ABSTRACT
Background
Growth hormone (GH) treatment preference and adherence are affected by delivery device convenience, injection-site pain, confidence in correct dose administration, and device satisfaction. This survey investigated if switching device to NordiFlex® improved treatment experience in pediatric patients in South Korea.
Design and methods
Patients aged 4–≤18 years were surveyed. Participants were NordiFlex® users who previously used NordiLet®/other devices. Participants compared preference, self-reported adherence, satisfaction, perceived ease of use, and device subjective benefits (across four domains: ease of use, self-efficacy, minimal disruption of daily life, positive feelings about injections) of NordiFlex® vs. previous device.
Results
Ninety-four patients were enrolled, of which 91.5% previously used NordiLet®. Significantly more patients preferred, and were more satisfied with NordiFlex® vs. previous device; mean score: 0.65 (95% confidence interval [CI]:0.41;0.88) and 0.61 (95% CI:0.36;0.85), respectively. Participants reported greater perceived ease of use (0.49 [95% CI:0.26;0.72]) and fewer missed injections (0.20 [95% CI:0.06;0.34], with NordiFlex® vs. previous device. Bivariate analysis showed significant associations between preference for NordiFlex® and higher scores on self-efficacy, ease of use, minimal disruption of daily life, and positive feelings about injection (all p < 0.001).
Conclusion
These results suggest that improvements in device features could be associated with improved treatment experience.
1. Introduction
Growth hormone (GH) therapy is approved to promote growth in children with short stature affected by conditions like growth hormone deficiency (GHD), idiopathic short stature (ISS), small for gestational age (SGA), Noonan syndrome (NS), SHOX deficiency, Prader-Willi syndrome (PWS), Turner syndrome, or chronic renal disease [Citation1–5].
Daily subcutaneous injections are required, often over many years, to promote linear growth. For example, in children with GHD, treatment may start in early infancy and continue until adult height is achieved [Citation4]. However, the necessity for daily injections may lead to suboptimal adherence, which may result in decreased efficacy and increased healthcare expenditure.
Non-adherence is documented in as many as 77% of adolescents [Citation6]; height velocity is compromised in patients who have suboptimal adherence [Citation6–9]. There are also economic costs associated with suboptimal adherence. These include direct costs including those owing to additional diagnostic procedures, wasted medicines, increased GH dosage (with the added increased risk of adverse effects), increased hospitalization, and/or additional drugs or the introduction of other therapeutic interventions [Citation10–12].
Inherent treatment burden that exists in the long-term administration of GH treatment (which could reduce the final efficacy of treatment) includes: the frequency and route of injections, the complexity of the injection regimen, injection pain, difficulties associated with the injection device (such as the need to reconstitute the GH), the patient’s or caregivers’ lack of understanding of the benefits of treatment, formulary/insurance changes, the administration burden of long-term therapy, and inadequate patient education [Citation6,Citation12–15].
There are several device-related factors that could potentially improve adherence to GH treatment. These include perceived device convenience, acceptability of and satisfaction with the device, reduction of injection pain or discomfort, storage flexibility, and confidence of having administered the right dose [Citation16–18]. To increase choice and satisfy patient needs, several pen devices for administering GH have been developed [Citation18–22], with features such as needle-free injection technology [Citation19], electronic injection [Citation20], and features to ease user experience, such as prefilled GH injection devices and liquid formulations that do not require reconstitution [Citation18].
In 2002, Norditropin® NordiLet® (Novo Nordisk A/S, Bagsvaerd, Denmark) was the first prefilled multidose GH injection pen device to be approved in Europe. It received regulatory approval in South Korea in 2004. Norditropin NordiFlex® (Novo Nordisk A/S, Bagsvaerd, Denmark; approved in South Korea in 2018) was designed to further improve on Norditropin® NordiLet®, with features to further alleviate injection burden and improve treatment experience (Appendix A). Like Norditropin® NordiLet®, Norditropin NordiFlex® is prefilled with a liquid formulation of GH to eliminate the need for reconstitution prior to use. Furthermore, Norditropin NordiFlex® is quick and easy to set up for injection, and requires less injection force than previous devices, enabling children to inject themselves [Citation23,Citation24]. All of these properties were designed to improve comfort and adherence to treatment, since patient autonomy has been found to be a positive factor in adherence to GH therapy [Citation13,Citation25].
Results from a Phase IV multicenter prospective study in France showed that Norditropin NordiFlex® was safe and easy to use, and most patients preferred it to their previous device [Citation24].
This questionnaire-based cross-sectional survey was conducted to evaluate patient-reported satisfaction and ease-of-use with Norditropin NordiFlex® compared with the last GH device used prior to switching in pediatric and adolescent patients who were prescribed treatment with Norditropin® ([GH] somatropin, Novo Nordisk A/S, Denmark) in routine clinical care in South Korea.
The primary objective of the survey was to examine if respondents preferred their current GH therapy device (Norditropin NordiFlex®) to the previous device (Norditropin® NordiLet® or other GH devices). Secondary objectives included examining the difference in self-reported adherence, satisfaction, and perceived ease of use with Norditropin NordiFlex® compared with the previous device.
2. Patients and methods
2.1. Study design
This was a cross-sectional survey of pediatric and adolescent patients (and/or their caregivers/legally authorized representatives [LARs]) who had been switched to GH therapy with Norditropin NordiFlex® from Norditropin NordiLet® or another GH injection device. The survey was conducted between January 2019 and July 2019 at eight outpatient pediatric clinics in South Korea. Patients were recruited via convenience sampling during regularly scheduled clinic visits.
During these visits, participating physicians identified potential participants, and at the end of the visit, the physician introduced the survey and explained its details to the patient and his/her caregiver/LAR. For children aged 4 to 6 years old, the caregiver/LAR signed the informed consent form. For those aged between 7 and 17 years old, written informed consent was obtained from the patient’s caregiver/LAR and the assent form or informed consent form was obtained from children who met the age requirement of each site’s Institutional Review Board guidelines. Written informed consent was obtained from participants who were aged 18 years.
Prior to study initiation, the protocol, amendments, informed consent and assent forms were reviewed and approved by the independent ethics committee of the respective study site. The study was conducted in accordance with the protocol and implemented according to the consensus ethical principles derived from international guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines, applicable International Council for Harmonization (ICH) Guidelines for Good Pharmacoepidemiology Practices, and other applicable laws and regulations.
2.2. Participants
Patients aged 4 to ≤18 years who were current users of Norditropin NordiFlex® for 4 to ≤12 weeks (initial protocol) at the time of enrollment, and had used another GH device (Norditropin® NordiLet® or any other GH device) for at least 3 months before switching to Norditropin NordiFlex® were eligible to participate.
Owing to the observational nature of the study, the majority of patients who were potentially eligible to participate in this survey had already been switched from their previous device to Norditropin NordiFlex® by the time that ethics committee approvals were received and the study sites were ready to enroll patients. Thus, these patients could not be enrolled into the study, limiting the overall number of patients that could participate in the survey. In order to mitigate this limitation and to ensure an appropriate level of statistical power, the protocol was amended to extend the use of Norditropin NordiFlex® from 4 to ≤12 weeks to 4–24 weeks. Enrollment was stopped when no additional patients met the expanded time-related inclusion criterion.
Patients aged 4 to 17 years old were to be accompanied by a LAR to give consent, and the caregiver who administered the injections, if different to the LAR.
Patients who had previously participated in this survey, or who had received any investigational drug within the past 6 months, or had a serious comorbidity or other circumstances that would interfere with adherence to GH therapy, or a physical or mental incapacity (as determined by physicians) interfering with capability to participate in the survey were excluded.
2.3. Assessments/data collection
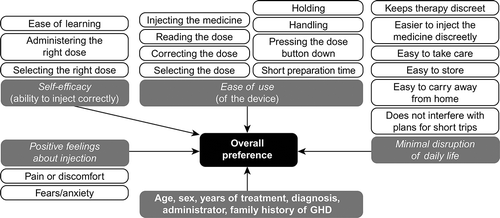
The survey comprised three parts: A, B, and C (Appendix B). Part A contained questions about the patient’s clinical and medical information, and was completed by the attending physician. Part B was a self-administered survey that contained questions about the demographic characteristics of the caregiver or LAR. Part C was a 25-item questionnaire (completed by the administrator of GH) assessing primary and secondary outcomes (preference for, satisfaction, perceived ease of use and adherence with Norditropin NordiFlex® compared with the previous GH device) as well as subjective benefits of GH device features, categorized under four domains: self-efficacy, ease of use, minimal disruption of daily life, and positive feelings about injection. These subjective benefits were based on a conceptual model of the factors influencing preference for GH delivery devices ().
Figure 1. The conceptual model used to identify factors associated with GH device preference GH, growth hormone; GHD, growth hormone deficiency
Except for two questions relating to participant classification and adherence on the basis of participants’ recall of missed injections in a typical month, participants responded on a 5-point Likert scale: 1, strongly disagree; 2, disagree; 3, no difference; 4, agree; 5, strongly agree. An option of ‘not sure’ (0) was also provided.
If a participant was unable to choose an option or provide an answer in the survey, the researcher did not coerce the participant to answer the question. All questionnaires were developed in English and were then translated to Korean. Face validity of the survey was assessed by two practicing investigators before rolling out to participants.
No qualitative data were collected in this study.
2.4. Data analysis
Primary and secondary outcome data were categorized as dependent variables. Participant clinical and/or demographic data, as well as data relating to the subjective benefits of GH device features, were categorized as independent variables.
All scores on the Likert scale were transformed from 1 to 5, to standardized scores ranging from −2 to 2 accordingly. ‘0’ responses were replaced with the median score. As the number of items in each domain varied from one another, the total scores were averaged by the number of items within each domain and reported as mean and standard deviation (SD) in order to facilitate interpretation. This resulted in a score of −2 to 2 per domain per patient. Scores from dependent variables were transformed into binary (>0 vs. ≤0) and ternary variables (>0 vs. 0 vs. <0).
As the study inclusion criteria extended the maximum use of Norditropin NordiFlex® from 12 weeks to 24 weeks, there was a potential for recall bias. This was examined with a sensitivity analysis by comparing preference for, and self-reported adherence with, Norditropin NordiFlex® between patients who used Norditropin NordiFlex® for 4 to ≤12 weeks vs. those who used it for >12 weeks.
STATA version 15 (StataCorp LLC, College Station, Texas, USA) was used to analyze the data.
2.5. Statistical methods
Sample size calculation: it was assumed that approximately 64% of users would prefer Norditropin NordiFlex®, based on findings from Tauber and colleagues [Citation24]. With a power of 80% and statistical significance set at 5%, and accounting for potential omitted responses, a sample size of 120 participants who had previously used Norditropin® NordiLet® and 120 participants who had previously used other GH devices were estimated.
Descriptive statistics were used to summarize the main participant demographic characteristics. The mean scores of the dependent variables were compared to the hypothesized value of zero using a one-sided one-sample t-test. Statistical significance level was set at p < 0.05 and power at 80%. Bivariate associations between the dependent and independent variables were performed. Continuous variables were reported as mean (SD) and median (interquartile range), where appropriate. Categorical variables were reported as n (%) and the differences were tested using a chi-square test.
Differences between participants who preferred Norditropin NordiFlex® and those who did not were tested using a t-test if the distribution was normal, or a Mann-Whitney U test if the data were not normally distributed.
The contribution of each independent variable on the preference for Norditropin NordiFlex® was assessed using multivariate logistic regression accounting for age, sex, height SDS at the point of the survey, and the ratio of duration of treatment with Norditropin NordiFlex® to total duration of GH treatment. As the majority of patients previously used Norditropin® NordiLet® (n = 86) and only eight patients used other devices before Norditropin NordiFlex®, preference was assessed using a multivariate model that included all participants.
Independent variables that had a statistical significance level of p < 0.1 were included in the multivariate logistic regression model using the backward stepwise removal method. Odds ratios were calculated with 95% CIs.
For the recall bias sensitivity analysis, the percentage of patients who preferred Norditropin NordiFlex®, as well as the percentage of the patients who missed fewer injections with Norditropin NordiFlex®, were compared between patients who used Norditropin NordiFlex® for 4 to ≤12 weeks and those who used the device for >12 to ≤24 weeks.
The internal consistency of the items within each domain was assessed using Cronbach’s alpha, except for items in the positive feelings about injections domain, which were assessed using kappa statistics. Cronbach’s alpha values were above the threshold of 0.70 for all three domains (range 0.81–0.92), showing high internal consistency. The two items in positive feelings about injections also had a good correlation, with a kappa of 0.79.
No transformation for missing data was required, as all participants provided responses to all survey items.
3. Results
3.1. Patient caregiver/LAR characteristics
A total of 94 patients were enrolled in the survey. Participant demographics and clinical characteristics are described in .
Table 1. Patient and caregiver/LAR demographics
3.2. Primary and secondary outcomes
A significantly greater proportion of participants preferred and were more satisfied with Norditropin NordiFlex® compared with the previous device, with mean-standardized scores of 0.65 (95% CI: 0.41;0.88) and 0.61 (95% CI: 0.36;0.85), respectively. Similarly, participants reported higher perceived ease of use and fewer missed injections with Norditropin NordiFlex® compared with the previous device, with mean standardized scores of 0.49 (95% CI: 0.26;0.72) and 0.20 (95% CI: 0.06;0.34), respectively.
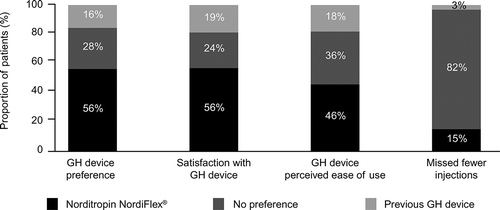
Categorization of outcome data as ternary variables showed that more than half of the participants showed a preference for, and had greater satisfaction with Norditropin NordiFlex® compared to the previous device (). Less than 20% of participants preferred or were more satisfied with their previous device. About half of participants perceived Norditropin NordiFlex® to be easier to use than their previous device. 15% of participants reported missing fewer injections with Norditropin NordiFlex® than with their previous device, and only 3% reported missing fewer injections with their previous device compared with Norditropin NordiFlex®.
3.3. Preference for Norditropin NordiFlex®
shows the bivariate associations between patient and caregiver/LAR characteristics and preference for Norditropin NordiFlex®. Patients who preferred Norditropin NordiFlex® had a longer duration of GH treatment before switching, compared with those who expressed no preference or preferred the previous device (2.5 years vs. 1.8 years, p = 0.040). Additionally, respondents were significantly more likely to prefer Norditropin NordiFlex® if patients were diagnosed with a more severe condition (GHD, SGA, NS) compared to those who were diagnosed with a less severe condition (ISS, familial short stature [FSS], precocious puberty, others) (76.2% vs. 50.7%, p = 0.038). No significant associations were identified for age, sex, height, and weight at diagnosis, height SDS and weight SDS at diagnosis, height and weight at the point of the survey, height SDS and weight SDS at the point of the survey, administrator of injection, last device used before Norditropin NordiFlex®, and duration of Norditropin NordiFlex® use. There were no significant associations between caregiver/LAR characteristics and preference for Norditropin NordiFlex®.
Table 2. Bivariate associations between patient and caregiver/LAR characteristics and preference for Norditropin NordiFlex®
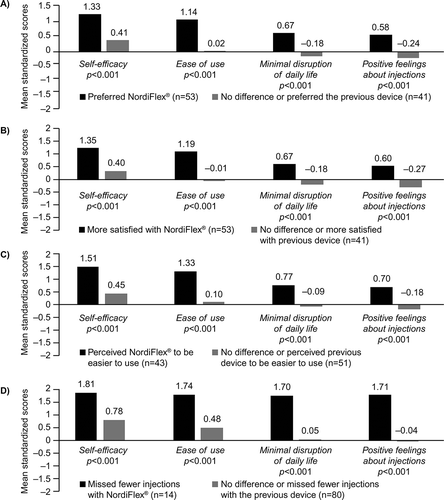
Respondents who preferred Norditropin NordiFlex® had higher standardized scores on self-efficacy (1.33 [0.69] vs. 0.41 [0.87]), ease of use (1.14 [0.75] vs. 0.02 [0.82]), minimal disruption of daily life (0.67 [0.88] vs. −0.18 [0.49]), and positive feelings about injection (0.58 [0.85] vs. −0.24 [0.63]) compared with those who reported no difference or preferred the previous device (all p < 0.001) ().
Figure 3. Associations between domains of subjective benefits and preference for (A), satisfaction with (B), perceived ease of use (C), and self-reported adherence (D) with Norditropin NordiFlex®
3.3.1. Subgroup analyses of participants who used Norditropin NordiFlex® for 4 to ≤12 weeks and those who used it for >12 weeks
There was no significant difference in GH device preference between those who used Norditropin NordiFlex® for 4 to ≤12 weeks and those who used it for >12 weeks (64.0% vs. 53.6%, p = 0.370).
3.3.2. Multivariate analysis of factors associated with preference for Norditropin NordiFlex®
Multivariate analysis (accounting for age, sex, height SDS at the point of the survey and the ratio of duration of treatment with Norditropin NordiFlex® to total duration of GH treatment) showed that higher scores of ease of use (adjusted odds ratio [OR] [95% CI]: 3.77 [1.04;13.57]; p = 0.042) and minimal disruption of daily life (adjusted OR [95% CI]: 5.05 [1.09;23.25]; p = 0.038) were significantly associated with preference for Norditropin NordiFlex®.
However, the observed associations with self-efficacy (adjusted OR [95% CI]: 1.10 [0.38;3.21]) and positive feelings about injection (adjusted OR [95% CI]: 1.63[0.44;6.01]) were no longer statistically significant (p > 0.05).
3.4. Satisfaction with Norditropin NordiFlex®
Patients who experienced greater satisfaction with Norditropin NordiFlex® had longer duration of GH treatment before switching, compared with those who experienced no difference or were more satisfied with the previous device (2.5 years vs. 1.8 years, p = 0.03). Additionally, patients with a relatively more severe diagnosis also showed significantly higher satisfaction with Norditropin NordiFlex® vs. the previous device (81.0% vs. 49.3%, p = 0.01). There were no significant associations between caregiver/LAR characteristics and satisfaction with Norditropin NordiFlex® ().
Table 3. Bivariate associations between patient and caregiver/LAR characteristics and satisfaction with Norditropin NordiFlex®
Respondents who were more satisfied with Norditropin NordiFlex® had higher standardized scores on self-efficacy (1.35 [0.66] vs. 0.40 [0.89]), ease of use (1.19 [0.67] vs. −0.01 [0.83]), minimal disruption of daily life (0.67 [0.86] vs. −0.18 [0.53]), and positive feelings about injection (0.60 [0.82] vs. −0.27 [0.65]) compared with those who reported no difference or were more satisfied with the previous device (all p < 0.001) ().
3.5. Perceived ease of use with Norditropin NordiFlex®
Similarly, patients who perceived Norditropin NordiFlex® to be easier to use were significantly more likely to have been receiving GH treatment for a longer period of time before switching, compared with those who perceived no difference or perceived the previous device to be easier to use (2.6 years vs. 1.9 years, p = 0.03) and diagnosed with a relatively more severe condition (66.7% vs. 39.7%, p = 0.029). There were no significant associations between caregiver/LAR characteristics and perceived ease of use with Norditropin NordiFlex® ().
Table 4. Bivariate associations between patient and caregiver/LAR characteristics and perceived ease of use with Norditropin NordiFlex®
Respondents who perceived Norditropin NordiFlex® to be easier to use vs their previous device had higher standardized scores on self-efficacy (1.51 [0.61] vs. 0.45 [0.82]), ease of use (1.33 [0.61] vs. 0.10 [0.82]), minimal disruption of daily life (0.77 [0.87] vs. −0.09 [0.59]), and positive feelings about injection (0.70 [0.87] vs. −0.18 [0.64]) compared with those who reported no difference or perceived the previous device to be easier to use vs Norditropin NordiFlex® (all p < 0.001) ().
3.6. Self-reported adherence with Norditropin NordiFlex®
Patients who missed fewer injections with Norditropin NordiFlex® (compared with those who reported no difference or missed fewer injections with the previous device) were more likely to be: i) a child rather than an adolescent (25.5% vs. 4.3%, p = 0.008); ii) diagnosed with a relatively more severe medical condition (33.3% vs. 9.6%, p = 0.007); iii) shorter and smaller at diagnosis (height 112.4 cm vs. 126.5 cm, p = 0.004; weight 21.4 kg vs. 28.4 kg, p = 0.018); and iv) shorter at the point of the survey (height 132.9 cm vs 142.7 cm, p = 0.014; height SDS −0.9 vs. −0.3, p = 0.027) ().
Table 5. Bivariate associations between patient and caregiver/LAR characteristics and self-reported adherence with Norditropin NordiFlex®
Patients missed fewer injections with Norditropin NordiFlex® if they had previously used other GH devices other than Norditropin® NordiLet® (Norditropin® NordiLet®: 9.3% vs. other GH devices: 75.0%, p < 0.001). Those that used Norditropin NordiFlex® for a shorter period (14.3 weeks vs. 17.6 weeks, p = 0.045) were also more likely to miss fewer injections. The difference in the number of injections missed in a typical month between the two groups was not statistically significant ().
With respect to caregivers/LARs, there were notable differences in education level and annual household income between those that reported missing fewer injections with Norditropin NordiFlex® and those that did not ().
Respondents who reported missed fewer injections with Norditropin NordiFlex® had higher standardized scores on self-efficacy (1.81 [0.39] vs. 0.78 [0.88]), ease of use (1.74 [0.47] vs. 0.48 [0.89]), minimal disruption of daily life (1.70 [0.46] vs. 0.05 [0.63]), and positive feelings about injection (1.71 [0.54] vs. −0.04 [0.61]) compared with those who reported no difference or missed fewer injections with the previous device (all p < 0.001) ().
3.6.1 Subgroup analyses of participants who used Norditropin NordiFlex® for 4 to ≤12 weeks and those who used it for >12 weeks
Patients who used Norditropin NordiFlex® for a longer period were less likely to report missing fewer injections (>12 weeks: 10.1% vs. 4–≤12 weeks: 28.0%, p = 0.032).
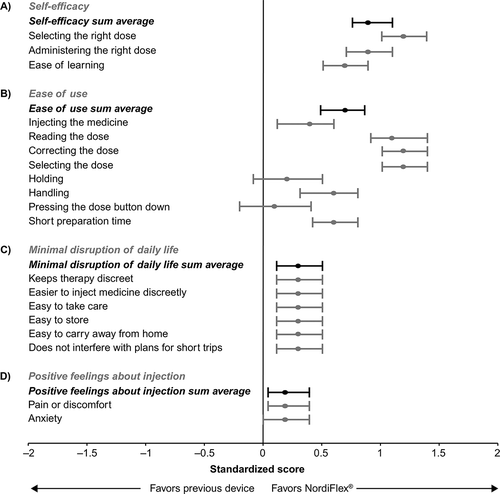
3.7. Mean standardized scores of subjective benefits of GH device features
shows forest plots of the mean standardized scores for individual subjective benefits of GH device features, as well as the overall mean score for each of the four domains of subjective benefits. Respondents’ opinions were in favor of Norditropin NordiFlex® across the majority of measures. For two subjective benefits, ‘NordiFlex® is easier to hold’ and ‘With NordiFlex®, it is easier to press the dose button,’ the difference was not statistically significant. Preference for Norditropin NordiFlex® was less pronounced on subjective benefits in the positive feelings about injection domain.
4. Discussion
A growing body of evidence suggests that device-related factors can play a role in improving adherence to GH treatment. Norditropin NordiFlex® introduced a number of key improvements compared with Norditropin® NordiLet®, including no requirement for dose conversion, a dose indicator window, finer dose increments, and a soft push button. Previous studies evaluating patients’ self-reported preference for Norditropin NordiFlex® compared to other devices, showed favorable outcomes for Norditropin NordiFlex® [Citation18,Citation23,Citation24,Citation26].
This survey assessed the patients’ and caregivers’ opinions regarding the subjective benefits of Norditropin NordiFlex® after switching from their previous device (primarily Norditropin® NordiLet®) in a real-world setting. To quantify the impact of this switch, a conceptual model (grounded on available evidence) that categorized subjective benefits under four domains (self-efficacy, ease of use, minimal disruption of daily life, and positive feelings about injection) was developed. Assessment of the subjective benefits showed that items within each domain had a high internal consistency.
Participants reported a greater preference for and satisfaction, improved perceived ease of use, and self-reported adherence with Norditropin NordiFlex®, compared with the previous GH device. This suggests that feature improvements in Norditropin NordiFlex® were associated with improved patient treatment experience. Furthermore, preference for Norditropin NordiFlex® was positively associated with the four domains of subjective benefits. Multivariate analysis showed that preference for Norditropin NordiFlex® was still significantly associated with ease of use and minimal disruption of daily life, while associations with self-efficacy and positive feelings about the injection were no longer statistically significant, after controlling for age, sex, height SDS at the point of the survey and the ratio of duration of treatment with Norditropin NordiFlex® to total duration of GH treatment.
Patients with a relatively more severe diagnosis (e.g. GHD, SGA, NS), showed a stronger preference for Norditropin NordiFlex® vs. patients with a less severe diagnosis (e.g. ISS, FSS, precocious puberty, others). Additionally, these patients (as well as those with a longer duration of GH treatment) reported a higher perceived ease of use, and greater satisfaction with Norditropin NordiFlex® compared with the previous device. These findings suggest that patients with a relatively more severe diagnosis may be more sensitive to (and gain a greater advantage from) device features that improve their treatment experience compared with patients with a relatively less severe diagnosis.
Furthermore, patients with a relatively more severe diagnosis as well as those with a longer duration of GH treatment or lower height SDS at the point of survey, may be more acutely aware of the impact of adherence on outcomes, and/or may experience challenges with their GH treatment device. Devices that can improve these patients’ treatment experiences are likely to be viewed favorably.
Standardized scores of the individual subjective benefits of GH device features showed that, overall, participants preferred Norditropin NordiFlex® to the previous device across the majority of measures. Respondents strongly favored Norditropin NordiFlex® on the ‘Read the dose,’ ‘Correct the dose’ and ‘Select the dose’ items within the ease of use domain, as well as the ‘Selecting the right dose’ and ‘Administering the right dose’ items within the self-efficacy domain. Dose selection can have a two-fold impact on patient experience. In terms of ease of use, device improvements that facilitate dose selection can make the injection process easier for the patient or caregiver. Similarly, from a self-efficacy perspective, device improvements that enhance confidence when selecting the correct GH dose can empower patients to self-inject. The large dose window with Norditropin NordiFlex® may have provided patients with additional dose clarity vs. their previous device. Bagnasco and colleagues [Citation17] found that among patients who reported being confident of having administered the correct GH dose, a significantly larger proportion of them was adherent to treatment. This suggests that features that increase a patient’s or caregiver’s confidence in administering the correct dose of GH could, in turn, have a positive impact on adherence.
Previous research has shown that refrigeration of GH products, especially when traveling away from home, was perceived to be a substantial burden by both patients and caregivers [Citation14]. In the same study, the majority of patients and caregivers indicated a wish for a treatment that could be stored outside the refrigerator for longer periods of time, while almost half of the patients and caregivers expressed a preference for a treatment that did not require reconstitution.
Both Norditropin NordiFlex® and Norditropin® NordiLet® are prefilled injector pens with liquid GH using histidine buffer that can be stored at room temperature for 3 weeks after the first use. These features are absent from most of the other devices previously used by the respondents in this survey. These devices typically require reconstitution (e.g., Genotropin® GoQuick®), refrigerated storage (e.g., Genotropin® GoQuick®), cartridge replacement (e.g., Saizen® easypod®), or extra care as it is electronic (e.g., Saizen® easypod®). Also, devices which are relatively large could be perceived as less discreet (e.g., Saizen® easypod®, Genotropin® GoQuick®). In this survey, respondents indicated only a marginal improvement in favor of Norditropin NordiFlex® on items within the minimal disruption of daily life domain, and had similar positive feelings towards injection for Norditropin NordiFlex® compared with their previous device. These domains are influenced by device features that are common to both Norditropin NordiFlex® and Norditropin® NordiLet®. It is plausible that these results are a reflection of the high proportion of patients who switched to Norditropin NordiFlex® from Norditropin® NordiLet®. Given the high proportion of patients who previously used Norditropin® NordiLet®, it is also plausible that the small improvement in the minimal disruption of daily life domain is directly associated with feature enhancements in Norditropin NordiFlex® compared with Norditropin® NordiLet®. It may be possible that a larger difference in these domains could have been observed had there been more participants switching from a device other than Norditropin® NordiLet®. This needs to be evaluated in future studies.
Some patients, particularly those with smaller hands, may have needed to grip Norditropin NordiFlex® from a higher position to compensate for the slightly larger dimensions (150 vs. 144 mm in length, and 15 vs. 13 mm in diameter, respectively), and longer push button travel at maximum dose (~33 vs. ~16 mm, respectively) compared with Norditropin® NordiLet® (Appendix A). However, the standardized scores of the individual subjective benefits observed in this survey suggest that the impact of these small differences in size may have been mitigated by the reduction in pressure required to press the push button of Norditropin NordiFlex® compared with the previous device. The two subjective benefits, ‘NordiFlex® is easier to hold’ and ‘With NordiFlex®, it is easier to press the dose button’ within the ease of use domain, were not statistically significantly in favor of Norditropin NordiFlex® or the previous device.
This survey assessed self-reported adherence as a relative measure of injections missed with Norditropin NordiFlex® vs. the previous device. As the survey design did not allow for adherence data to be collected before and after patients switched devices, adherence was assessed on the basis of patients’ recall of missed injections with the current device in comparison with the previous device. The conceptual model suggested that treatment experience improvement across the four domains of subjective benefits could have an indirect effect on adherence.
Self-reported adherence results showed that patients who missed fewer injections with Norditropin NordiFlex® vs. the previous device were more likely to be children (rather than adolescents) diagnosed with a relatively more severe condition, be shorter in height at diagnosis and at the point of survey, have a lower weight at diagnosis, and have a lower height SDS at the point of survey. Considering the relatively low number of the patients who used other devices before Norditropin® NordiLet®, it is difficult to make a meaningful interpretation of this finding. The results also showed that the difference in the number of injections missed in a typical month between the two groups was not statistically significant. However, it is not possible to meaningfully interpret this observation, as only eight patients reported suboptimal adherence. Differences were observed in the educational level and annual household income between caregivers/LARs who reported missing fewer injections with Norditropin NordiFlex® and those that did not. A meaningful interpretation of this finding was not possible owing to the large proportion of caregivers/LARs who chose not to disclose their education level and household income.
Results from the subgroup analysis suggest that patients who used Norditropin NordiFlex® for 4 to ≤12 weeks were less likely to report missing fewer injections (vs. the previous device) compared with patients who used Norditropin NordiFlex® for >12 weeks. This observation could be potentially accounted for by two reasons. First, previous studies have reported different effects of duration of treatment on adherence; some studies [Citation7,Citation17,Citation27] reported a negative impact, while others [Citation28,Citation29] did not observe any relation. Bagnasco et al. [Citation17] also reported the impact of ‘Duration of use of current device’ on adherence where the non-adherence was significantly more in patients using their current device for more than one year compared to less than 6 months. While one cannot confidently exclude the impact of duration of using Norditropin NordiFlex® on the observed difference, the size of this impact might be minimal in light of the evidence from Bagnasco et al. [Citation17]. Secondly, and more plausibly, patients or caregivers/LARs could have experienced a recall bias with respect to adherence levels with the previous device.
As self-reported adherence was based solely on recollection, it is possible that patients or caregivers/LARs could have erroneously attributed better levels of adherence with the previous device than was actually the case. Furthermore, respondents who used Norditropin NordiFlex® for a longer period of time could have had less recollection about the level of adherence with their previous device compared with those who used NordiFlex® for a shorter period. Further research with a larger sample size will be required to clarify this result.
Bivariate analysis revealed a statistically significant positive association between all four domains of subjective benefits and self-reported adherence. A multivariate analysis was not performed owing to the small number of participants who reported missing fewer injections with Norditropin NordiFlex®. The adherence results from this survey suggest that minimal improvement in device features (that are not related to drug formulation) may have a positive effect on adherence. Furthermore, they are qualitatively similar to those reported by Bagnasco and colleagues (2010), who found that factors, such as level of confidence in administering the right dose, as well as convenience and overall satisfaction with the GH device, were associated with adherence.
It was not possible to establish whether other factors affected adherence in this study, as the design of the survey was developed around the primary outcome of ‘preference.’ Nevertheless, treating physicians should consider other factors (e.g. psychosocial factors [Citation30], motivation [Citation31], the patient’s or carer’s knowledge and understanding of the condition, the quality of the HCP-patient relationship [Citation32], as well as education on the positive effects of GH treatment [Citation33]) that may influence adherence to treatment.
4.1. Study limitations
This survey was affected by some limitations. It was not possible to assess the satisfaction level of patients who were unaccompanied by their caregiver/LAR, owing to informed consent requirements. This group of patients could exhibit greater independence compared with patients that were accompanied by their caregiver/LAR.
This study relied largely on participants’ self-reported data and could be potentially biased by social desirability. Nonetheless, questions related to the evaluation of device features are unlikely to be affected by this bias. Adherence to treatment was also self-reported as the majority of the devices used by the study participants did not have electronic monitoring capability, which could have potentially provided more objective adherence data.
Factors that could have played a role in device preference (e.g., disease awareness, patient-physician relationship) were not measured in this survey. Additionally, as the study had a cross-sectional design, the temporal association could not be established.
As the majority of patients used Norditropin® NordiLet® before switching to Norditropin NordiFlex®, it was not possible to compare the impact of some GH product-specific characteristics (such as storage flexibility and liquid formulation), or to perform exploratory subanalyses based on prior device group.
It was not possible to collect data or draw conclusions on the potential impact of factors that could influence device preference or acceptance, like the clinic setting or time of the appointment. This was due to limitations that are intrinsic to the design of survey-driven studies and differences between study centers that could not be controlled for. In this study, it was only possible to enroll 94 patients. Thus, it was not possible to stratify patients by previous device. Therefore, although number of participants allowed for the analysis of the primary endpoint, it was not possible to perform further comparative analyses between Norditropin NordiLet® and the other previously used devices.
Finally, a larger study with a prospective design to test the observed relations with a comprehensive multivariate model could have provided broader insight into the impact of GH device improvements on patients’ treatment experience and clinical outcomes.
4.2. Study strengths
This is one of the very few studies conducted in Asia that assesses the preference of GH devices for pediatric patients with growth disorders. The multi-center design allowed for a wider range of patients, thereby increasing the generalizability of the results.
The potential risk of recall bias among participants [Citation34] was mitigated by limiting the recall period to 24 weeks. Additionally, a sensitivity analysis was undertaken to compare the data on preference between those who used Norditropin NordiFlex® for 4 to ≤12 weeks and those who used it for >12 weeks.
The medicinal product and formulation were identical between the new device (Norditropin NordiFlex®) and the previous device in the majority of participants (Norditropin® NordiLet®). This allowed the detection of device-related impact on treatment experience while minimizing any potential confounds related to differences in formulation.
5. Conclusions
Participants in this survey reported greater preference and satisfaction, improved perceived ease of use, and self-reported adherence to Norditropin NordiFlex®, compared to the previous device. Of the subjective benefits of device features, ease of use, and minimal disruption of daily life domains were significantly associated with preference for Norditropin NordiFlex®.
Author contributions
All authors were involved in the conduct of the study and data collection, drafting, critical revision, and final approval of the version of the paper to be published. All authors agree to be accountable for all aspects of the work.
Geolocation information
Republic of Korea.
Declaration of interest
Medical writing and editorial support were provided by Gilbert Bezzina and Beverly La Ferla, Watermeadow Medical, part of the Ashfield Group (funded by Novo Nordisk Health Care AG). YJ Lee is an employee of Novo Nordisk Korea. Navid Nedjatian is an employee of Novo Nordisk Health Care AG. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed they are a consultant and an investigator in clinical trials for Novo Nordisk but do not receive any direct reimbursement for my services as reimbursement is provided to their University. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
ierd_a_1864324_sm9993.docx
Download MS Word (180.1 KB)Acknowledgments
The authors would like to thank all the investigators, their staff, and the patients and carers involved in this survey. The authors thank Junice Yi Siu Ng and Evie Achilla, IQVIA (funded by Novo Nordisk Health Care AG), for their contribution to data management and data analysis. We also thank Jinsook Jeong and Gina Yeonjoo Choi from Novo Nordisk Pharma Korea Ltd, for review and input to the manuscript.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author (WYC). The data contain information that could compromise research participant privacy/consent are therefore not publicly available.
Additional information
Funding
References
- Mahan JD, Warady BA. Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatr Nephrol. 2006;21(7):917–930.
- Clayton PE, Cianfarani S, Czernichow P, et al. Management of the child born small for gestational age through to adulthood: a consensus statement of the international societies of pediatric endocrinology and the growth hormone research society. J Clin Endocrinol Metab. 2007;92(3):804–810.
- Deal CL, Tony M, Hoybye C, et al. GrowthHormone research society workshop summary: consensus guidelines for recombinant human growth hormone therapy in prader-willi syndrome. J Clin Endocrinol Metab. 2013;98(6):E1072–E1087.
- Christiansen JS, Backeljauw PF, Bidlingmaier M, et al. Growth hormone research society perspective on the development of long-acting growth hormone preparations. Eur J Endocrinol. 2016;174(6):C1–C8.
- Gravholt CH, Andersen NH, Conway GS, et al. Clinical practice guidelines for the care of girls and women with turner syndrome: proceedings from the 2016 cincinnati international turner syndrome meeting. Eur J Endocrinol. 2017;177(3):G1–G70.
- Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143–154.
- Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93(2):147–148.
- Cutfield WS, Derraik JGB, Gunn AJ. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PloS One. 2011;6(1):e16223.
- van Dommelen P, Koledova E, Wit JM. Effect of adherence to growth hormone treatment on 0-2 year catch-up growth in children with growth hormone deficiency. PloS One. 2018;13(10):e0206009.
- Haverkamp F, Johansson L, Dumas H, et al. Observations of nonadherence to recombinant human growth hormone therapy in clinical practice. Clin Ther. 2008;30(2):307–316.
- National institute for health and clinical excellence (NICE) [internet]. NICE clinical guideline 76 (CG76). Medicines Adherence: involving patients in decisions about prescribed medicines and supporting adherence 2009 [ cited Dec 2009]. Available from: https://www.nice.org.uk/guidance/cg76/evidence/full-guideline–242062957
- Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Hormone Res Paediatrics. 2013;79(4):189–196.
- Norgren S. Adherence remains a challenge for patients receiving growth hormone therapy. Pediatr Endocrinol Rev. 2009;6(Suppl 4):545–548.
- Kremidas D, Wisniewski T, Divino VM, et al. Administration burden associated with recombinant human growth hormone treatment: perspectives of patients and caregivers. J Pediatr Nurs. 2013;28(1):55–63.
- Acerini CL, Wac K, Bang P, et al. Optimizing patient management and adherence for children receiving growth hormone. Front Endocrinol (Lausanne). 2017;8:313.
- Meinhardt U, Eiholzer U, Seitz L, et al. Parent preference in Switzerland for easy-to-use attributes of growth hormone injection devices quantified by willingness to pay. Expert Rev Med Devices. 2014;11:31–38.
- Bagnasco F, Di Iorgi N, Roveda A, et al. Prevalence and correlates of adherence in children and adolescents treated with growth hormone: A multicenter Italian study. Endocr Pract. 2017; 23(8): 929–941.
- Rohrer TR, Horikawa R, Kappelgaard AM. Growth hormone delivery devices: current features and potential for enhanced treatment adherence. Expert Opin Drug Deliv. 2017;14(11):1253–1264.
- Brearley C, Priestley A, Leighton-Scott J, et al. Pharmacokinetics of recombinant human growth hormone administered by cool.click 2, a new needle-free device, compared with subcutaneous administration using a conventional syringe and needle. BMC Clin Pharmacol. 2007;7:10.
- Tauber M, Payen C, Cartault A, et al. User trial of Easypod, an electronic autoinjector for growth hormone. Ann Endocrinol (Paris). 2008;69(6):511–516.
- Rapaport R, Saenger P, Schmidt H, et al. Validation and ease of use of a new pen device for self-administration of recombinant human growth hormone: results from a two-center usability study. Med Devices (Auckl). 2013;6:141–146.
- Fidotti E. A history of growth hormone injection devices. J Pediatr Endocrinol Metab. 2001;14(5):497–501.
- Hokken-Koelega A, Keller A, Rakov V, et al. Patient acceptance, ease of use, and preference for Norditropin NordiFlex with NordiFlex penmate: results from an open-label, user survey of everyday use. ISRN Endocrinol. 2011;2011:803948.
- Tauber M, Jaquet D, Jesuran-Perelroizen M, et al. User assessment of Norditropin NordiFlex((R)), a new prefilled growth hormone pen: a Phase IV multicenter prospective study. Patient Prefer Adherence. 2013;7:455–462.
- Oyarzabal M, Aliaga M, Chueca M, et al. Multicentre survey on compliance with growth hormone therapy: what can be improved? Acta Paediatr. 1998; 87(4): 387–391.
- Rohrer TR, Winter F, Qvist M, et al. Comparison of intuitiveness, ease of use and preference among three prefilled, disposable growth hormone injection pens. Expert Opin Drug Deliv. 2013;10(12):1603–1612.
- De Pedro S, Murillo M, Salinas I, et al. Variability in adherence to rhGH treatment: socioeconomic causes and effect on children’s growth. Growth Horm IGF Res. 2016;26:32–35.
- Leiberman E, Pilpel D, Carel CA, et al. Coping and satisfaction with growth hormone treatment among short-stature children. Horm Res. 1993;40(4):128–135.
- Mohseni S, Heydari Z, Qorbani M, et al. Adherence to growth hormone therapy in children and its potential barriers. J Pediatr Endocrinol Metab. 2018;31(1):13–20.
- Haverkamp F, Gasteyger C. A review of biopsychosocial strategies to prevent and overcome early-recognized poor adherence in growth hormone therapy of children. J Med Econ. 2011;14(4):448–457.
- Cassorla F, Cianfarani S, Haverkamp F, et al. Growth hormone and treatment outcomes: expert review of current clinical practice. Pediatr Endocrinol Rev. 2011;9(2):554–565.
- Graham S, Weinman J, Auyeung V. Identifying Potentially modifiable factors associated with treatment non-adherence in paediatric growth hormone deficiency: a systematic review. Hormone Res Paediatrics. 2018;90(4):221–227.
- Kreitschmann-Andermahr I, Siegel S, Unger N, et al. Motivation for and adherence to growth hormone replacement therapy in adults with hypopituitarism: the patients’ perspective. Pituitary. 2020;23(5):479–487.
- van den Brink M, Bandell-Hoekstra EN, Abu-Saad HH. The occurrence of recall bias in pediatric headache: a comparison of questionnaire and diary data. Headache. 2001;41(1):11–20.