1. Introduction
In the last decades, the widespread use of percutaneous coronary intervention (PCI) with drug-eluting stent (DES) implantation and the introduction of novel anti-ischemic drugs represented major steps forward for the treatment of coronary artery disease (CAD). However, despite these improvements refractory angina remains an important healthy problem [Citation1]. Indeed, it has been estimated that there are >500,000 Canadians and up to 1.8 million Americans living with refractory angina [Citation1]. Moreover, clinical trials and registries clearly demonstrated that 20–30% of patients undergoing percutaneous or surgical revascularization might continue to experience persistence of angina despite a successful revascularization [Citation2]. Thus, each year, as many as 525,000 patients develop refractory angina in continental Europe and in the USA [Citation3], and an additional 500,000 who undergo either PCI or coronary bypass surgery continue to suffer from angina, which means that an additional 1 million patients could benefit annually from new targeted therapies [Citation4].
According to the 2013 ESC Guidelines on Stable coronary artery disease (CAD), refractory angina is defined as a ‘chronic condition caused by clinically established reversible myocardial ischemia in the presence of CAD, which cannot be adequately controlled by a combination of medical therapy, angioplasty and coronary artery bypass graft’ [Citation3]. Refractory angina represents a very disabling condition, which determines a very poor quality of life. It has also an important clinical impact on public health resources determining a large number of hospitalizations and exceeding instrumental examinations.
Current guidelines recommend the introduction of an optimized medical therapy with ß-blockers, calcium-channel blockers or nitrates as first-line approach, and ivabradine, ranolazine, nicorandil or trimetazidine as second-line [Citation3]. In addition, in case of failure of medical therapy alone, the use of non-pharmacological approaches, such as enhanced external balloon counterpulsation (EECP), spinal-cord stimulation (SCS) and transcutaneous electric nerve stimulation (TENS) [Citation3] may be considered. However none of these devices has become a standard of care, and despite their initial promise, many trials demonstrated only modest improvements in exercise capacity and in angina relief [Citation5]. Thus, new treatments for refractory angina are needed.
2. History and rationale of coronary sinus intervention. From Claude Beck to coronary sinus Reducer
The hypothesis that an increased coronary sinus pressure could have antianginal effects was proposed by Claude Schaeffer Beck, a pioneer American cardiac surgeon, that in 1950’s and 1960’s obtained a significant relief of angina symptoms in patients suffering from severe disabling angina, by performing a 60–70% narrowing of coronary sinus [Citation6]. Other surgeons confirmed his results in the next years stimulating the research on devices able to update Beck’s hypothesis on new technologies. The physiological mechanism behind the antianginal effect of coronary sinus intervention is that coronary sinus increasing pressure provides a direct retrograde access route to ischemic myocardium for blood flow. Indeed, in patients with epicardial coronary disease there is a dysfunction in normal physiological compensatory mechanism of blood redistribution from subepicardial layers to subendocardial ones during stress. In this condition the normal ratio between subendocardial and subepicardial blood flow is altered, in favor of second one, so that subendocardium perfusion is compromised during stress, causing angina symptoms [Citation7]. It has been supposed that an increased pression in the coronary venous system could determine a vasodilatation of the arterioles, reducing resistances in the subendocardium vessels and favoring a blood redistribution from subepicardial layers to subendocardium, with a reduction of subendocardium ischemia and, consequently, angina symptoms [Citation7,Citation8].
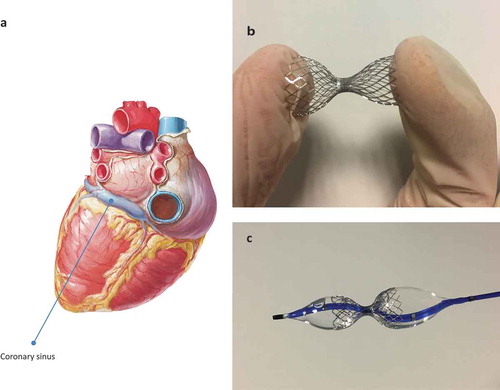
Keeping in mind this pathophysiological background, the Coronary Sinus Reducer () is a balloon-expandable stainless steel mesh, pre-mounted on an hourglass shaped balloon catheter, that represents a modern percutaneous approach to increase coronary sinus venous pressure creating a narrowing in the coronary sinus (CS) lumen [Citation9]. The narrowing within the CS, and consequently the pressure gradient in the narrowed central part of the device, are not achieved in the acute peri-procedural phase, but are established 4–6 weeks after implantation, when the metal mesh will be covered by tissue in-growth generated from oversizing of the wide ends of the device (causing injury-induced tissue proliferation) [Citation7]. Procedure is performed via right internal jugular vein and represents a quite safe procedure. Acute complication related to CS Reducer implantation may be CS perforation or dissection, device migration, CS thrombosis [Citation7].
3. Clinical evidence
Preclinical studies demonstrated safety and efficacy of CS Reducer implantation in mice [Citation9], opening the avenue for device implantation in humans. Banai et al. conducted a small prospective multicentre first-in-man study to evaluate the safety and feasibility of the Coronary Sinus Reducer in 15 patients with severe refractory angina and reversible ischemia [Citation10]. In this study they demonstrated, after 6 months and then after 3 years from CS Reducer implantation, that its use is safe and effective in reducing angina and ischemia. Konigstein et al confirmed these results in an open-label trial involving 23 patients with refractory angina treated with CS Reducer [Citation11]. In this study no adverse event were reported at discharge and at 6 months from implantation. Efficacy was demonstrated with a reduction in Canadian Cardiovascular Society (CCS) angina class, a prolongation in exercise duration and a reduction of ischemia (evaluated with thallium SPECT).
Actually, the most largest study evaluating CS reducer is COSIRA trial, a randomized double-blind sham-controlled trial enrolling 104 patients with severe refractory angina and evidence of myocardial ischemia [Citation12]. Primary end point was the improvement of CCS angina class at 6 month after implantation. In this trial, the device was successfully implanted in 50 of the 52 patients (96%). Quality of life improved in the treatment group, with 35% of patients having an improvement of at least two CCS classes when compared to 15% in the control group, and with an improvement of at least one CCS angina class in 71% of patients in the device-group, as compared with 42% of those in the control group. About safety, only one case of periprocedural myocardial infarction was registered in the treatment group. Moreover, this trial showed a trend for ischemia reduction in the device-group, although this end point did not reach statistical significance. Abawy et Al. [Citation13] confirmed the COSIRA trial results in a real-world study, showing safety and effective of CS Reducer in CCS angina class improvement (achieved in 70% of patients). Recently, Giannini et al, in a single-center experience, enrolled 50 patients with refractory angina and documented myocardial ischemia undergoing to CS Reducer implantation. Of interest, they demonstrated an improvement of at least one CCS angina class in 80% of patients, an improvement in quality of life, 6-min walking test distance, without any device-related adverse events during the procedure or at follow-up [Citation14].
4. Future perspectives
Despite these interesting results, data reveal that 15–30% of patients do not gain clinical benefit from CS Reducer implantation. The reason is currently unknown. However, the presence of alternative venous drainage systems to the CS could explain this phenomenon. The measurement of difference between coronary sinus wedge pressure and right atrial pressure has been proposed as a predictor of alternative venous drainage systems, suggesting a reduced benefit deriving from CS reducer implantation [Citation15].
CS Reducer received the CE mark and is currently implanted in European countries, but it is not still approved for marketing in the United States by the FDA. In the future more information will be obtained about safety and effectiveness of CS Reducer implantation by the COSIRA-II pivotal trial. This study is a multicentre, double-blinded sham-controlled randomized trial, enrolling 380 patients in North America in order to get the FDA approval for marketing of the device in the United States [Citation16]. In Europe, a large observational post-market study, the REDUCER-1 (NCT02710435), aiming at enrolling up to 400 patients with refractory angina, is providing encouraging preliminary results (with 115 patients enrolled), indicating that 81% of patients experienced an improvement of at least one CCS angina class after 6 months [Citation16]. Complete results will be important in order to confirm these data.
In conclusion, in patients with chronic angina, refractory to medical therapies, CS Reducer demonstrated to be effective in about 70–80% of patients in reducing symptoms of angina, myocardial ischemia and improving quality of life and it is candidate to become the standard of care for these patients. Identifying the target population that can get benefit from CS implantation will be the next purpose. Further studies are needed to solve these open issues.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- McGillion M, Arthur HM, Cook A, et al. Management of patients with refractory angina: canadian cardiovascular society/canadian pain society joint guidelines. Can J Cardiol. 2012;28(2 Suppl.):S20–S41.
- Niccoli G, Montone RA, Lanza GA, et al. Angina after percutaneous coronary intervention: the need for precision medicine. Int J Cardiol. 2017;248:14–19.
- Montalescot G, Sechtem U, Achenbach S, et al. ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J. 2013;34:2949–3003.
- Mannheimer C, Camici P, Chester MR, et al. The problem of chronic refractory angina; report from the ESC joint study group on the treatment of refractory angina. Eur Heart J. 2002;23:355–370.
- Giannini F, Aurelio A, Jabbour RJ, et al. The coronary sinus reducer: clinical evidence and technical aspects. Expert Rev Cardiovasc Ther. 2017;15:47–58.
- Beck CS, Leighninger DS. Scientific basis for the surgical treatment of coronary artery disease. J Am Med Assoc. 1955;159:1264–1271.
- Konigstein M, Giannini F, Banai S. The Reducer device in patients with angina pectoris: mechanisms, indications, and perspectives. Eur Heart J. 2018;39:925–933.
- Ido A, Hasebe N, Matsuhashi H, et al. Coronary sinus occlusion enhances coronary collateral flow and reduces subendocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H1361–H1367.
- Banai S, Ben-Muvhar S, Tsehori J, et al. Transcatheter coronary sinus narrowing with the Neovasc Reducer - an alternative treatment approach for patients with disabling angina who are not candidates for revascularization procedures - a preclinical study. Abstract presentation at the 2004 annual conference of the Israel Heart Society.
- Banai S, Ben Muvhar S, Parikh KH, et al. Coronary sinus reducer stent for the treatment of chronic refractory angina pectoris: a prospective, open-label, multicenter, safety feasibility first-in-man study. J Am Coll Cardiol. 2007;49:1783–1789.
- Konigstein M, Meyten N, Verheye S, et al. Transcatheter treatment for refractory angina with the coronary sinus Reducer. EuroIntervention. 2014;9:1158–1164.
- Verheye S, Jolicoeu R, Behan MW, et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med. 2015;372:519–527.
- Abawi M, Nijhoff F, Doevendans PA, et al. Safety and efficacy of a device to narrow the coronary sinus for the treatment of refractory angina: a single-centre real-world experience. Neth Heart J. 2016;24(9):544–551.
- Giannini F, Baldetti L, Ponticelli F, et al. Coronary sinus Reducer implantation for the treatment of chronic refractory angina: a single-center experience. JACC Cardiovasc Interv. 2018 Apr 23;11(8):784–792.
- Baldetti L, Colombo A, Banai S, et al. Coronary sinus reducer non-responders: insights and perspectives. EuroIntervention. 2018;13:1667–1669.
- Verheye S. REDUCER-1 post-market study and US IDE. Washington (DC): TCT Congress; 2017 Oct 30.