum concentration/time curve from 0 h to the last detectable concentration (AUC0-t); other important factors including the maximum serum concentration (Cmax), the time to Cmax (Tmax). PK parameters were calculated using the PhoenixTM WinNonlin (Version 8.1, Certara USA, Inc., New Jersey, US) noncompartmental model. The main PK parameters (area under the curve (AUC) and Cmax) were transformed by natural logarithm, and then the difference was tested by analysis of variance. The 90% confidence interval (CI) of the difference value of the natural logarithm means of the main PK parameters of the test drug and the reference drug was calculated, and then the antilog was taken to obtain the ratio of the geometric mean of the PK parameters and the 90% CI.
Pharmacodynamics (PD): the concentration of testosterone 28 days after administration (Cday29), the last detectable testosterone concentration (Clast), the time to castration level (Tlag), the duration of castration level (Tcastr) and the concentration of LH and FSH. The Kaplan-Meier method was used to estimate the median Tlag and Tcastr values, and the log-rank test was used for comparisons between groups.
Safety: Adverse events (AEs), laboratory examinations, vital signs, 12-lead electrocardiogram results, local tolerance and physical examinations were recorded and summarized with appropriate descriptive statistics.
3. Results
3.1. Baseline characteristics
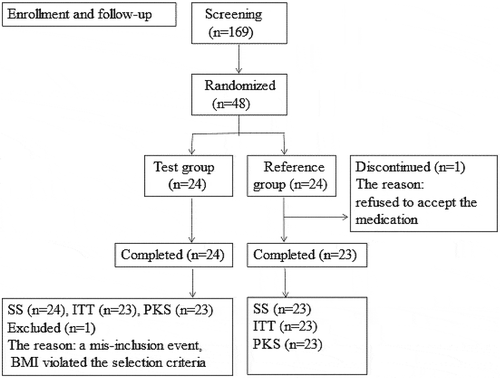
A total of 169 male volunteers were screened; 48 subjects were randomly enrolled, with 24 cases per group. The 24 subjects enrolled in the test group all completed the test. One subject in the reference group refused to accept the prescribed medication and testing and dropped out of the trial without medication. Therefore, 23 subjects in the reference group completed the trial. The 47 subjects who received the medication were all included in the SS (safety set), including 24 in the test group and 23 in the reference group. In the test group, one subject (random number 37) whose BMI did not meet the selection criteria was not included in the intentional to treat (ITT) and pharmacokinetic set (PKS), so ITT and PKS included 46 subjects, 23 each group (). The baseline characteristics of the volunteers are shown in .
Table 1. Baseline characteristics (data set: ITT)
3.2. Pharmacokinetics
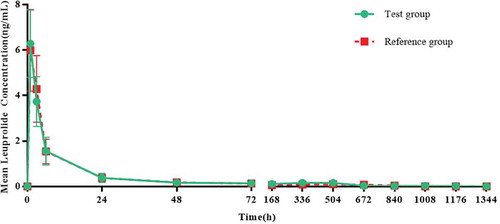
After the test group and the reference group were separately administered their respective drugs, the mean (SD) drug concentration-time profiles of the two groups of leuprolide were similar (). The inferential analysis of leuprolide PK parameters showed that there was no significant difference in Cmax, AUC0-t and AUC0-48 of leuprolide between the two groups. The geometric mean ratios of the PK parameters of the test group and the reference group of leuprolide were 99.32%, 122.41%, and 93.88%, and the 90% CIs were 87.49%~112.74, 97.15%~ 154.25%, and 80.85%~109.01% ().
Table 2. The inferential analysis, the geometric mean ratio and the 90% CI of leuprolide pharmacokinetic parameters of the test group and the reference group (data set: PKS)
3.3. Pharmacodynamics
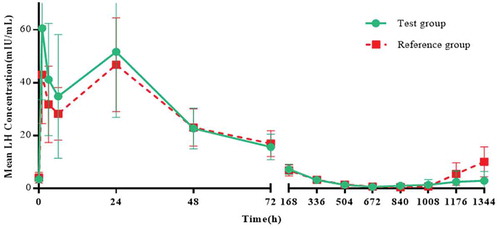
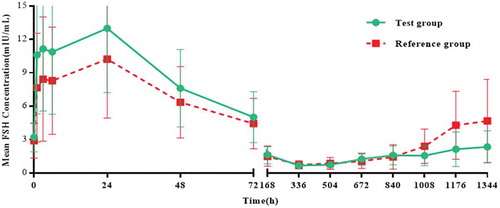
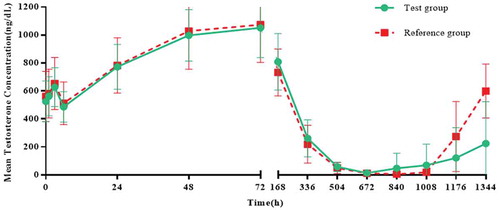
The mean (SD) drug concentration-time curves of LH, FSH and testosterone in the two groups were similar. The mean (SD) LH concentrations in the test group and the reference group reached 51.6 (24.81) and 46.8 (17.72), respectively on the second day; the mean (SD) FSH concentrations in the two groups reached their maximum on the second day, 13.0 (6.63) and 10.2 (5.28), respectively. The mean (SD) testosterone concentrations in the two groups reached their maximum on day 4, 1052.0 (213.93) and 1073.4 (269.48), respectively, followed by reductions in LH, FSH and the concentration of testosterone (). Twenty-eight days after administration (4 weekends), 23 subjects (data set: ITT) of both groups reached 100.0% castration level (The level of castration is defined as the testosterone concentration below 0.5 ng/mL [Citation21]), and comparison between groups is not applicable.
Testosterone level parameters: In the test group, the mean values of Cday29 and Clast were 24.6 ng/dL and 132.6 ng/dL, respectively. Moreover, in the reference group, the mean values of Cday29 and Clast were 24.5 ng/dL and 126.7 ng/dL, respectively. The mean Tlag of testosterone parameters in the test group was 25.3 days and in the reference group was 24.7 days. The median Tlag of both groups was 22 days, and the difference between groups was not statistically significant. The mean Tcastr of the testosterone parameter in the two groups was 26.5 days, and the difference between groups was statistically significant, indicating that compared with the reference group, the test group had more subjects who maintained a longer castration time ().
Table 3. Testosterone parameters (data set: ITT)
3.4. Safety
In the 24 subjects in the test group (data set: SS), the number of AEs was 68, the number of cases was 23, and the incidence was 95.8%, all of which belonged to treatment emergent adverse events (TEAEs). The number of TEAEs related to the study drug was 66, the number of cases was 23, and the incidence was 95.8%. In the 23 subjects in the reference group (data set: SS), the number of AEs was 35, the number of cases was 15, and the incidence was 65.2%. All of them were TEAEs and were all related to the study drugs. At a two-tailed test level of 0.05, the incidence of AEs, TEAEs, and TEAEs related to the study drug were compared between the two groups, and the results showed that the differences were statistically significant. In terms of severity, 100% of treatment-related adverse events were mild and moderate events. There were no serious TEAE, no TEAEs that led to withdrawal from the trial, and no TEAEs that led to death in the two groups ().
Table 4. Summary of adverse events (data set: SS)
Common TEAEs in the two groups included erectile dysfunction, decreased libido, night sweats, fever, limb pain, headache and rash, which are all related to the pharmacological effects of leuprolide acetate and are also expected to be undesirable events. These reversible adverse reactions will subside after the medication is stopped. Although laboratory test values and electrocardiogram data with clinically significant changes were diagnosed as abnormal and clinically significant, there were no significant clinical symptoms or signs, and they could be cured without intervention ().
Table 5. Summary of the relationship between TEAE and study drugs (data set: SS)
After the administration of the respective treatment in the two groups, no vital sign-related changes were found with the change in observation time; there was no local redness or local induration at any time point, and the pain score at the injection site showed a gradually decreasing trend.
4. Discussion
In this study, the PK characteristics of the new prolonged-release leuprorelin acetate microspheres for injection (3.75 mg) and Enantone® (3.75 mg) were consistent. The geometric mean ratios of Cmax, AUC0-t and AUC0-48 of the test group and the reference group were 99.32%, 122.41%, and 93.88%, and the 90% CIs were 87.49%~112.74%, 97.15%~154.25%, and 80.85%~109.01%.
Leuprolide acetate acts as an effective inhibitor of gonadotropin secretion by downregulating the pituitary LHRH receptor, which can cause serum testosterone to drop to levels close to surgical castration [Citation2,Citation5]. In a previous phase I trial, Lutrate® 3.75 mg and 7.5 mg depot (GP-Pharm S.A., Barcelona, Spain) and the marketed reference drugs Lucrin® 3.75 mg (Abbott AG, Baar/Zug, Switzer-land) and Procrin® 7.5 mg depot (Abbott Labo-ratories S.A., Madrid, Spain) were compared in terms of effectiveness in suppressing testosterone levels in 20 healthy male volunteers. Studies have shown that these two doses of Lutrate® can inhibit testosterone levels to achieve ‘chemical castration’, 4/5 volunteers reached the level of castration, and the duration of castration and recovery time of castration were longer than that of the reference drugs [Citation19]. In a multicenter, open-label, phase III study, Lutrate® 3.75 mg depot (GP-Pharm S.A., Barcelona, Spain) was used in prostate cancer patients to evaluate its effectiveness and safety, and the results show that Lutrate® 3.75 mg depot can inhibit testosterone levels and keep it below castrate levels [Citation20]. In this study, considering potential racial and ethnic differences, the inhibitory effects of the new prolonged-release leuprorelin acetate microspheres for injection (3.75 mg) and Enantone® (3.75 mg) on testosterone were compared among healthy Chinese male volunteers. Based on statistical evaluation, both groups reached a 100.0% castration level 28 days after administration. The two groups exhibited similar inhibitory effects on testosterone, and more subjects in the test group maintained a longer castration time. Similar to other LHRH agonists [Citation7,Citation9,Citation22], after administration, the concentrations of LH, FSH and testosterone increase sharply in the initial stage, and then these hormones are suppressed. The overall trends of changes in LH, FSH and testosterone in the two groups were roughly the same, which is similar to the results of previous studies [Citation19,Citation20].
In general, leuprolide acetate is a safe and tolerable drug. Its oral bioavailability is low, and it is administered by parenteral routes, such as intravenous injection and subcutaneous injection. Compared with subcutaneous injection, intramuscular injection may reduce local adverse reactions. Most of the adverse reactions, including hot flashes, night sweats, pain at the injection site, etc., will subside after the medication is stopped and are within the expected range, consistent with changes in hormone levels [Citation6,Citation9,Citation10,Citation19,Citation20,Citation23] . In this study, the route of administration of the test drug was intramuscular injection, and the route of administration of the reference drug Enantone® was subcutaneous injection. There was no local redness or local induration at any time point, and the pain score at the injection site showed a gradually decreasing trend in two groups. The high incidence of decreased libido and night sweats in this study is related to the pharmacological effects of the study drug, and both of these side effects are expected AEs. No hot flashes were found in this study, probably because the subjects only received a single 3.75 mg injection of the new prolonged-release leuprorelin acetate microspheres or a single 3.75 mg injection of the reference drug Enantone®. The severity of AEs in the test group was mild to moderate. Laboratory test values and electrocardiogram data showed clinically significant changes. Although the diagnosis was abnormal and clinically significant, there were still no significant clinical symptoms or signs, and can be cured without intervention.
5. Conclusions
The new prolonged-release leuprorelin acetate microspheres for injection is consistent with the PK characteristics of Enantone®. The two groups exhibited similar inhibitory effects on testosterone, and more subjects in the test group maintained a longer castration time. The safety of the test drug is good and tolerable. It is suggested that the single intramuscular injection dose of the new prolonged-release leuprorelin acetate microspheres for injection in phase II and III clinical trials is 3.75 mg. Leuprolide acetate is used to treat a variety of sex hormone-related disorders [Citation9–13]. The new prolonged-release leuprorelin acetate microspheres for injection may also present a good effect on the treatment of endometriosis, precocious puberty, etc. It is worthy of further research. This trial also has some limitations. First, this trial involved only a single dose, and thus, it was impossible to obtain information about the changes in leuprolide and sex hormones under multiple doses. Second, the subjects in this trial were healthy male volunteers who could tolerate the test drug. The patient’s response to the test drug may be different due to their physical fitness and other reasons, which needs further research.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2020;70(1):7–30.
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505.
- Dreicer R. The evolving role of hormone therapy in advanced prostate cancer. Clev Clin J Med. 2000;67(10):720–2, 725–6.
- Mcleod DG. Hormonal therapy: historical perspective to future directions. Urology. 2003;61(2 Suppl 1):3–7.
- ÉD S, Ferreira U, Matheus W, et al. Goserelin versus leuprolide in the chemical castration of patients with prostate cancer. Int Urol Nephrol. 2012;44(4):1039–1044.
- Lepor H. Comparison of single-agent androgen suppression for advanced prostate cancer. Rev Urol. 2005;7 Suppl 5(Suppl5):S3–S12.
- Lepor H, Shore ND. LHRH agonists for the treatment of prostate cancer: 2012. Rev Urol. 2012;14(1–2):1–12.
- Limonta P, Marelli MM, Moretti RM. LHRH analogues as anticancer agents: pituitary and extrapituitary sites of action. Expert Opin Investig Drugs. 2001;10(4):709–720.
- Plosker GL, Brogden RN. Leuprorelin. A review of its pharmacology and therapeutic use in prostatic cancer, endometriosis and other sex hormone-related disorders. Drugs. 1994;48(6):930–967.
- Wilson AC, Meethal SV, Bowen RL, et al. Leuprolide acetate: a drug of diverse clinical applications. Expert Opin Investig Drugs. 2007;16(11):1851–1863.
- Wang ST, Johnson SJ, Mitchell D, et al. Cost–effectiveness of elagolix versus leuprolide acetate for treating moderate-to-severe endometriosis pain in the USA. J Comp Eff Res. 2019;8(5):337–355.
- Vatopoulou A, Roos E, Daniilidis A, et al. Long-term effects of treatment of central precocious puberty with gonadotropin-releasing hormone analogs every three months. Gynecol Endocrinol. 2020;36(12):1124–1126.
- Klein KO, Freire A, Gryngarten MG, et al. Phase 3 trial of a small-volume subcutaneous 6-month duration leuprolide acetate treatment for central precocious puberty. J Clin Endocrinol Metab. 2020;105(10):e3660–e3671.
- Crawford ED, Phillips JM. Six-month gonadotropin releasing hormone (GnRH) agonist depots provide efficacy, safety, convenience, and comfort. Cancer Manag Res. 2011;3:201–209.
- Shore ND, Guerrero S, Sanahuja RM, et al. A new sustained-release, 3-month leuprolide acetate formulation achieves and maintains castrate concentrations of testosterone in patients with prostate cancer. Clin Ther. 2019;41(3):412–425.
- Shore N, Mincik I, DeGuenther M, et al. A phase 3, open-label, multicenter study of a 6-month pre-mixed depot formulation of leuprolide mesylate in advanced prostate cancer patients. World J Urol. 2020;38(1):111–119.
- Freitas CSMD, Soares AN. Efficacy of Leuprorelide acetate (Eligard®) in daily practice in Brazil: a retrospective study with depot formulations in patients with prostate cancer. Int Braz J Urol. 2020;46(3):383–389.
- Bruneu-Avierinos Y, Monestier S, Tasei A-M. Granulomas induced by injections of leuprorelin acetate (Enantone®). Ann Dermatol Venereol. 2011;138(1):35–37.
- Leitner JM, Mayr FB, Spiel AO, et al., The pharmacokinetics and pharmacodynamics of a new sustained-release leuprolide acetate depot compared to market references. Int J Clin Pharmacol Ther. 2008;46(8):407–414.
- Marberger M, Kaisary AV, Shore ND, et al., Effectiveness, pharmacokinetics, and safety of a new sustained-release leuprolide acetate 3.75-mg depot formulation for testosterone suppression in patients with prostate cancer: a phase III, open-label, international multicenter study. Clin Ther. 2010;32(4):744–757.
- Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015;26(8):1589–1604.
- Bubley GJ. Is the flare phenomenon clinically significant? Urology. 2001;58(2 Suppl 1):5–9.
- Yasukawa K, Sawamura D, Sugawara H, et al. Leuprorelin acetate granulomas: case reports and review of the literature. Brit J Dermatol. 2005;152(5):1045–1047.