ABSTRACT
Introduction: Recent developments in implantable cardioverter-defibrillators (ICDs) and smartphone technology have increased the possibilities for remote monitoring. It is the purpose of this review to give an overview of these new possibilities.
Areas covered: Remote monitoring in ICD allows for early detection of lead fractures and remote follow-up of patients. Possible limitations are the lack of standardization and the possible unsafety of the data stored on the ICD. Secondly, remote monitoring of health parameters using smartphone compatible wearables and smartphone medical apps is addressed. Possible limitations include the fact that the majority of smartphone apps are unregulated by the regulatory authorities and privacy issues such as selling of app-generated data to third parties. Lastly, clinical studies with smartphone apps are discussed.
Expert commentary: New technologies in ICDs and smartphones have the potential to be used for remote monitoring. However, unreliability of smartphone technology, inadequate legislation and lack of reimbursement impede implementation.
1. Introduction
In the Netherlands, highly specialized care is centralized in a number of University Medical Centers and other large hospitals [Citation1]. If a patient needs high specialized care, he is referred from a local hospital to one of these specialized centers [Citation1,Citation2]. After treatment, a patient is referred back to the local hospital [Citation3]. In this structure, involving more than one treating physician and relatively large distances, adequate data exchange, doctor–doctor communication tools, doctor–patient communication tools, and remote vital sign monitoring could enhance safety, efficiency, and patient satisfaction of care [Citation4].
The introduction of the iPhone, allowing users to use the Internet on their telephone, marked the beginning of massive adaptation of smartphone usage [Citation5]. The iPhone, as well as independently released Android phones, allowed users to build and use health and fitness applications. Concordant with the adaptation of smartphones, smartphone-compatible devices that can measure various health parameters such as heart rate (HR), blood pressure (BP), and weight have been introduced on the consumer market [Citation6,Citation7]. These devices are small, handheld, relatively cheap, do not necessitate the assistance of health-care staff, and allow for automatic transferring of generated data, making them suitable for remote monitoring (RM) [Citation8]. These trends have increased the interest in mobile health ().
However, these new possibilities are also subject of several constraints, such as data validity, data safety, and patient’s privacy [Citation8,Citation9]. It is therefore the purpose of this review to give an overview of the current possibilities and constraints of new technologies for RM in cardiology.
2. RM of implanted devices
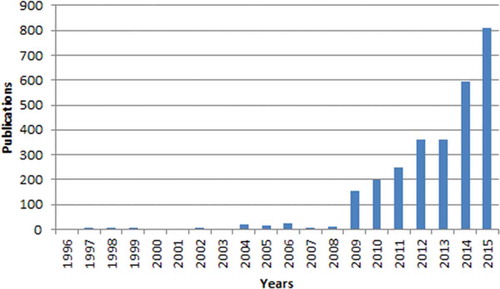
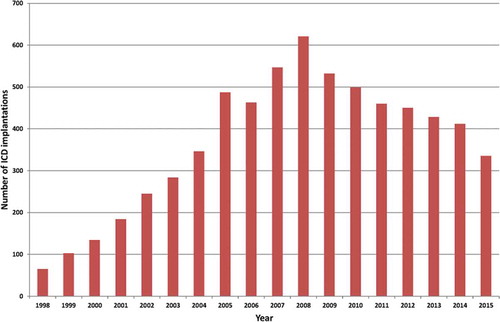
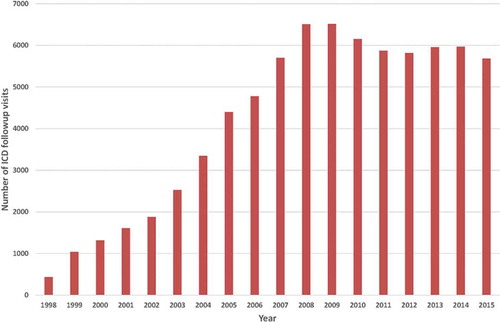
The implementation of the results of large randomized trials showing the effectiveness of implantable cardioverter-defibrillators (ICDs) in clinical practice has led to an exponential rise in the number of implanted ICDs [Citation10,Citation11] (). The growing number of ICD recipients has resulted in a rapidly increasing workload with respect to the follow-up of these patients [Citation12] ().
Remote follow-up of implanted pacemakers or ICDs can offer a solution to the problem of overcrowded outpatient clinics and will bring considerable convenience to the patients since they will have to visit to the outpatient clinic less frequently [Citation12]. The clinical and health economics impact of RM however is still under discussion [Citation13]. An RM system makes it possible to alternatively schedule a remote follow-up between in-clinic follow-up [Citation14]. Furthermore, RM may allow early detection of ICD or lead failures without requiring any patient intervention [Citation12]. Furthermore, RM enables early detection of arrhythmias such as atrial fibrillation or confirm either appropriate or inappropriate shock delivery while the patient is still at home [Citation12].
RM of ICDs can also aid in early detection of technical problems, including device problems such as battery depletion, but also lead problems such as fracture [Citation15]. For instance, the major defibrillator lead problems experienced with Medtronic’s Sprint Fidelis lead have proven to be more predictable using RM; in an observatory study which was part of the effectiveness and cost of ICD follow-up schedule with telecardiology (ECOST) trial, involving 40 patients with an ICD who were home monitored, it was shown that RM allowed the early and reliable detection of lead fractures, without a notification by a patient [Citation15–Citation17].
2.1. Remote follow-up versus continuous RM
A recent consensus document has proposed terms to standardize the description of the different functions of remote patient management in patients with implanted devices [Citation18]. Remote follow-up involves scheduled automatic device interrogation, which replaces in-office visits for assessing device function; RM involves automatic unscheduled transmission of event alerts; finally, patient-initiated interrogations are nonscheduled follow-ups initiated by the patient as a result of a real or perceived clinical event [Citation19].
2.2. Effect of RM on outcomes
Until recently, large randomized trials of RM of patients with ICDs and heart failure (HF) showed no significant difference in mortality [Citation20,Citation21]. However, a study by Inglis et al. [Citation22]. in 2011 has shown that RM appears to have a substantial impact on reducing mortality. This Cochrane Review article included 25 peer-reviewed articles with 11 articles describing randomized clinical trials (RCTs) about telemonitoring (11 articles and 2710 participants) with two analyzing both interventions. In these articles, participants were randomized to either telemonitoring or usual care. All-cause mortality was calculated. The review showed that telemonitoring reduced all-cause mortality (risk ratio (RR): 0.66, P <.001). Telemonitoring also reduced hospitalizations (RR: 0.79, P = 0.008) [Citation22]. Furthermore, in an important recent study by Hindricks et al. [Citation23], where 664 patients with mild-to-moderately symptomatic chronic HF and a recent dual-chamber ICD or Cardiac Resynchronization Therapy Device implant were randomly assigned to either automatic daily implant-based monitoring in addition to usual care or usual care alone, the authors showed that after 12 months of follow-up, there was a significantly lower mortality in the RM group (10 vs. 27 deaths, 12 months hazard ratio 0.36, 95% confidence interval: 0.17–0.74). They conclude that automatic, daily, implant-based telemonitoring of rhythm and technical parameters had a significantly beneficial effect on the composite clinical score and all-cause mortality [Citation23].
In a recent study by Klersy et al. [Citation24], the authors have investigated the effect of RM of implanted cardiac devices on health-care utilization. In a systemic review and meta-analysis of 11 RCTs on RM in a total of 5702 patients with HF, RM was compared to standard care. The authors conclude that RM is associated with a marked reduction in planned hospital visits and overall costs, without compromising survival or markedly increasing unplanned hospitals visits [Citation24].
3. Privacy and security of ICDs
In a December 2012 episode of the popular fictional television series Homeland, the vice president of the United States was assassinated when a terrorist organization wirelessly hacked the implanted pacemaker and induced a tachycardia resulting in a myocardial infarction [Citation25]. Although this scenario may seem far-fetched, there has been a recent demonstration of networked medical device vulnerability: in order to test the vulnerability of security breaches by hackers accessing devices with wireless capability, a group of researchers at the University of Washington, Seattle, WA, USA, performed laboratory tests on a Medtronic Maximo DR ICD (Medtronic, Minneapolis, MN, USA). After having partially reversed the ICD’s communication protocol, they performed several software radio-based attacks that were able to retrieve encrypted personal patient data, as well as change device settings (including commanded shocks) [Citation26]. Therefore, security and privacy of implantable medical devices remain important issues that need more research [Citation27].
4. Integration of RM into clinical practice
All of the major manufacturers have developed proprietary methods for data transfer from a patient’s device to the health-care professional [Citation28]. Although there are some operational differences between manufacturers, the general flow of information is similar with all systems [Citation28]. At regular intervals (depending on the setup of the specific RM system), the implanted device will connect to a receiving system at the patient’s home and then send data on the status of the device and of the patient to the central database system, operated by the device company. The physician can log into a secure website and check the data from the remote follow-up for each patient [Citation12,Citation28]. However, so far, it has not been possible to integrate the data from RM system into the local electronic health record (EHR) system, which potentially may create patient safety issues. In other words, data are stored on different systems and may not be accessible for all health-care providers [Citation12]. This may potentially result in patient safety issues as it may be difficult to keep track of all information available and information which may be only accessible for certain doctors or technicians. Ideally all information should be available in the EHR system [Citation12,Citation28].
4.1. Need for standardized data exchange
Since all vendors have developed proprietary solutions for collecting and storing the data from the implanted devices, there is a strong need to be able to import data from the RM database system and to integrate the data into the local EHR in a standardized way [Citation12]. To obtain this goal, there is a need for a standard set of observations, communicated in standard messages, such as therapy settings, events, and device self-monitoring. Furthermore, there should be a consistent presentation of data from all devices [Citation29].
4.2. Integrating the Healthcare Enterprise Implantable Device Cardiac Observation
To address the requirement of integrating RM data in the local EHR, the Integrating the Healthcare Enterprise (IHE) Implantable Device Cardiac Observation (IDCO) profile has been developed [Citation30]. IHE is a shared initiative by health-care professionals and industry to improve the way computer systems in health care share information [Citation31]. The IHE IDCO profile defines a standards-based transfer of device interrogation information from the interrogation system into the information management system. Features of the IHE IDCO profile are standard set of observations, communicated in standard messages, consistent presentation of data from all devices, and direct link between interrogating device and local EHR [Citation32,Citation33].
4.3. Cardiac device outpatient follow-up
The IHE IDCO profile not only brings a solution to the problem of data in the RM database that is not available locally in the Cardiology Information System (CIS) [Citation29]. The profile also brings a solution to the following problem: during outpatient clinic device follow-up, the measurements are performed with the use of a so-called programmer. Such a programmer system can connect wirelessly to the device implanted in the patient and then extracts the device data (e.g. settings, status, and events) from the device. Furthermore, it can also be used to reprogram the settings of the device, if necessary. However, after the measurements are performed, the information needs to be typed in by hand into CIS from a paper report printed on the programmer. The IHE IDCO profile also brings a solution to this problem, by defining standards for this specific data exchange [Citation31].
4.4. Nomenclature
An important part of the IHE IDCO profile is the nomenclature, which is the definition of the variables that are exchanged. Companies that implement the IHE IDCO profile not only need to exchange data in a standard way, but also should make the data available using uniquely defined data definitions [Citation34].
The Institute of Electrical and Electronics Engineers’ (IEEE) Standards Association is defining sets of terminology for ‘point-of-care’ medical device communication. One of these sets is IEEE 11073-10103 which supports terminology for implantable cardiac devices [Citation35].
4.5. Device vendor involvement and implementation
All large cardiovascular implantable device vendors are involved in the development of the IHE IDCO profile and in the development of the IEEE 11073-10103 nomenclature standard [Citation35]. All companies have already partially or completely implemented the IHE profile and IEEE standard and have a hardware/software solution available which can be used to communicate with an EHR or data management system [Citation36].
The implementation from Biotronik (Biotronik SE & Co. KG, Berlin, Germany), Boston Scientific (Marlborough, MA, USA), St. Jude Medical (St. Paul, MN, USA), and Sorin (Clamart, France) is freely available, but Medtronic has only implemented the IHE IDCO profile to communicate with their proprietary solution Paceart™. Biotronik, St. Jude Medical, and Sorin have also already implemented the possibility for the data exchange between a programmer and the EHR. More details also on the implementation have been described previously by Van der Velde et al. [Citation12].
5. RM with smartphone applications and compatible wearables
5.1. Electrocardiogram devices
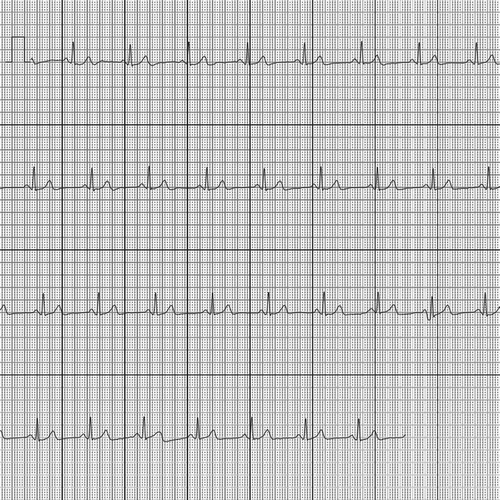
There are various devices available for over-the-counter sale that allows their user to make a single-lead electrocardiogram (ECG) [Citation37]. These devices are easy-to-use, handheld, and do not require the presence of health-care professionals. One of these devices is the AliveCor [Citation6]. This device, having the size of a credit card, has two electrodes [Citation38]. Upon placing fingers of one hand against the one electrode and fingers of the other hand against the other electrode, the device sends an ultrasound signal. This is picked up by the smartphone’s microphone, filtered, and digitalized. Subsequently, a live single-lead ECG can be seen on the smartphone screen [Citation39].
After 30 s of measurement, the AliveCor algorithm gives a diagnosis, varying from ‘normal,’ ‘possibly abnormal’ to ‘undetermined.’ This algorithm is based on R-R intervals irregularity. In a validation study by Lau et al., it showed a 97% sensitivity and 98% specificity for atrial fibrillation detection [Citation39]. The ECGs are stored on the users’ smartphone and on AliveCors secured servers [Citation38] ().
A patient’s account can be linked to a physician’s account, allowing the physician to view the ECGs made by the patient. The AliveCor is compatible with Android operating system (OS) and iOS [Citation38].
5.2. BP cuffs
Several smartphone-compatible BP cuffs are available for over-the-counter sale. These are all automated oscillometric Bluetooth-enabled cuffs, which can be applied without the presence of health-care staff [Citation40–Citation42].
Three examples are the iHealth BP5 [Citation40], QardioArm [Citation41], and Withings BP monitor [Citation42]. These cuffs are placed around the upper arm of the patient. After automated inflation and deflation, the systolic BP, diastolic BP, and HR can be viewed on the smartphone or tablet screen. Results are shown in a device dedicated app. The Qardio and Withings apps are both Android OS and iOS compatible [Citation41,Citation42]. The iHealth app is only iOS compatible [Citation40]. An advantage of these devices is that data are automatically stored and transferred, saving the patient’s time of writing down his measurements and preventing errors in copying the data.
5.2.1. Mobile applications
Adequate measurement of vital signs such as (but not limited to) ECG, BP, HR, and weight can be done via smartphone-compatible external hardware [Citation40,Citation42]. There are, however, apps in the App store or Play Store that claim to allow the smartphone user to measure HR or BP, without the need of external hardware [Citation43].
These apps rely on photoplethysmography, which is based on the principle that the absorbency of infrared light differs among various types of tissue [Citation44]. The amount of absorbed infrared light determines the amount of infrared light detected by the photodetector. The amount of detected light is determined by the volume of blood crossing the photodetector. Less light is detected when a larger volume of blood is crossing the photodetector. Thus, the photodetector is able to detect the pulsatile flow in the arteries [Citation45,Citation46]. The HR can subsequently be calculated by an algorithm [Citation44].
In these apps, the finger has to be placed in front of the smartphone camera. The flashlight is used to detect volume differences. The HR is subsequently calculated by an algorithm in the app [Citation44]. This method has been validated against ECG and oximetry-derived HR by Gregoski et al. [Citation44]. They investigated 14 healthy subjects. They measured HR via an ECG and via the smartphone app simultaneously. All subjects measured their HR during sitting, reading, and playing a videogame. The authors compared the HRs and calculated Pearson’s correlation coefficients and standard errors of the estimate (SEE). Correlation coefficients of 0.99 were found with an SEE of 0.59 (sitting), 0.94 (reading), and 0.66 (video game). It was therefore concluded that the app provided reliable HRs [Citation44]. However, the clinical value of these apps may be limited, as these apps are not suitable for continuous HR monitoring.
Apps which measure BP are available for download as well. These apps claim to be able to measure BP using only the smartphone’s camera [Citation47]. One of these apps is ‘Quick BP measure and monitor.’ This app claims to ‘let you measure your BP using only your iPhone – no cuff required.’ However, it also claims that it is not a medical device and that the accuracy is still being improved [Citation47]. Furthermore, it is unclear which technique the app is based on. Several articles have described methods for noninvasive continuous BP monitoring; however, it has been recognized that these methods are still prone to errors [Citation48–Citation51].
Measuring BP with only the smartphone might improve health care, as it does not require trained health-care staff and is patient friendly and as it does not require the inflation of a cuff. However, scientific articles which underline the accuracy of these apps are scarce. The reliability of these apps has been questioned in both scientific and nonscientific literature [Citation52,Citation53].
6. Legislation
There are, both in the USA and the European Union (EU), strict regulations for medical devices. A medical device has an intended use, which is the primary purpose for which a medical device is manufactured. All medical devices have to get approval by the US FDA for their intended use before they can be sold and prescribed in the USA or receive a Conformité Européenne-mark before they can be sold and prescribed in the EU [Citation54,Citation55].
The FDA and the EU have similar approaches to mobile apps. If a mobile app falls under the definition of medical device, then it needs to be cleared by the certified body (either the FDA in the USA or the European Medical Agency in the EU).
The FDA recognizes that 99% of all health apps are not considered medical devices and are therefore not regulated by the FDA [Citation54,Citation55].
According to the EU regulations, mobile apps are considered medical devices if they give a medical diagnosis, if they give a therapeutic advice, or if the app is inevitable for the device to function. If an app is considered a medical device, it needs to undergo the same testing and certifying procedures as any other medical device. Selling or prescribing a non-CE-marked (EU) or non-FDA-cleared (USA) app is an offense and can lead to claims for the doctor or manufacturer [Citation56].
Nevertheless, the fact that a mobile app is not a medical device does not mean that a mobile app is not collecting medical data. Therefore, apart from the regulation, the privacy of patients has to be taken into account when developing, selling, or prescribing mobile medical apps.
7. Privacy and security of mobile apps
An important constraint for implementation of mobile apps in health care is privacy and security of the mobile app-generated data [Citation9]. Mobile health apps, by definition, generate data about at least part of the user’s health. These mobile health-generated data are therefore sensitive information and subject of privacy regulations. However, various reports have identified that the majority of mobile apps suffer serious privacy concerns [Citation57,Citation58]. In a recent article, 79 health apps were evaluated for data safety principles. The results show that the majority did not encrypt data sent over the Internet. A total of 20% did not have a privacy policy [Citation57].
The privacy statements made by mHealth apps have been reviewed by Sunyaev et al. [Citation58]. Of 600 commonly used apps, only 30.5% had privacy policies. The average reading grade level of these privacy policies was found to be 16 (2.9 standard deviation), which corresponds to college-level literacy. This may inhibit the public’s understanding of the privacy risks of mobile applications [Citation58].
A major concern in collection of data by third parties is the selling of data to third parties. A recent study by Zang et al. [Citation59]. investigated the 110 most downloaded free apps. They investigated if the data were transferred to a third-party domain (i.e. a domain that did not primarily belong to the application) and categorized that information to identify transmission of sensitive data, personal identifiable data, behavior data, and location data. It found that of the 10 most downloaded health and fitness apps, 9 apps transmitted personal identifiable data to a third-party domain [Citation59].
This selling can even be used for ethically justifiable purposes: Strava, an application that uses global positioning system to track speed and distance during a workout, sells data to city planners, which use it to decide where new bike paths can be built [Citation60].
However, there are also purposes which might require further evaluation by privacy and security experts. One concern is that the person’s employer or health insurance company will buy the data. Health insurance companies might raise health insurance premiums based on data generated by activity trackers [Citation61]. Recently, the self-insured company British Patrol gave 14,000 employees a Fitbit Zip (Fitbit Inc., San Francisco, CA, USA). If an employee walked more than 1 million steps, he received points that could lower their insurance premiums [Citation61]. Concerns about the usage of data generated by wearables might inhibit adaptation of mobile health apps in clinical practice [Citation62].
Many mHealth apps target a global community, which makes the manufacturers having to deal with security and privacy laws worldwide. Martinez-Pérez et al. [Citation63]. reviewed the standards and certifications about security and privacy in North America and the European countries. They state that ‘In practice, these laws are too open and too old, and need to be revised and reformulated taking into account the current technologies, industries and healthcare fields, focusing especially on mHealth and the mobile apps industry, which is continuously expanding.’[Citation63] Mamlin et al. [Citation64]. also point out that the laws, in the United States, need to be updated to match the current methods for recording and transmitting data [Citation64]. Privacy and security issues need to be addressed adequately because otherwise it may adversely affect the trust in mHealth [Citation65]. However, there is no one-size-fits-all approach to security and privacy [Citation66]. mHealth technology should be tailored to deal with the heterogeneous types of information, comfort, skills, and concerns of the end users [Citation67].
8. Clinical value
Hypertension, lack of physical activity, obesity, and smoking are well-established risk factors for coronary artery disease (CAD) [Citation68]. A combination of these risk factors exponentially raises the chance for the development of CAD [Citation69]. Both medication and lifestyle interventions have been proven to lower BP, lower body mass index, and improve lipid profile. In certain clinical studies, lifestyle interventions have been proven to be non-inferior to medication or even stenting [Citation70,Citation71]. Nevertheless, as the European Society of Cardiology guidelines on cardiovascular disease prevention in clinical practice note, changing lifestyle is very difficult [Citation68]. Successful lifestyle interventions often require feedback and training provided by trained health-care workers and is therefore expensive and small scaled [Citation68]. mHealth can potentially bring training programs to patients on a large scale.
A recent review by Piette et al. [Citation72]. identified studies that involved mHealth in weight management, physical activity, or smoking cessation. They found that there were numerous trials positively correlating an mHealth intervention and lower BP, lower BMI, higher physical activity, and more smoking cessation [Citation72].
An example of an RCT with a lower BP is described by Margolis et al. [Citation73]. In this RCT, 450 patients were randomized to either home BP measurements (intervention group) or usual care (control group). In the intervention group, patients measured their BP six times a week and sent the data to their pharmacist. The control group received usual care, meaning regular visits to the general practitioner. The results showed that the percentage of patients with controlled BP in the intervention group at 12 months was significantly higher than in the control group. This study is of considerable interest, as it indicates that increasing the frequency of monitoring and subsequent treatment adjustments might improve quality of care [Citation73].
Burke et al. [Citation74]. published a scientific statement on mHealth in cardiology. They reviewed clinical studies in which mobile phones were used to address one or more of the American Heart Association’s Life’s Simple 7 program health indicators: healthy weight, enough physical activity, quitting smoking blood glucose, and BP control as well as lipids to target levels. For each category, an RCT describing a significant difference between intervention and control groups was found, although several RCTs not finding a significant difference were included as well [Citation74]. Several constraints were applicable to almost every category: whatever tracking device was used, a BP monitor, an ECG apparatus, or an activity tracker, it did not stand alone. Concordant coaching, by short message service, Interactive Voice Recording, a website, or face-to-face, was obligatory in almost all trials in order for the intervention to be clinically effective. The major drawback of this approach is that an mHealth intervention with interactive coaching has seldom been proven to be cost-effective, which inhibits its implementation in health care [Citation72,Citation74].
Another limitation for clinical implementation is the difficulty to act evidence based. The methods of all randomized controlled trials differ significantly, making it difficult to extrapolate an evidence-based working method from the literature. Furthermore, there are a lot of so-called ‘pilot studies’ or ‘feasibility studies,’ studies which are typically characterized by a non-randomized design and small sample size.
The final limitation is that in an RCT, one app or mobile technology intervention is studied. Often, this one app is targeting one risk factor for CAD [Citation74]. However, patients at risk for CAD often have more than one risk factor for CAD [Citation69]. So far, very few randomized controlled trials have addressed an intervention in which several apps were applied to the patient at the same time. It can be hypothesized that, given the previously described difficulties to adhere to one app and concordant doctor–patient interaction [Citation74], adhering to several apps addressing several cardiovascular risk factors will lower the overall clinical effectiveness of the intervention.
Another important question that still needs to be answered in the scientific literature is the long-term effect of mHealth lifestyle interventions. Very few studies have studied the effect of an mHealth intervention after coaching is quitted.
Therefore, more research needs to be done in which several apps are applied at the same time. Furthermore, the methods of mHealth intervention need to be standardized for comparison purposes. Last, long-term effects need to be carefully monitored.
9. Expert commentary
Mobile health and RM is still in its disruptive phase. The basic tools for remote medicine (i.e. remote diagnosis, remote treatment, and remote communication) have been available for less than 10 years [Citation5]. Currently, there are a couple of factors essentially inhibiting this new way of delivering care: first, the quality of the available mobile technology is often not sufficient for medical practice. This is the case with most mobile applications for vital sign monitoring. Second, the legislation is still based on traditional medicine. Partly caused by the insufficient quality described above, current policy makers are hesitating to change legislation and allow remote medicine. Third, there is a lack of reimbursement which inhibits active participation in implementation by health-care professionals. Finally, the long-term effects of remote medicine, especially remote coaching, have not been scientifically assessed. Traditionally, legislation and reimbursement regulations are only changed if an intervention has been proven scientifically, clinically cost-effective. As heterogeneity of currently available RCTs and lack of long-term evidence inhibit unambiguously scientific conclusions, we expect that remote medicine cannot be fully implemented on a short notice.
10. Five-year view
We expect that remote medicine, despite the mentioned constraints, will change the way health care is delivered. Currently available outpatient clinics, wards, and general ways to deliver health care require too much human and financial resources to be sustainable in the future, especially with the aging population. In 5 years, there will be more wearables available to measure vital signs. These wearables will be more accurate. Overall, technological constraints will be easily overcome.
With the adjusted legislation, remote medicine will be available to those who prefer to be diagnosed and treated remotely. It is however difficult to expect that remote medicine will be fully implemented in the upcoming 5 years, especially since medicine is traditionally a slow moving field, keeping in mind that serious legal changes have to be made to speed up the implementation of remote medicine.
Key issues
Mobile technology has accelerated remote medicine possibilities
Remote monitoring has the ability to decrease outpatient clinic visits and improve quality of care. The remote monitoring of ICD patients serves as example
A key issue for mobile health to succeed is the integrating of various mobile technology information systems. Therefore, standardization will be a great topic in the nearby future
Mobile access to cardiovascular images, enabling a rapid diagnosis, is still limitedly used since the obliged quality cannot be delivered on mobile phones so far
Mobile apps have the potential to help patient manage their own cardiovascular risk factors, such as blood pressure, weight and activity
The beneficial short term effects of remote monitoring have been demonstrated in the literature
Limited literature exists however on the long term effects of mobile apps in remote monitoring and lifestyle interventions
The heterogeneity of randomized clinical trials makes it difficult to draw unambiguous conclusions and to practice evidence based medicine.
Declaration of interest
E.T. van der Velde is a member of the steering committee of Integrating the Healthcare Enterprise (IHE) The Netherlands. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- NFU. Erkende Expertisecentra Zeldzame Aandoeningen. 2015. [cited 2016 May 09]. Available from http://www.nfu.nl/img/pdf/Erkende_expertisecentra_zeldzame_aandoeningen_TOTAAL_FINAL_05-06-2015.pdf
- NFU. Minister erkent expertisecentra zeldzame aandoeningen. 2015. [cited 2016 May 09]. Availablefrom: http://www.nfu.nl/actueel/minister-erkent-expertisecentra-zeldzame-aandoeningen
- NFU. Betere samenwerking rond de patiënt met zeldzame aandoening. 2016. [cited 2016 Jul 11]. Available from: http://www.nfu.nl/actueel/betere-samenwerking-rond-de-patient-met-zeldzame-aandoening
- Treskes RW, Van Der Velde ET, Atsma DE, et al. Redesigning healthcare: the 2.4 billion euro question? Connecting smart technology to improve outcome of patients. Neth Heart J. 2016 Apr 6;24:441–446.
- Statista. [cited 2016 Mar 10]. Global Apple iPhone sales from 3rd quarter 2007 to 1st quarter 2016 (in million units). Available from: http://www.statista.com/statistics/263401/global-apple-iphone-sales-since-3rd-quarter-2007/.
- AliveCor. [cited 2016 Mar 26]. Available from: http://www.alivecor.com/
- Withings. Available from: http://www.withings.com/eu/en/
- Clark PA, Capuzzi K, Harrison J. Telemedicine: medical, legal and ethical perspectives. Med Sci Monit. 2010;16:RA261–RA272.
- Anderson JG. Social, ethical and legal barriers to e-health. Int J Med Inform. 2007 May–Jun;76:480–483.
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015 Nov 1;36:2793–2867.
- Goldenberg I, Gillespie J, Moss AJ, et al. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the multicenter automatic defibrillator implantation trial II. Circulation. 2010 Sep 28;122:1265–1271.
- Van der Velde ET, Atsma DE, Foeken H, et al. Remote monitoring of patients with implanted devices: data exchange and integration. Eur J Prev Cardiol. 2013 Jun;20:8–12.
- Parthiban N, Esterman A, Mahajan R, et al. Remote monitoring of implantable cardioverter-defibrillators: a systematic review and meta-analysis of clinical outcomes. J Am Coll Cardiol. 2015 Jun 23;65:2591–2600.
- Varma N, Epstein AE, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T safely reduces routine office device follow-up (TRUST) trial. Circulation. 2010 Jul 27;122:325–332.
- Borleffs CJ, Van Erven L, van Bommel RJ, et al. Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2:411–416.
- Guedon-Moreau L, Chevalier P, Marquie C, et al. Contributions of remote monitoring to the follow-up of implantable cardioverter-defibrillator leads under advisory. Eur Heart J. 2010;31:2246–2252.
- Guedon-Moreau L, Lacroix D, Sadoul N, et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. 2013;34:605–614.
- Dubner S, Auricchio A, Steinberg JS, et al. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs). Europace. 2012;14:278–293.
- Burri H. Remote follow-up and continuous remote monitoring, distinguished. Europace. 2013 Jun;15(Suppl 1):i14–i16.
- Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010 Dec 9;363:2301–2309.
- Desai AS. Home monitoring heart failure care does not improve patient outcomes: looking beyond telephone-based disease management. Circulation. 2012 Feb 14;125:828–836.
- Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011 Sep;13:1028–1040.
- Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–590.
- Klersy C, Boriani G, De Silvestri A, et al. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta-analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail. 2016;18:195–204.
- Homeland, season 2, episode 10 “Broken Hearts. [cited 2016 May 10]. Available from: http://www.sho.com/sho/homeland/season/2/episode/10#/index
- Heydt-Benjamin T, Halperin D, Ransford B, et al. Pacemakers and implantable cardiac defibrillators: software radio attacks and zero-power defenses. In: Proceedings of the 2008 IEEE Symposium on Security and Privacy. 2008. p. 129–142,
- Halperin D, Heydt-Benjamin TS, Fu K, et al. Security and privacy for implantable medical devices. IEEE Pervasive Comput. 2008;7:30–39. Available from: https://spqr.eecs.umich.edu/papers/b1kohFINAL2.pdf
- Burri H, Senouf D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace. 2009 Jun;11:701–709.
- I. t. H. Enterprise. PCD implantable device cardiac observation. 2015. [cited 2016 Jul 11]. Available from: http://wiki.ihe.net/index.php/PCD_Implantable_Device_Cardiac_Observation
- I. t. H. Enterprise. IHE cardiology. [cited 2016 Mar 10]. Available from: http://www.ihe.net/Cardiology/
- I. t. H. Enterprise. Main page: integrating the healthcare enterprise. [cited 2016 Mar 10]. Available from: http://wiki.ihe.net/index.php?title=Main_Page
- Heidbuchel H. Telemonitoring of implantable cardiac devices: hurdles towards personalised medicine. Heart. 2011 Jun;97:931–939.
- Slotwiner D, Varma N, Akar JG, et al. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12:e69–e100.
- De Cock CC, Elders J, van Hemel NM, et al. Remote monitoring and follow-up of cardiovascular implantable electronic devices in the Netherlands: an expert consensus report of the Netherlands Society of Cardiology. Neth Heart J. 2012;20:53–65.
- I. S. Association. 11073-10103-2012-health informatics–point of care medical device communication Part 10103: nomenclature. 2012. [cited 2016 Mar 10]. Available from: https://standards.ieee.org/findstds/standard/11073-10103-2012.html
- Kasparick M, Schlichting S, Golatowski F, et al. New IEEE 11073 standards for interoperable, networked point-of-care medical devices. Conf Proc IEEE Eng Med Biol Soc. 2015 Aug;2015:1721–1724.
- Grier J. Comparison and review of portable, handheld, 1-lead/channel ECG/EKG recorders. [cited 2016 May 09]. Available from: https://www.ndsu.edu/pubweb/~grier/Comparison-handheld-ECG-EKG.html
- AliveCor. [cited 2016 Jan 12]. Available from: www.alivecor.com.
- Lau JK, Lowres N, Neubeck L, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165:193–194.
- iHealth. iHealth wireless blood pressure monitor. [cited 2016 May 09]. Available from: https://ihealthlabs.com/blood-pressure-monitors/wireless-blood-pressure-monitor/
- Qardio. QardioArm. [cited 2016 May 09]. Available from: https://www.getqardio.com/qardioarm-blood-pressure-monitor-iphone-android/
- Withings. Wireless blood pressure monitor. [cited 2016 May 09]. Available from: https://www.withings.com/eu/en/products/blood-pressure-monitor?
- I. Cardiio. Cardiio – heart rate monitor. [cited 2016 May 09]. Available from: https://itunes.apple.com/us/app/cardiio-heart-rate-monitor/id542891434?mt=8
- Gregoski MJ, Mueller M, Vertegel A, et al. Development and validation of a smartphone heart rate acquisition application for health promotion and wellness telehealth applications. Int J Telemed Appl. 2012;2012:696324.
- Reisner A, Shaltis PA, McCombie D, et al. Utility of the photoplethysmogram in circulatory monitoring. Anesthesiology. 2008 May;108:950–958.
- Millikan. The oximeter, an instrument for measuring continuously the oxygen saturation of arterial blood in man. Rev Sci Instrum. 1942;13:434.
- Ren J. Quick blood pressure measure and monitor. [cited 2016 May 09]. Available from: https://itunes.apple.com/nl/app/quick-blood-pressure-measure/id890706381?mt=8
- Janelle GM, Gravenstein N. An accuracy evaluation of the T-Line Tensymeter (continuous noninvasive blood pressure management device) versus conventional invasive radial artery monitoring in surgical patients. Anesth Analg. 2006 Feb;102:484–490.
- Challoner AV, Ramsay CA. A photoelectric plethysmograph for the measurement of cutaneous blood flow. Phys Med Biol. 1974 May;19:317–328.
- Ruiz-Rodriguez JC, Ruiz-Sanmartin A, Ribas V, et al. Innovative continuous non-invasive cuffless blood pressure monitoring based on photoplethysmography technology. Intensive Care Med. 2013;39:1618–1625.
- Nitzan M, Adar Y, Hoffman E, et al. Comparison of systolic blood pressure values obtained by photoplethysmography and by Korotkoff sounds. Sensors (Basel). 2013;13:14797–14812.
- Husain I. Top 10 downloaded iPhone health app can cause significant patient harm. 2014. [cited 2016 Feb 20]. Available from: http://www.imedicalapps.com/2014/07/iphone-health-app-patient-harm/#
- Bruining N, Caiani E, Chronaki C, et al. Acquisition and analysis of cardiovascular signals on smartphones: potential, pitfalls and perspectives: by the task force of the e-Cardiology Working Group of European Society of Cardiology. Eur J Prev Cardiol. 2014;21:4–13.
- United States Food and Drug Administration. Mobile medical applications: guidance for industry and Food and Drug Administration staff. 2015 Feb 9 [cited 2016 Jul 12]. Available from: http://www.fda.gov/downloads/MedicalDevices/…/UCM263366.pdf.
- E. Union. Green paper on mobile health (“mHealth”). 2014. Available from: https://ec.europa.eu/digital-single-market/en/news/green-paper-mobile-health-mhealth
- Meulen VE, van der S. Dutch DPA finds fitness app violates data protection law. E health Law & Policy. Cecile Park Publishing Ltd. 2015 Dec [cited 2016 Jul 12]. Available from: http://www.axonlawyers.com/wp-content/uploads/2015/12/EHLP-December-2015-pg-3-4.pdf .
- Huckvale K, Prieto JT, Tilney M, et al. Unaddressed privacy risks in accredited health and wellness apps: a cross-sectional systematic assessment. BMC Med. 2015;13:214.
- Sunyaev A, Dehling T, Taylor PL, et al. Availability and quality of mobile health app privacy policies. J Am Med Inform Assoc. 2015 Apr;22:e28–e33.
- Zang DK, Graves J, Lisker P, et al. Who knows what about me? A survey behind the scenes personal data sharing to third parties by mobile apps. JOTS Tech Sci. 2015. [cited 2016 Feb 20]. Available from: http://techscience.org/a/2015103001/.
- Klosowski. Lots of health apps are selling your data. Here’s why. 2014. [cited 2016 Mar 20]. Available from: http://lifehacker.com/lots-of-health-apps-are-selling-your-data-heres-why-1574001899.
- Olson P. Wearable tech is plugging into health insurance. 2014. [cited 2016 May 10]. Available from: http://www.forbes.com/sites/parmyolson/2014/06/19/wearable-tech-health-insurance/#3bdf8b7d5ba1
- Cusano DBJ, Starrs A, Viale E, et al. Digital insurance era: stretch your boundaries. 2015 Available from: https://www.accenture.com/us-en/insight-technology-vision-insurance-2015
- Martinez-Pérez B, de la Torre-Diez I, Lopez-Coronado M. Privacy and security in mobile health apps: a review and recommendations. J Med Syst. 2015 Jan;39:181.
- Mamlin BW, Tierney WM. The promise of information and communication technology in healthcare: extracting value from the chaos. Am J Med Sci. 2016 Jan;351:59–68.
- Hale TM, Kvedar JC. Privacy and security concerns in telehealth. Virtual Mentor. 2014;16:981–985.
- Baig MM, GholamHosseini H, Connolly MJ. Mobile healthcare applications: system design review, critical issues and challenges. Australas Phys Eng Sci Med. 2015 Mar;38:23–38.
- Atienza AA, Zarcadoolas C, Vaughon W, et al. Consumer attitudes and perceptions on mHealth privacy and security: findings from a mixed-methods study. J Health Commun. 2015;20:673–679.
- Perk J, De BG, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701.
- Aktas MK, Ozduran V, Pothier CE, et al. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA. 2004 Sep 22;292:1462–1468.
- Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. Br J Sports Med. 2015 Nov;49:1414–1422.
- Hambrecht R, Walther C, Mobius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004 Mar 23;109:1371–1378.
- Piette JD, List J, Rana GK, et al. Mobile health devices as tools for worldwide cardiovascular risk reduction and disease management. Circulation. 2015 Nov 24;132:2012–2027.
- Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56.
- Burke LE, Ma J, Azar KM, et al. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2015;132:1157–1213.