ABSTRACT
Introduction: Although there is much attention for proper sizing of pre-operative anatomy before (thoracic) endovascular aneurysm repair ([T]EVAR), standardized assessment of endograft position and apposition at postoperative imaging is seldom addressed in the international guidelines. The highly detailed three-dimensional computed tomography angiography (CTA) volumes contain valuable information about the apposition of the endograft with the arterial wall and the position of the device relative to anatomical landmarks in the proximal and distal landing zones, which is currently hardly used. With proper assessment on CTA of the endograft after EVAR, the risk for future endograft-related complications may be determined, allowing patient-tailored, risk-stratified surveillance.
Areas covered: This systematic review identified three standardized methods for assessing apposition or position of the endograft in the proximal or distal landing zone on CTA after (T)EVAR. Quantification of apposition and position, validation of measurement precision, and association with endograft-related complications were extracted. Short (<10 mm apposition length) and decreasing (>0 mm) apposition were associated with endograft-associated complications.
Expert commentary: Standardized assessment of apposition and position of the endograft in the proximal and distal landing zones on CTA should be incorporated in post-(T)EVAR surveillance. A risk-stratified CTA surveillance protocol is proposed.
1. Introduction
Endovascular aneurysm repair (EVAR) has been widely adopted as a treatment for patients with abdominal or thoracic aortic aneurysms (AAA/TAA). Concern for secondary aneurysm sac rupture as a result of endoleak makes life-long surveillance mandatory[Citation1]. Contrary to assessment of the pre-operative anatomy on computed tomography (CT) angiography (CTA) volumes, which is incorporated in international guidelines and manufacturers’ indications for use, standardized assessment of the endograft on postoperative CTA scans is underreported.
International guidelines advise a CTA scan within 30 days after EVAR, on which the presence of endoleak and >10 mm endograft apposition length to the proximal and distal arterial wall should be assessed [Citation2,Citation3]. When risk of failure is present due to challenging anatomy or doubt exists about the adequacy of sealing, a second postoperative CTA is advised. Recent European guidelines also advise a very late CTA scan at 5 years to evaluate sealing in the long term, which was not included in the previous guidelines. The optimal time period and frequency for CTA surveillance is, however, still debated. When no endoleak or aneurysm growth is detected, further surveillance by annual Doppler ultrasound is advised.
However, the three-dimensional (3D) CTA volumes contain much more valuable information in addition to sensitive detection of aneurysm growth and endoleaks, which are currently seldom used and not detectible on duplex ultrasound or x-ray imaging. Accurate measurement of the endograft position relative to anatomical landmarks and proximal and distal endograft apposition with the arterial wall on the first postoperative CTA scan can stratify the patient’s risk for future complications [Citation4–Citation7]. Comparing these endograft properties during follow-up reveals subtle changes that may precede later complications[Citation8].
Dedicated workstations are available, and standardized methods for assessing the 3D aortic morphology have been described and validated[Citation9]. However, an overview of standardized measurement methods for postoperative assessment of the endograft position and apposition is lacking and is not included in the guidelines.
This review provides an overview of standardized methods to assess the position of the endograft relative to an anatomical landmark and the apposition of the endograft onto the arterial wall at the proximal and distal landing zones on the CTA scan after EVAR and thoracic EVAR (TEVAR). Validation of measurement precision and association of measured variables with endograft-associated complications are reviewed.
2. Methods
The literature review conformed to the Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) statement standards [Citation10,Citation11]. The review protocol was prospectively registered in the PROSPERO database (No. 133,794).
A search in MEDLINE of the literature published between April 2009 and April 2019 was performed between April 1 and 10, 2019, using the following keywords: endovascular repair, aortic aneurysm, apposition, position, postoperative standardized method, computed tomography. Two authors (RS, PdR) independently screened the titles and abstracts for eligibility. Discrepancies between the authors during the search, selection, quality assessment, and data extraction were resolved by discussion. In case of disagreement, a third author (JPdV) was consulted. A filter for language was not applied to the search, so studies with an English translation could be included. The search terms for MEDLINE are provided in . Search terms within each category were combined by Boolean OR, and search categories were combined by Boolean AND.
Table 1. Search terms in MEDLINE database.
2.1. Selection criteria
Studies were included if they reported the quantitative assessment on postoperative CTA of endograft apposition with the arterial wall or endograft position relative to anatomical landmark(s) in the proximal and/or distal landing zone in patients with a TAAA or AAA treated by EVAR. Studies reporting patients treated for an aneurysm in an artery other than the aorta or patients treated for aortic dissection were excluded. Studies reporting on complex repair, such as fenestrated or branched EVAR, chimney EVAR, or endovascular sealing were excluded. Case reports, reviews, commentaries, conference abstracts, letters to the editor, studies without human subjects, studies reporting on fewer than 10 patients, studies in other than the English language, and studies without full-text availability were excluded. End points were the reported endograft apposition and/or position, validation of measurement precision, and association with endograft-associated complications.
2.2. Data collection and quality assessment
Two authors (RS, PdR) independently performed data extraction. Data extraction included study period, study design, sample size, inclusion criteria, exclusion criteria, follow-up duration of CTA, follow-up duration of clinical outcome, assessed landing zone(s), determination of endograft apposition with the arterial wall, determination of endograft position relative to an anatomical landmark, the measurement method(s), validation of measurement precision, and association with endograft-associated complications. Quantitative analysis was performed if no significant heterogeneity was present. Continuous variables are presented as the median and interquartile range [quartile 1, quartile 3] or as the mean ± standard deviation.
Uniform terminology has been used to increase the readability of this review. The apposition of the endograft fabric with the arterial wall, which is also called seal, sealing, attachment, or contact, is referred to as ‘apposition.’ The position of the endograft edge relative to an anatomical landmark, which is also called deployment accuracy or distance to (target) vessel or artery, is referred to as ‘position.’
Risk of bias for the association between endograft apposition and/or position and endograft-associated complications was assessed for those studies designed for comparison (i.e., case-control or cohort studies). The quality of the studies was assessed with the Newcastle-Ottawa Scale for assessing the quality of non-randomized studies, which is recommended by the Cochrane Collaboration. The Newcastle-Ottawa Scale includes bias analysis of selection of the study groups (four items), the comparability of the groups (two items), and the ascertainment of the exposure or outcome of interest for case-control or cohort studies (three items)[Citation12].
Quality of the described measurement methods was assessed by reproducibility and validation of measurement precision. Reproducibility of the method was deemed valid when the study reported the software that was used for the measurement and potential post-image processing, the steps that were taken to perform the measurement, and the choices that were made for steps that are open to interpretation. Measurement precision was deemed valid when a quantitative measure was given of the intra- and/or interobserver variability, such as the mean difference (MD) and the repeatability coefficient (RC). The measurement methods used in the studies were marked as high quality – reproducible and validated; medium quality – reproducible, but not validated; or poor quality – not reproducible.
3. Results
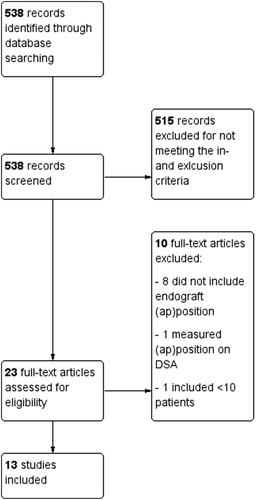
The search resulted in 538 items, which were screened for eligibility. After a detailed screening of titles and abstracts, 515 were removed for not meeting the inclusion and exclusion criteria. After the 23 full-text papers were read for eligibility, 10 additional studies were removed because eight studies did not include measurement of endograft position relative to an anatomical landmark or endograft apposition with the arterial vessel wall, one study measured these parameters from 2D digital subtraction angiography images, and one study reported measurements on fewer than 10 patients. The remaining 13 studies were included in this review [Citation4–Citation8,Citation13–Citation20]. A flowchart of the selection procedure is shown in . The characteristics of included studies are summarized in . Quantitative analysis was not performed because of the heterogeneity of study design, patient inclusion, end points, follow-up, and measurement methods.
Table 2. Overview of measurement methods in studies that met the inclusion criteria in chronological order.
Table 2. (Continued.)
Five studies assessed the endograft in the proximal landing zone in the aortic neck after EVAR [Citation8,Citation14,Citation17,Citation19,Citation20], three studies assessed the distal landing zone in the common iliac arteries (CIAs) after EVAR [Citation6,Citation7,Citation16], and three studies assessed proximal and distal landing zones [Citation4,Citation5,Citation15]. One study assessed the proximal landing zone after TEVAR[Citation13], and one assessed the distal landing zone after TEVAR[Citation18].
3.1. Quality assessment
Two cohort studies were designed to compare patients with or without exposure to insufficient proximal or distal apposition (<10 mm) after EVAR [Citation4,Citation5], and one cohort study was designed to compare patients with or without exposure to inaccurate landing in the distal landing zone (>5 mm) after TEVAR (Supplementary Table 1)[Citation18]. Risk of bias for selection was low (mean score, 3.7 of 4) but was high for comparability (mean score, 0.7 of 2) and for outcome (mean score, 1.3 of 3). Seven studies were designed to compare patients with endograft-associated complications, AAA-associated complications, or re-intervention to controls with endograft apposition or position as an end point (Supplementary Table 2) [Citation4,Citation6–Citation8,Citation13,Citation15,Citation19]. Risk of bias for selection was low (mean score, 3.6 of 4) but high for comparability (0.3 of 2) and moderate for exposure (1.6 of 3).
3.2. Measurement methods
The studies described three methods to quantify endograft apposition and position:
Method A determines the length over the centerline between two orthogonal boundary planes that include a proximal and a distal point of reference ()). The method has been used for the determination of apposition and position of the endograft within the artery and requires a dedicated vascular workstation that facilitates a centerline reconstruction with orthogonal planes. Precision of the measurements has only been validated for the distance between the distal edge of the endograft limb and the iliac artery bifurcation[Citation7]. The method has been used to assess proximal endograft apposition in the aortic neck [Citation4,Citation5,Citation14,Citation15,Citation19], proximal endograft position in the aortic neck relative to the lowest renal artery [Citation4,Citation5] or relative to the superior mesenteric artery (SMA)[Citation15], distal endograft apposition in the CIAs [Citation4–Citation7,Citation15,Citation16], distal endograft limb position in the CIAs relative to the iliac bifurcation [Citation6,Citation7,Citation15,Citation16], proximal position of the thoracic endograft relative to the left subclavian artery (LSA)[Citation13], and distal position of the thoracic endograft relative to the celiac trunk[Citation18].
Method B determines the distance over the arterial wall between two 3D coordinates that are located on the arterial wall ()). The method has been used for the determination of apposition and position of the endograft within the artery. It requires 3D coordinates, a segmentation of the aortic lumen, and dedicated post-processing software for automated geometric calculations. Measurement precision has been validated for proximal apposition in the aortic neck and for the distance between the proximal edge of the endograft fabric and the renal arteries [Citation17,Citation20]. The method has been used to determine endograft apposition in the aortic neck [Citation8,Citation17] and endograft position in the aortic neck relative to the renal arteries [Citation8,Citation17,Citation20].
Method C determines the surface area over the arterial wall between two boundary planes to determine endograft apposition with the arterial wall ()). When the total area of the potential landing zone is also determined, the percentage of landing zone coverage can also be assessed. The method requires 3D coordinates or vectors to define the planes, a segmentation of the aortic lumen, and dedicated post-processing software for automated geometric calculations. Measurement precision has been validated for the apposition surface area in the aortic neck[Citation17]. The apposition surface area has been determined in the aortic neck in three studies [Citation8,Citation14,Citation17].
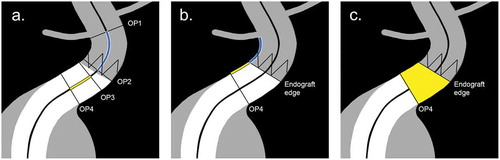
Figure 2. Schematic representation of published methods for determination of endograft apposition with the arterial wall, and position relative to the target vessels. The figure displays the proximal landing zone for EVAR, but the methods also apply to both proximal and distal landing zones for EVAR and TEVAR. Method A defines the length over the centerline between two orthogonal planes. Endograft position is measured from the orthogonal plane through the orifice of the target vessel (OP1) and the orthogonal plane through the top of the endograft fabric (OP2). Endograft apposition is measured from the orthogonal plane with full proximal (OP3) and distal (OP4) circumferential apposition. Method B defines the length between 3D coordinates over the arterial wall with dedicated post-processing software. Endograft position is calculated as the shortest distance between the target vessel and the endograft fabric edge circumference. Endograft apposition is calculated as the shortest distance between the endograft fabric edge circumference and the distal apposition circumference in the orthogonal plane (OP4). Method C defines the apposition surface area between the endograft fabric edge circumference and the distal apposition circumference in the orthogonal plane (OP4), which is calculated automatically by dedicated post-processing software.
3.3. Validation of measurement precision
Precision of the measurements was evaluated in four studies [Citation4,Citation7,Citation17,Citation20]. The first study validated interobserver variability for determination of apposition and position lengths with use of method B and apposition surface area by method C in the proximal aortic neck in a series of 24 elective EVAR patients on the first postoperative CTA scan[Citation17]. Mean differences (MD) of length calculations with method B were 0.2 to 0.7 mm, with RCs of 4.1 to 5.7 mm. These levels corresponded with the precision of determining endograft position in the aortic neck in a second study with 24 patients (MD, 0.2–0.3 mm; RC, 3.2–3.4 mm)[Citation20]. Precision of measuring position or apposition length in the aortic neck by method A has not been validated, but neck length was measured with a similar method and validated in a study with 30 patients that reported similar precision (MD, 1.5 mm; RC, 5.5 mm)[Citation4]. Precision of measuring position of the distal endograft edge relative to the CIA bifurcation by method A was validated in a study with 30 patients that reported slightly higher precision (MD, 0 mm; RC, 2 mm)[Citation7]. Precision of apposition surface area calculations in the aortic neck by method C was also assessed (MD, 9 mm2; RC, 307 mm2)[Citation17]. In addition, accuracy of the surface area calculations was tested in vitro, which showed an error of 2.8%. Measurement precision of methods A, B, and C has not been validated for the proximal and distal landing zones of the thoracic aorta, and methods B and C have not been validated for the distal landing zone in the CIAs[Citation17].
3.4. Arterial wall apposition and position relative to anatomic landmarks
Apposition of the endograft with the arterial wall and/or position relative to an anatomical landmark was assessed in 10 studies. Three studies reported a quantitative value of apposition length in specific patient populations (either with or without specific complications or in a consecutive cohort) [Citation8,Citation14,Citation19]. A fourth study did not measure apposition but did measure fixation length, which included the bare stent[Citation15]. Four studies assessed the distal apposition in the CIAs in specific patient groups (either with or without specific complications or a consecutive cohort) [Citation6,Citation7,Citation15,Citation16]. The findings of these studies are reported in .
Table 3. Apposition length findings.
Two studies reported the distance between the proximal edge of the endograft fabric and the lowest renal artery or both renal arteries in a specific patient population [Citation8,Citation20]. Four studies assessed the distance between the covered distal edge of the endograft limbs and the iliac bifurcation [Citation6,Citation7,Citation15,Citation16]. One study reported the position in the proximal landing zone relative to the LSA post-TEVAR[Citation13], and one study reported the position in the distal landing zone relative to the celiac trunk post-TEVAR[Citation18]. The findings of these studies are reported in . In addition, two studies categorized good (>10 mm) or bad apposition in the proximal and distal landing zones in specific patient groups [Citation4,Citation5]. One study categorized good or bad position within 5 mm from the target renal artery in the proximal landing zone after EVAR[Citation4]. No studies reported proximal or distal apposition after TEVAR.
Table 4. Position length findings.
3.4.1. Reported values of apposition and position
Schuurmann et al. determined changes in apposition and position on CTA after EVAR to detect early caudal displacement of the device in order to predict type IA endoleak[Citation8]. Apposition length, apposition surface area, and distance between the endograft fabric and both renal arteries were measured with methods B and C on the first postoperative CTA scan and a late (>1 year) CTA scan of patients with and without endograft-related complications (type IA endoleak and migration >10 mm). The late scan was the latest scan without reported complications for the type IA endoleak and migration groups and the latest available scan for the control patients (>1 year). Apposition had decreased significantly during follow-up in the complication groups but had increased significantly in the control patients as a result of aneurysm shrinkage. The shortest distance between the graft fabric and the renal artery had increased significantly in the complication groups and increased to lesser extent, but also significantly in the control group.
Wang et al. quantified the influence of proximal neck anatomy on contemporary outcomes in a cohort of AAA patients with highly angulated aneurysm necks treated with the Aorfix endograft (Lombard Medical, Oxfordshire, UK)[Citation19]. Apposition length was measured with method A on the first postoperative CTA scan, with and without endograft-associated complications (migration >10 mm, sac expansion >5 mm, and type IA endoleak) and appeared not to be significantly different. Definition of the distal end of the apposition was unclear, so the measurements were not reproducible.
Welborn et al. evaluated the clinical outcome and imaging findings of the AFX endograft (Endologix, Irvine, CA)[Citation14]. They used methods A and C to measure the apposition length and surface of patients treated with the AFX; however, definition of the surface area calculation was not clear, so these measurements were not reproducible. Apposition length exceeded the neck length by 5.1 ± 13 mm. The average surface area was 19 ± 13 cm2, and greater apposition surface area was associated with sac regression.
Waasdorp et al. investigated the importance of iliac fixation in the proximal and distal landing zones of the Talent endograft (Medtronic, Santa Rosa, CA) on the first postoperative CTA scan[Citation15]. The proximal fixation length included the 15-mm bare stent, and distal fixation length was the same as apposition length. The proximal and distal fixation lengths were both significantly shorter in patients with migration (≥10 mm) than in the controls. The distance between the distal edge of the endograft and the iliac bifurcation was not significantly different.
Schuurmann et al. reported the 3D deployment accuracy of the endograft relative to both renal arteries on the first postoperative CTA scan[Citation20]. Partial coverage of the renal artery orifice occurred in 30%, and low deployment (>3 mm distally from the lowest renal artery) occurred in 26%. Measurements of these distances by method A and method B were compared in a subset of 24 patients. Distance measurement between the contralateral (highest) renal artery and the endograft over the centerline by method A underestimated the distance over the (outer) curve versus method B by 20%.
Bastos Gonçalves et al. evaluated the dynamics of the iliac attachment zone after EVAR and the association with clinical events[Citation7]. Apposition length in CIAs with seal complications was significantly shorter in patients with seal complications (type IB endoleak or need of iliac limb extension), and the distance between the distal edge of the endograft and the iliac bifurcation was significantly longer. Longer apposition length was associated with fewer iliac seal complications (odds ratio, 0.94 per mm increase) in multivariate analysis.
Roos et al. determined underlying causes of type IB and type III endoleaks and identified anatomical factors associated with iliac re-intervention after EVAR[Citation6]. Apposition length in the CIAs that underwent re-intervention of the iliac endograft was significantly shorter than in the contralateral, non-treated limbs. The distances between the distal edges of the endograft and the iliac bifurcations were not significantly different.
Taneva et al. evaluated the chronological distal sealing zone changes after EVAR in 52 CIAs[Citation16]. During a follow-up of 3.1 ± 1.4 years, the apposition length decreased from 32.1 ± 14.7 mm to 30.8 ± 15.1 mm. The distance between the distal edge of the endograft and the iliac bifurcation increased from 19.5 ± 11.9 mm to 21.2 ± 12.8 mm. The significance of these changes was not assessed.
Kotelis et al. identified morphologic factors affecting type IA and IB endoleak formation and bird-beak configuration after TEVAR[Citation13]. The distance between the top of the fabric and the LSA on the first postoperative CTA was significantly shorter in patients with bird-beak configuration. Comparison of endograft position in patients with and without endoleak was incomplete.
Berezowski et al. investigated the accuracy of endograft deployment in the distal landing zone during TEVAR in 59 patients with a landing zone <40 mm where the aim was to deploy the endograft just above the target vessel, which was the celiac trunk (74%), SMA (24%), or renal artery (2%)[Citation18]. The median distance was 10.0 [6.5, 16.0] mm, and the target vessel was covered in three patients (one complete coverage, two partial). Accurate landing (<5 mm from target vessel) was achieved in only 10 patients. Primary type IB endoleak occurred less frequently in patients with accurate landing (0% vs. 33%). The difference between accurate and inaccurate landing was not significant for late-type IB endoleaks occurrence (10% vs. 14%).
3.4.2. Categorized short apposition (<10 mm) and suboptimal deployment (>5 mm from renal artery) into cohorts
Bastos Gonçalves et al. evaluated the predictive value of the first postoperative CTA characteristics for aneurysm-associated adverse events as a means of patient selection for risk-adapted surveillance[Citation4]. Apposition length in the proximal and distal landing zones and position relative to the target renal artery in the proximal landing zone were measured with method A on the first postoperative CTA scan in a series of 131 patients treated with the Excluder endograft (W. L. Gore, Flagstaff, AZ). They identified 18 patients (14%) with short proximal apposition length, 22 patients (17%) with short distal apposition, and 38 patients (29%) with suboptimal position (>5 mm). Short seal length and presence of endoleak on the first postoperative CTA scan were associated with AAA-related complications (endoleak type IA, IB, III, or undefined; AAA growth >5 mm, migration >10 mm, device failure, and AAA-associated death, rupture, or re-intervention) during 4.1 [2.1, 6.1] years of follow-up. Suboptimal position was not associated with AAA-associated complications. Patients were categorized as high risk (n = 69) and low risk (n = 62). High-risk patients were considered to have short proximal and/or distal apposition length and/or any endoleak on the first postoperative CTA scan. Neck length was similar between the high-risk (32.9 ± 14.2 mm) and low-risk (32.4 ± 15.3 mm) groups. High-risk patients had increased risk for AAA-related complications. Short proximal and/or distal apposition length was associated with a hazard ratio of 3.89.
Baderkhan et al. also examined whether it may be possible to identify patients at low risk of complications based on their first postoperative CTA[Citation5]. Apposition length in the proximal and distal landing zones was measured with method A on the first postoperative CTA scan in a series of 326 patients. Patients were categorized as high risk (n = 114) or low-risk (n = 212) and were considered high risk when they had short proximal and/or distal seal zone and/or any endoleak on the first postoperative CT scan. Aortic neck length was similar between the high-risk (23.8 ± 13.8 mm) and low-risk (22.0 ± 13.9 mm) patients. High-risk patients had increased risk for AAA-related complications (migration >10 mm, AAA growth >5 mm, rupture, or endoleak type IA, IB, III, or undefined) and AAA-associated re-intervention during 4.8 ± 3.2 years of follow-up. Short proximal apposition length was associated with an odds ratio of 26.6, and short distal apposition length was associated with an odds ratio of 37.6 for AAA-related events.
4. Discussion
This systematic review provides an overview of the studies that described a standardized method for assessing endograft apposition with the arterial wall or position of the endograft relative to the target vessel(s) in the proximal and distal landing zones after (T)EVAR and the association with AAA-related complications.
Standardized methods that have been described in the literature can be grouped into three categories. Method A requires a dedicated vascular workstation with a centerline reconstruction. The advantage of this method is that physicians who are familiar with pre-operative sizing on a workstation can assess the postoperative CTA scans in a similar and reproducible way. The major disadvantage of this method is that the 3D orientation of the endograft within complex aortic morphology is simplified to a cylinder-shaped reconstruction, which may under- or overestimate the true apposition and position lengths of the endograft within the artery. In highly curved anatomy, the lengths will be underestimated in the outer curve and overestimated in the inner curve (). An average underestimation of 20% can be expected in the outer curve[Citation20]. Tilted deployment of the endograft edge toward the axis of the aorta is also not accounted for with method A.
Methods B and C determine the lengths and surface area over the aortic wall and, therefore, appreciate the 3D shape of the artery and the tilted deployment of the fabric edge. The downside is that specialized geometric post-processing software is required, such as Vascular Image Analysis prototype software (VIA; Endovascular Diagnostics BV, Utrecht, The Netherlands). Methods B and C may also be more intuitive, since the computer-assisted calculations in 3D are clearly visualized. The average total measurement time was estimated at <5 min for method A and was 8.0 ± 1.5 min for method B [Citation4,Citation20]. Comparison between methods A, B, and C was not possible because of heterogenic study design, patient inclusion, end points, and follow-up.
Measurements in the infrarenal aortic neck have been validated for methods A, B, and C and in the CIA for method A, with similar outcomes. The mean difference between observers is less than 1 mm, and 95% of paired observations are within 6 mm. The measurements have not yet been validated for the thoracic aorta.
A short proximal apposition length (<10 mm) has been associated with AAA-associated complications, including type IA, IB, III, or undefined endoleak, aneurysm growth, migration, device failure, and AAA-related death, rupture, or re-intervention [Citation4,Citation5]. However, the study by Schuurmann et al. found no significant differences in proximal apposition on the first postoperative CTA scan of patients who developed late (>1 year) type IA endoleak or migration versus patients without these complications[Citation8]. These different findings are probably caused by differences in patient selection and end points. On a follow-up CTA scan, the apposition and position of the endograft differed significantly between patients who later developed type IA endoleak or migration compared with patients without endograft-related complications[Citation8]. This may emphasize the need for more than one CT scan during EVAR follow-up in the first 5 years after the primary procedure.
A short apposition length (<10 mm) in the distal landing zone on the first postoperative CTA scan has been associated with AAA-related complications after EVAR (defined as type IA, IB, III, or undefined endoleak, aneurysm growth, migration, device failure, AAA-related death, rupture, or re-intervention) [Citation4,Citation5], and distal apposition was significantly shorter in patients with complications (defined as limb retraction, CIA dilatation, type IB endoleak, iliac extension, re-intervention, or migration) [Citation6,Citation7,Citation15]. The distance between the edge of the endograft and the iliac bifurcation on the first postoperative CTA scan was longer in patients with distal complications (defined as limb retraction, CIA dilatation, type IB endoleak, or iliac extension) [Citation7] but was not associated with endograft-related complications (defined as type IA, IB, III, or undefined endoleak, aneurysm growth, migration, device failure, AAA-related death, rupture, or re-intervention) in other studies [Citation4,Citation6,Citation15]. These different findings could be caused by shorter clinical follow-up of the studies that found no association, and by different end points.
Only one study reported endograft-related complications after TEVAR. Primary type IB endoleak was associated with inaccurate landing (>5 mm from target vessel)[Citation18]. Late-type IB endoleak was not associated with inaccurate landing, possibly due to the low number of complications (n = 8).
4.1. Limitations
All of the studies were retrospective observational studies, which is a limitation and should be taken into account when considering the recommendations in this review. Some studies were case series and not designed for group comparison. Several studies were limited by small numbers, especially of patients who developed complications, or by limited follow-up. Studies assessing apposition after TEVAR were lacking. Only two studies assessed position after TEVAR, of which the measurements were not validated. More research is needed to define adequate apposition and position in the proximal and distal landing zones of the thoracic aorta and the association with TEVAR-related complications. The studies were too heterogeneous in design, patient inclusion, follow-up, and end points, so pooling of data was not possible.
5. Conclusion
This systematic review has described three standardized methods to assess apposition of the endograft with the arterial wall and position relative to the target vessels on post-(T)EVAR CTA, each with pros and cons, but similar precision. Proper EVAR surveillance should include an assessment of the endograft apposition on postoperative CTA scans to stratify risk for later endograft-related complications. We should use the information of the CT scans to their full extent, so we can justify the exposure to contrast and radiation. Proximal and distal apposition length <10 mm is insufficient, and a decrease of apposition during follow-up predicts later seal failure. If these signs are determined on a post-EVAR CT scan, it is advised to consider treatment or perform regular CTA follow-up instead of duplex ultrasonography, especially in high-risk patients. The literature on TEVAR is insufficient for proper advice on postoperative surveillance.
6. Expert opinion
Standardized post-EVAR surveillance of apposition and position of the endograft in the proximal and distal landing zones is feasible and can be derived from arterial phase CTA scans. With use of these calculations, some EVAR-associated complications may be detected in an early stage or may even be predicted before they occur. This detailed information is not available from duplex ultrasound or x-ray imaging, which mainly focus on detection of complications such as significant device migration, aneurysm growth or endoleaks.
Bastos Gonçalves et al. proposed a risk-stratified surveillance protocol after EVAR in 2013 based on sufficient apposition (>10 mm length) and no endoleak on the first postoperative CTA scan[Citation4]. The use of proper measurement of apposition on the first postoperative CTA scan was backed up by Baderkhan et al. in 2018[Citation5]. High-risk patients with insufficient proximal and/or distal apposition and/or endoleak are at increased risk for later AAA-associated complications, and annual CTA seems mandatory. Low-risk patients were advised only to have CTA at 5 years and to have duplex ultrasound surveillance unless clinical suspicion occurred. This should reduce high surveillance costs and radiation exposure associated with EVAR. Schuurmann et al., however, showed in 2018 that late-type IA endoleak and migration (>1 year) were not associated with shorter apposition on the first postoperative CTA scan but that significant changes did appear on a later CTA scan that preceded eventual failure of effective seal[Citation8].
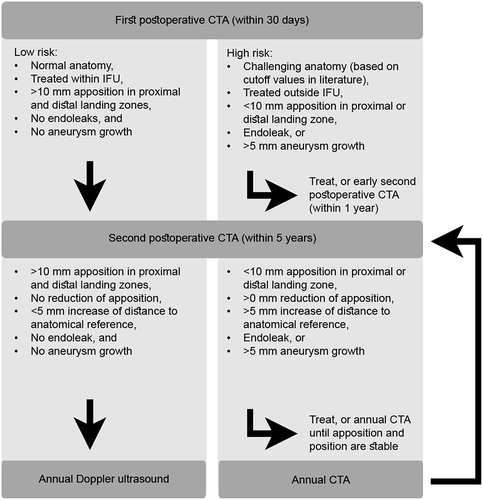
We therefore propose to modify the previous risk-stratified surveillance protocol, because patients with increased risk may benefit from a timely second postoperative CTA scan. These high-risk patients include those with challenging pre-operative anatomy, such as short neck length (<10 mm), large neck diameter (>30 mm), large aortic curvature (>50 m−1), large aneurysm sac diameter (>65 mm), or large CIA diameter (>19 mm) [Citation21–Citation26], those treated outside indications for use[Citation27], those with any endoleak or insufficient proximal or distal seal (<10 mm) on the first postoperative CTA [Citation4,Citation5], and those where suspicion of complications arises during follow-up, such as >5 mm aneurysm growth. In these patients, a second postoperative CTA is advised within 2 years, on which endograft apposition and position should be re-assessed and compared with the baseline values on the first postoperative CTA scan to allow detection of continuous (subtle) deterioration of apposition over time. In any event, apposition and position should be re-assessed on any consecutive CTA scan and compared with previous scans to detect eventual changes. Comparison with only the previous scan may not be sufficient when subtle changes appear within short periods of follow-up. The proposed adjusted risk-stratified surveillance protocol is shown in . Patients with persistent decrease of apposition may benefit from early re-intervention before an actual type I endoleak will appear. However, the pros and cons of such reinterventions must be debated and should be subject of future prospective studies before robust conclusions can be drawn.
Article highlights
Current international guidelines lack advice for proper assessment of the apposition of endografts on computed tomography angiography (CTA) surveillance after endovascular aneurysm repair.
Endograft apposition with the arterial wall and position relative to anatomic landmarks can adequately be assessed with three standardized methods:
Method A. Distance along the centerline between two orthogonal planes;
Method B. Distance over the arterial wall between two three-dimensional coordinates;
Method C. Surface area between two boundary planes.
Patients with a short apposition length (<10 mm) on the first postoperative CTA scan are at increased risk for endograft-associated complications. Change of apposition and position on further CTA follow-up can also identify patients at risk for later endograft-associated complications.
Risk-stratified post-EVAR surveillance includes postoperative CTA scans for patients at risk for endograft failure and should not be duplex ultrasound only.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclosure statement
JPPM de Vries and RCL Schuurmann are co-founders of the company “Endovascular Diagnostics B.V. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Patel R, Sweeting MJ, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388(10058):2366–2374.
- Wanhainen A, Verzini F, Van Herzeele I, et al. European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2019;( 2018. ; . 57:8–93.
- Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2–77.e2.
- Bastos Gonçalves F, van de Luijtgaarden KMV, Hoeks SE, et al. Adequate seal and no endoleak on the first postoperative computed tomography angiography as criteria for no additional imaging up to 5 years after endovascular aneurysm repair. J Vasc Surg. 2013;57(6):1503–1511.
- Baderkhan H, Haller O, Wanhainen A, et al. Follow-up after endovascular aortic aneurysm repair can be stratefied based on first postoperative imaging. Br J Surg. 2018;105:709–718.
- Roos H, Sandstrom C, Koutouzi G, et al. Predisposing Factors for Re-interventions with Additional Iliac Stent Grafts After Endovascular Aortic Repair. Eur J Vasc Endovasc Surg. 2017;53(1):89–94.
- Bastos Gonçalves F, Oliveira NF, Josee van Rijn M, et al. Iliac Seal Zone Dynamics and Clinical Consequences After Endovascular Aneurysm Repair. Eur J Vasc Endovasc Surg. 2017;53(2):185–192.
- Schuurmann RCL, van Noort K, Overeem SP, et al. Determination of Endograft Apposition, Position, and Expansion in the Aortic Neck Predicts Type Ia Endoleak and Migration After Endovascular Aneurysm Repair. J Endovasc Ther. 2018. DOI:10.1177/1526602818764616.
- Ghatwary TMH, Patterson BO, Karthikesalingam A, et al. A systematic review of protocols for the three-dimensional morphologic assessment of abdominal aortic aneurysms using computed tomographic angiography. Cardiovasc Intervent Radiol. 2013;36(1):14–24.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9.
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions : checklist and Explanations. Ann Intern Med. 2015 Jun 2;162(11):777-784. doi: 10.7326/M14-2385.
- Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Published 2013. [cited 2019 Apr 29]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Kotelis D, Brenke C, Wörz S, et al. Aortic morphometry at endograft position as assessed by 3D image analysis affects risk of type I endoleak formation after TEVAR. Langenbecks Arch Surg. 2015;400:523–529.
- Welborn MB, Mcdaniel HB, Johnson RC, et al. Clinical outcome of an extended proximal seal zone with the AFX endovascular aortic aneurysm system. J Vasc Surg. 2014;60(4):876–884.
- Waasdorp EJ, De Vries JPPM, Sterkenburg A, et al. The Association between Iliac Fixation and Proximal Stent-graft Migration during EVAR Follow-up : mid-term Results of 154 Talent Devices. Eur J Vasc Endovasc Surg. 2009;37(6):681–687.
- Taneva GT, Garcia AG, Arribas Diaz AB, et al. Evolution and clinical relevance of common iliac artery seal zone after endovascular aortic aneurysm repair. Vascular. 2019;1–6.
- Schuurmann RCL, Overeem SP, van Noort K, et al. Validation of a New Methodology to Determine 3-Dimensional Endograft Apposition, Position, and Expansion in the Aortic Neck After Endovascular Aneurysm Repair. J Endovasc Ther. 2018. DOI:10.1177/1526602818764413
- Berezowski M, Morlock J, Beyersdorf F, et al. Inaccurate aortic stent graft deployment in the distal landing zone : incidence, reasons and consequences. Eur J Cardio Thoracic Surg. 2018;53:1158–1164.
- Wang S, Hicks CW, Malas MB. Neck diameter and inner curve seal zone predict endograft-related complications in highly angulated necks after endovascular aneurysm repair using the Aorfix endograft. J Vasc Surg. 2018;67(3):760–769.
- Schuurmann RCL, Overeem SP, Ouriel K, et al. A Semiautomated Method for Measuring the 3-Dimensional Fabric to Renal Artery Distances to Determine Endograft Position After Endovascular Aneurysm Repair. J Endovasc Ther. 2017;24(5):698–706. .
- Schuurmann RCL, Van Noort K, Overeem SP, et al. Aortic Curvature Is a Predictor of Late Type Ia Endoleak and Migration After Endovascular Aneurysm Repair. J Endovasc Ther. 2017;24(3):411–417.
- Schuurmann RCL, Ouriel K, Muhs BE, et al. Aortic curvature as a predictor of intraoperative type Ia endoleak. J Vasc Surg. 2016;63(3):596–602.
- Bastos Goncalves F, Hoeks SE, Teijink JA, et al. Risk factors for proximal neck complications after endovascular aneurysm repair using the endurant stentgraft. Eur J Vasc Endovasc Surg. 2015;49(2):156–162.
- Jordan WD, Ouriel K, Mehta M, et al. Outcome-based anatomic criteria for defining the hostile aortic neck. J Vasc Surg. 2015;61(6):1383–1390.
- Wyss TR, Dick F, Brown LC, et al. The influence of thrombus, calcification, angulation, and tortuosity of attachment sites on the time to the first graft-related complication after endovascular aneurysm repair. J Vasc Surg. 2011;54(4):965–971.
- Oliveira NFG, Bastos Gonçalves FM, Van Rijn MJ, et al. Standard endovascular aneurysm repair in patients with wide infrarenal aneurysm necks is associated with increased risk of adverse events. J Vasc Surg. 2017;65(6):1608–1616.
- Abbruzzese TA, Kwolek CJ, Brewster DC, et al. Outcomes following endovascular abdominal aortic aneurysm repair (EVAR): an anatomic and device-specific analysis. J Vasc Surg. 2008;48(1):19–28.