ABSTRACT
Introduction: Subcutaneous cardiac rhythm monitors (SCRMs) provide continuous ambulatory electrocardiographic monitoring for surveillance of known and identification of infrequent arrhythmias. SCRMs have proven to be helpful for the evaluation of unexplained symptoms and correlation with intermittent cardiac arrhythmias. Successful functioning of SCRM is dependent on accurate detection and successful transmission of the data to the device clinic. As the use of SCRM is steadily increasing, the amount of data that requires timely adjudication requires substantial resources. Newer algorithms for accurate detection and modified workflow systems have been proposed by physicians and the manufacturers to circumvent the issue of data deluge.
Areas covered: This paper provides an overview of the various aspects of ambulatory rhythm monitoring with SCRMs including indications, implantation techniques, programming strategies, troubleshooting for issue of false positive and intermittent connectivity and strategies to circumvent data deluge.
Expert opinion: SCRM is an invaluable technology for prolonged rhythm monitoring. The clinical benefits from SCRM hinge on accurate arrhythmia detection, reliable transmission of the data and timely adjudication for possible intervention. Further improvement in SCRM technology is needed to minimize false-positive detection, improve connectivity to the central web-based server, and devise strategies to minimize data deluge.
1. Introduction
Ambulatory monitoring for arrhythmias is a routine clinical practice. The subcutaneous cardiac rhythm monitors (SCRMs) is a relatively newer technology, and its use is steadily increasing for surveillance of known arrhythmias such as atrial fibrillation (AF) or identification of infrequent tachy and brady arrhythmias in patients with a risk of sudden cardiac death [Citation1,Citation2].
Currently, there are four manufacturers who have SCRMs available for routine clinical use. Some of the changes in these devices over the years have included miniaturization and introduction of several algorithms to improve the specificity of arrhythmia detection. Despite an improvement in discrimination algorithms and miniaturization of SCRMs, there are some existing challenges with these devices. A high incidence of false-positive (FP) transmissions is an Achilles’ heel of ambulatory rhythm monitoring with SCRM. Although the introduction of newer algorithms has shown some modest improvement in the reduction of FP, the incidence continues to be substantial and ranges from 46% to 86% based on the indication of implantation [Citation3]. A high incidence of FP increases the workload for the device clinic personnel as well as the supervising physician (electrophysiologist or cardiologist) who must then adjudicate all remote transmissions to identify true positive (TP) episodes [Citation4]. More importantly, FP transmissions may lead to an incorrect diagnosis and initiation of therapy, such as anticoagulation in vulnerable patients.
The issue with FP and its impact on the workflow of device clinics is increasingly being recognized as the number of SCRMs being implanted is increasing. After the issue with high FP transmissions was recognized, several approaches with the collaboration of physicians, device clinic personnel, and industry have been introduced into clinical practice to curtail the high incidence of FP. These measures targeted several components of rhythm detection with SCRMs. These approaches can be implemented system-wide by introducing custom programming at the time of implantation and during alert notification setup. Certain changes can be implemented by custom programming at the individual patient level by modifying device sensitivity and detection criteria based on the patient’s body habitus and amplitude of detection signal.
This review is intended to elaborate on various components of arrhythmia detection with SCRMs. The fundamental differences between four commercially available SCRMs with respect to implantation techniques, detection parameters, and data transmission are discussed. Improved understanding of various components of ambulatory monitoring with SCRMs will allow the implementation of various approaches to streamline the workflow in the device clinic.
2. Historical perspective and available scrm
Currently, there are four commercially available SCRMs. The SCRM manufactured by Medtronic (Minneapolis, MN, USA), called Reveal™ XT, obtained approval in 2007. In subsequent years, several improvements have been made. Importantly, the size of the SCRM has been significantly miniaturized in the form of the Reveal™ LINQ that was approved in 2017. The Reveal LINQ II™ device was approved by the FDA in 2020 with the unique ability to allow for remote programming by the managing physician. Medtronic has also implemented several algorithms to improve the specificity of arrhythmia detection
The second SCRM introduced to clinical practice was BioMonitor™ (Biotronik, Berlin, Germany). With the original BioMonitor shaped like a pacemaker, its next generation device, BioMonitor II™ differed from the original devices with the presence of an extended antenna to allow improved detection of the R-wave. The newest version of this device, BioMonitor III™, is comparable in size to the other preceding devices; however, it continues to have an extended antenna. With the advent of newer devices, the Reveal XT™ and BioMonitor™ are no longer commercially available.
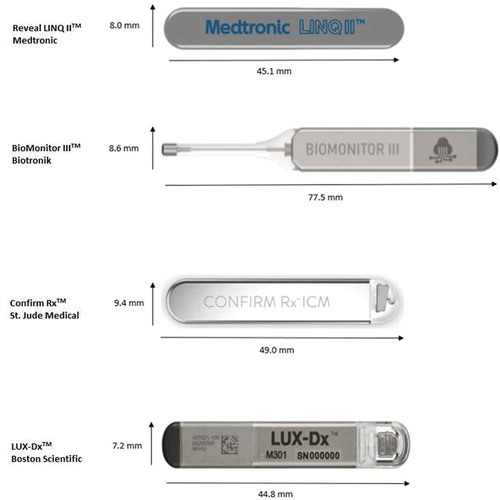
The third SCRM in clinical practice is Confirm Rx™ (Abbott, Abbott Park, IL, USA). Confirm Rx™ was the first SCRM that was smartphone compatible and incorporated BlueTooth technology. The latest version of ConfirmRx ™ was approved in 2019 and incorporated new SharpSense technology aimed at the reduction of FP. The newest SCRM is LUX-Dx (Boston Scientific, Minneapolis, MN, USA) which is similar in size to Reveal™ LINQ and Confirm Rx™. outlines important features of clinically available SCRMs. displays the four commercially available SCRM’s.
Table 1. Comparison of various features in the available SCRMs
3. Diagnostic value of SCRMs
SCRM provides the advantage of prolonged continuous arrhythmia monitoring as opposed to the Holter or event monitor which monitors for much shorter amounts of time. Depending on the vendor, the battery life of an SCRM can be between 2 and 4.5 years. They are generally well tolerated and explantation is rare [Citation5]. Indications for an SCRM include workup of cryptogenic stroke, syncope, palpitation and AF monitoring. In we describe specific indication and guideline statements pertaining to the various indications for SCRM implantation.
Table 2. Indications for SCRM implantation and associated guidelines
The diagnostic value of SCRMs and their unique ability for prolonged monitoring have been demonstrated in several studies. In syncope, meta-analysis of randomized clinical trials have shown SCRM’s to have a high diagnostic yield with one study finding patients were 3.6 times more likely to obtain a diagnosis when they had a SCRM implanted compared to patients treated with conventional care [Citation6]. Other studies have shown a reduced time to diagnosing and treating syncope with SCRM usage [Citation7]. Similarly, when attempting to diagnose the etiology of palpitations, SCRM’s have been found to have higher diagnostic yield than other types of monitoring [Citation8]. In cryptogenic stroke (CS), the CRYSTAL AF was the largest randomized control trial of its kind that demonstrated the benefit of prolonged monitoring via SCRM in the detection of silent AF in patients following a cryptogenic stroke [Citation9].
While there is no established guideline on the usage of SCRM’s in AF, they can be very useful in the management of this arrythmia. Several studies have demonstrated the importance of AF burden documentation with ICM prior to AF ablation, as not uncommonly there is inadequate AF burden to warrant an ablation [Citation10–12]. The ABACUS trial was a prospective randomized control trial that was able to demonstrate the use of SCRM’s led to more actionable events and higher rates of antiarrhythmic discontinuation when compared with other forms of rhythm monitoring in a post AF ablation population [Citation13].
4. Implantation technique
There are some standard guidelines for the implantation of SCRMs. Primarily, the SCRM is implanted between the fourth and sixth intercostal space on the left chest. The angle of implantation can vary from 0° to 90° based on operator’s preference and patient’s body habitus and gender. The authors use Lidocaine and 1% Epinephrine for local anesthesia and typically allow 2–4 minutes after injection for maximum vasoconstriction at the implantation site to minimize the risk of oozing after implantation. A long needle for local anesthesia is often preferred to allow for greater depth of analgesic injection. For incision closure, a subcuticular suture can be used if there is significant oozing, especially in patients with anti-platelet medications such as those with cryptogenic stroke. The angle of implantation can vary based on the operator’s preference and patients’ body habitus and gender. In females, the SCRM can be implanted in parallel to the long axis of the sternum to avoid an invasion of breast tissue. A device inserted in the breast tissue tends to float, which may cause unstable R-wave sensing. Additionally, an oblique angle of insertion in females results in higher incidence of premature SCRM explanation due to discomfort [Citation5]. Mapping prior to the selection of sites can be performed to identify the area of acceptable R wave amplitude. However, a recent study showed similar R wave amplitude irrespective of angle of SCRM insertion [Citation14]. The importance of an adequately sensed R wave has been documented in the literature [Citation15]. Studies have shown higher BMI to be associated with lower R wave amplitudes [Citation2]. Different vendors recommended different minimum R wave voltages for their given device as mentioned in . While SCRM implantation commonly occurs in the EP lab, it should be noted this procedure can be done in an office environment, provided that proper precautions are taken [Citation16].
5. Device programming at implantation
After implantation of the device, two parameters need to be set for initial programming, R wave sensing and arrhythmia episode detection criteria. R wave sensing can be set up manually by the operator. If the patient has a small R wave, one could consider re-programming to a more sensitive value. Arrhythmia episode detection criteria will be set based on ‘patient age’ and ‘Reason for Monitoring’ information entered into the device programmer. The operator can change these parameters if desired. The automatic detection and ECG storage of tachycardia (ventricular tachycardia, supraventricular tachycardia), pause (asystole), bradycardia, and AF episodes are turned on when the device is activated after implantation. An automatically detected episode starts when it meets the detection criteria for that episode type. describes the specific detection criteria for each individual arrhythmia, both manufacturers recommended ‘out-of-the-box’ nominal programming, as well as our own institution recommended custom programming. shows the nominal arrhythmia detections settings for a given ‘Reason for Monitoring.’ The reason for monitoring and individual parameters can also be changed during follow-up sessions or remotely for some newer SCRMs.
Table 3. Comparison of manufacturer-recommended nominal programming and institutional custom programming
Table 4. Proposed programming for alert settings to minimize data deluge from subcutaneous cardiac rhythm monitors
Studies have shown that a higher incidence of FP transmissions is attributed to nominal programming [Citation4]. The increased incidence of FP with nominal programming prompted the introduction of custom programming, which includes turning off detection for clinically irrelevant arrhythmias and extension of duration to the clinically acceptable maximum. This approach has resulted in a substantial drop in the number of episodes that required adjudication and thus has resulted in a significant reduction in the workload of device clinic personnel and supervising physicians [Citation4]. The ability to customize the programming of SCRMs is available in all current devices.
6. Device data collection
SCRMs stratify arrhythmias into four categories, tachycardia, bradycardia, pause, and atrial tachycardia (AT)/AF. The SCRM declares an episode as tachycardia when the patient’s heart rate increases to a rate that is higher than the programmable tachycardia rate (typically 230 – age) for a certain minimum duration. The tachycardia rate and duration criterion are programmable in all four SCRMs. The device detects bradycardia when the patient’s heart rate falls to a rate that is lower than the programmable brady threshold for a minimum duration and is also programmable. Pause (asystole) is detected when no ventricular events are sensed for a programmable period of time. Similarly, AT/AF is diagnosed when pre-set criteria is met. All SCRMs detect an episode of AF by analyzing the irregularity of ventricular rhythm using an automatic algorithm based on Lorenz plot.
Overall, the programming characteristics of various SCRMs are similar with minor exceptions. Every SCRM allows the programmer to adjust, tachycardia threshold, bradycardia threshold, minimum duration of episode (for both tachy and brady), pause duration and AT/AF detection. Additionally, they all have a parameter to adjust the sensitivity of AF detection to some sort of low, medium, or high sensitivity setting. LINQ IITM and BioMonitor IIITM have ectopy rejection parameters via detection of short then long R-R intervals. This can be turned ‘on’ or ‘off’ based on the indication for SCRM placement. For tachycardia, LINQ IITM and Confirm also have a programmable ‘sudden onset’ feature which can be turned on or off. Supraventricular tachycardia and ventricular tachycardia usually are abrupt onset tachyarrhythmias while sinus tachycardia tends to be slower onset. LINQ II and Confirm RxTM implement this ‘sudden onset’ feature to distinguish between the two. Confirm RxTM also has an additional parameter called ‘Delta Onset’ where the degree of sudden onset needed for a detection can be programmed.
The SCRMs will store episode data and ECGs for each episode type where detection is programmed ‘on.’ When it is ‘on,’ arrhythmia episode data is stored in an episode log. This includes up to a variable number of episodes, specific for each manufacturer. When the log is full, data from the most recent episode may overwrite the oldest stored episode data of that type. EKGs recorded before and during an episode can also be stored in device memory. The SCRMs have variable storage per day for automatically detected episodes. When the available memory for automatically detected episodes is full, a new ECG recording will overwrite the oldest stored ECG recording, provided that a minimum number of episodes of each type remain in memory. After the device has reached the storage limit for individual arrhythmia type, new episodes will not be stored. Some SCRMs uses different form of memory management to preserve clinically significant episodes more effectively as opposed to a simple first in-first out protocol.
7. AF diagnostics
AF surveillance and identification is the most common reason for use of SCRMs. The rate and irregularity of R waves serve as the fundamental principle during rhythm identification of AF with SCRMs. A positive AF episode is defined when device detected AF episode is noted to be true AF after adjudication by device clinic personnel and/or a supervising physician. For identification of an AF episodes, a minimum duration of R-R irregularity is needed. For the most part, a minimum duration of 30 seconds of AF meets the criteria for AF episode. Devices can be programmed to save episodes that last for a certain period of time. The minimum duration of AF is variable among various vendors. In the literature the length of a clinically significant episode of AF is up for debate, however the HRS defines an AF episode of 30 seconds or greater to be clinically significant [Citation17]. Length of episode parameters can be adjusted to make the device more sensitive or specific depending on the indication for the SCRM. Nominal settings for duration of AF in the most common implantation indications are similar between vendor using an episode length of 2 minutes. One exception being LINQ IITM using an AF duration of 10 minutes when implanted for syncope.
Since the inception of SCRMs, several algorithms have been introduced to improve the diagnostic accuracy for arrhythmias. Reveal LINQTM is probably the one SCRM with multiple iterations for improvement in AF diagnostics. For detection of AF, the current generation of Reveal™ LINQ uses a 3-step process. In the first step, the algorithm looks for patterns of incoherence in a Lorenz plot for the difference in RR intervals and computes an AF evidence score every 2 min. In the second step, a P-wave evidence score (P-Sense) is calculated based on the detection of p waves [Citation18]. In the third and final step, the algorithm uses the presence of P wave during RR irregularity as evidence of sinus arrhythmia or ectopy and adaptively optimizes sensitivity for AF detection [Citation19]. Before comparison with a threshold value to diagnose AF, the AF evidence score derived from the Lorenz plot is reduced by the P-wave evidence score. The enhancements in the ILR algorithm were prompted by a higher rate of FP detection in the earlier studies [Citation13,Citation20,Citation21]. Data for Confirm RxTM in pediatric patients showed a reduction of FP from 55% to 0 with introduction of newer algorithms in a small number of patients receiving SCRM for variable indications [Citation22]. A similar algorithm is used in other SCRMs [Citation23]. The BioMonitor IIITM AF algorithm works by detecting R-R interval variability by a programmable value. Additionally, this variability must occur for a programmable amount of time to identify a positive episode. BioMonitor IIITM also incorporates its own RhythmCheck technology to reject ectopic beats. Confirm RxTM also uses a similar AF algorithm in which 64 beat windows are evaluated for irregularity, R-R interval variance and if there is a sudden onset of initiation. Additionally, Confirm RxTM has incorporated SharpSense technology to use additional discriminators to verify the absence or presence of P waves. The verification of the absence of P waves then allows the device to store the episode. The Lux DxTM ICM also uses a 2-step algorithm in which 2-min windows analyze heart rate and R-R variability. A second step then applies additional criteria to ensure the episode is not a result of under or over sensing.
8. Data transmission
The transmission of data from the patient to the physician is accomplished via two approaches. In the first approach, the data is transmitted as alert notifications. An alert notification consists of a device determined abnormal rhythm and is transmitted to the web-based central server once a day at a particular time. The alert notification consists of a total count of all episodes in the preceding 24 hours and consists of a snapshot of the arrhythmia electrogram (30 seconds to 2 minutes, depending on the type of SCRM) at the onset of an episode from the longest episode. This approach is based on the assumption that the electrograms from the transmitted episode are representative of all the episodes. The second approach is the manual download of the data that is prompted by the patient via a home monitoring system or cellphone-based application. Often, these downloads are performed at scheduled intervals (every 31 or 90 days). During a manual transmission of data, all stored episodes from the device will be communicated to the central server, thus accessible by the device clinic.
8.1. Data transmission from non-blue tooth enabled SCRMs (LINQ ITM and BiomonitorTM)
The transmission of data from non-blue tooth enabled SCRMs involves multiple steps. The data for all episodes is stored in the device and transmitted via home monitor to the central web-based server, specific for each manufacturer. The device sends the data to the home monitor using RF technology at a fixed interval every night if the patient is in close proximity to the monitor. Multiple transmissions of the data from the device to the home monitoring system are sent to ensure that the data is transmitted. This nightly transmission sends only those episodes that have been programmed as an alert notification. Only a limited number of episodes are sent during alert transmission.
8.2. Data transmission from Bluetooth enabled devices (ConfirmRx, LINQ II, Lux-DM)
SCRMs with Bluetooth technology are capable of real-time data transmission from the patient to the device clinic and involve similar steps as described above with some distinct advantages. With Bluetooth technology, there is no need for a home monitoring system. The data is stored in the device and is transmitted to the central server each night at a fixed time. In the event if the patient is having an episode and wishes to transmit to the clinic, the patient must follow the prompts on the phone-based application and the data is transmitted to the central server without any delay. This means episodes can be detected in real time at practically any location rather than older technology where the patient must be in close proximity to their vendor provided home monitor for data transmission. For a scheduled transmission, the patient can initiate a complete download that consists of all the episodes stored in the device since the last complete download. The storage of episodes is prioritized based on pre-selected criteria. Device‐detected episodes are scheduled to transmit every night at fixed time. Theoretically, there are security concerns, however the phone-based applications act as a ‘pass through’ where information is merely transmitted and never saved on to the phone itself. No major security breaches in SCRM data have been reported with Bluetooth monitoring.
8.3. Patient triggered episodes
Each of the SCRM offers the ability for the patient to trigger episodes. Patient activated episodes have similar triggering mechanisms between the different vendors. The patient is given a remote monitor which then the patient places over the SCRM to record a triggered episode. Giving the patient the option to trigger episodes may be useful in certain clinical scenarios. For example, if the SCRM was placed for palpitations, patient activated episodes would be especially useful as the symptom-rhythm correlation is the gold standard for diagnosis. For other scenarios i.e. identification of AF following a cryptogenic stroke, allowing the patient to trigger episodes may lead to data deluge. In this author’s experience there can be pitfalls with giving the patient the option to trigger episodes. Certain patients may overuse the device and inappropriately trigger episodes. Given that each one of these episodes must be analyzed, patient triggered episodes may create a large amount of unnecessary workload for the managing clinic. Additionally,with limited storage capacity, patients who inappropriately overuse the activating option are at risk for causing the deletion of clinically important arrhythmias.
8.4. Suggested approaches for routine data transmission
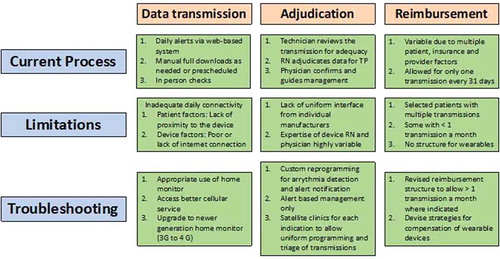
There are some limitations with each approach of data transmission. Some manufacturers have suggested that only alert notifications should be used for data transmission. This approach minimizes the resource utilization for adjudication of the data. However, intermittent connectivity of the device with the home monitoring system due to patient or internet factors may lead to truncation of data. Adequate patient education and surveillance for connectivity is helpful for data transmission using alerts only. Data transmission using scheduled download is associated with significant workload for the device clinic personnel. A hybrid approach using an alert-based system and as needed manual download may prove to be the optimal strategy for data transmission from the SCRM to the device clinic. In we summarize the current process of data transmission, adjudication and reimbursement.
9. Challenges during SCRM monitoring and troubleshooting
9.1. Intermittent connectivity
Poor cellular service or the patient not being in close proximity to the device can create challenges in the management of SCRMs. Even if the patient had more than 1 episode in the preceding 24 hours, only the longest episode is selected for the transmission of electrograms. Once the patient regains connection with the home monitoring system, the alert notification occurs for the episodes that happened in the preceding 24 hours. However, for the episodes that occurred during days when there was a lack of connection with the home monitoring system, a complete manual download will be required that contains all the missing data from days when nightly communication did not occur. This can create a significant workload on the interpreting electrophysiologist.
9.2. Frequent FP episodes
The high incidence of false positive (FP) episodes is an Achilles heel of ambulatory monitoring with SCRMs. Manufacturer sponsored studies have shown that implementation of new and improved algorithms lead to AF detections with high positive predictive value. However, these studies were based on implementing the algorithm on known rhythm datasets [Citation19], in populations with known AF [Citation24] or during bench testing [Citation25,Citation26] which do not allow the results of these studies to always be generalizable. Real world studies have shown that SCRMs have a high incidence of FP detections with some studies citing between a 46–86% FP rate depending on the indication for implantation [Citation3]. Furthermore, individual patients can generate large amounts of FP transmission. One study found that in 7% of patients, under sensing episodes triggered more than 100 FP transmission with one patient transmitting 3294 FP episodes [Citation27]. Not only can this be a source of significant resource utilization but may also lead to incorrect management in vulnerable patient if the episodes are not correctly adjudicated. Additionally, if an individual patient has a high volume of FP episodes, irrelevant episodes may overwrite the clinically significant episodes once the memory of the device reaches maximal storage capacity.
The reasons for FP are variable. Bradycardia and pause episodes can be caused by signal dropout from a sudden loss of the R-wave amplitude secondary to postural changes or a high BMI. For tachycardia and AF, atrial and ventricular ectopy, oversensing P and/or T waves and artifactual noise can result in FP episodes. Though different vendors use different algorithms, there are no head-to-head trials evaluating performance between different vendors. In this author’s institution, FP transmissions are similar among different vendors.
9.3. Data deluge
To minimize the data deluge from the SCRM transmission, various modifications in the diagnostics and device clinic workflow can be done. The first step is the custom programming at the time of implant. Educating the patient on the importance of being near the home monitoring device or cellphone at night can help with the issue of intermittent connectivity. Data deluge can also be reduced by recognizing patients with a high false-positive transmission rate and appropriately reprogramming their device, whether that be in the clinic or remotely. Providers should also consider turning off alerts for arrhythmias there is no clinical interest in (i.e. nocturnal bradycardia in patients with AF). Once a patient is diagnosed with an arrhythmia or the initial indication of the SCRM is fulfilled, the device can be programmed to turn off the detection and similarly, alert notifications can also be programmed off in order to reduce SCRM data deluge. outlines our suggested programming for alert settings in order to reduce data deluge.
10. Cost effectivness
The cost-effectiveness of SCRM’s is up for debate. Most of the literature about cost has been done in the evaluation of syncope, likely due to the high cost and diagnostic difficulty associated with syncope. A Cochrane review of two randomized control trials of SCRM’s vs conventional therapy in syncope, found costs were higher when including all patients with SCRM’s. However, if only patients with a diagnosis were analyzed, patients randomized to the SCRM group had lower cost than those with conventional care [Citation28]. Another prospective, randomized control trial concluded early SCRM implantation resulted in decreased costs as earlier diagnosis and higher diagnostic yield in the SCRM group led to fewer, advanced cardiac tests. However, it should be noted this study did not include the cost of the SCRM implantation itself [Citation29]. A separate similar trial did include costs of the SCRM implantation and found the initial high costs of implantation were offset by decreased advanced investigations and reduced hospitalization days resulting in a net savings when SCRM was implanted for syncope. It should be noted these studies were done in France and UK, respectively, where cost and reimbursement structures differ from those in the USA [Citation30]. Other studies have found similar results [Citation31]. Other studies looking at cost per quality-adjusted life (QALY) year have concluded SCRM implantation can be a cost-effective approach in infrequent syncope. These studies were also limited as they had wide range of cost per QALY based on sensitivity analysis and were not compared against diagnostic tools, such as Holter or event monitor [Citation32,Citation33]. For palpitations, one study found the cost effectiveness of continuous monitoring decreases significantly after 2 weeks, suggesting SCRM’s may not be cost-effective in this setting [Citation34]. Overall studies examining the cost-effectiveness of SCRM’s are difficult due to variable cost structures, differing definitions of ‘conventional care’ and differences in the patient populations studied. However, continued research in this area is warranted to optimize cost-effectiveness of SCRM’s for various indications.
11. Summary
The use of SCRMs is steadily increasing. The amount of data provided by these devices is significant and requires extensive resources from the device clinic and electrophysiologist for appropriate adjudication of the data. The incidence of FP episodes continues to be substantial and requires individualized device programming. The high incidence of FPs can be minimized by the introduction of indication-based programming at the time of device implantation and follow up. Understanding the indications, workflow and strategies of managing SCRM’s allow providers to yield the benefits of high diagnostic utility while minimizing the data deluge that can come with the implantation of SCRM’s.
12. Expert opinion
The SCRM is an effective tool in diagnosis and monitoring of arrhythmia. Indications for an SCRM include workup of cryptogenic stroke [Citation35], syncope [Citation36], palpitations [Citation37] and AF management. The SCRM is a small device, usually less than 5 cm3 and is typically implanted between the fourth and sixth intercostal space. While these devices provide great diagnostic utility, they can create a high workload for the clinics that manage them. This high resource burden comes from data deluge that comes in the form of false-positive transmissions, unnecessary scheduled downloads, intermittent connectivity, and nonactionable alerts.
Several barriers exist to optimizing the performance of SCRMs, however, we have strategies to overcome these barriers and maximize the use of this helpful diagnostic tool. False-positive transmissions have been recognized as a problem in recent years. At implantation, these devices have ‘out of the box’ or nominal programming. However, these nominal settings are at risk for transmitting high rates of false-positive transmissions. We recommend customizing the settings and tailoring them to each patient’s body habitus, clinical indication and R wave amplitude as doing this has been shown to reduce false-positive transmission rates. Additionally, new AF algorithms, such as the reveal LINQ have recently created a new 3 step-algorithm to optimize sensitivity for AF detection.
Another part of data deluge comes from the transmission of non-actionable events, i.e. receiving a transmission for nocturnal bradycardia for a patient with SCRM placed for AF monitoring. We recommend assessing and recognizing these types of situations and programming transmissions for peer review only non-actionable arrhythmias off. Data transmission from the SCRM comes in two forms, alert notifications and scheduled downloads. Alert notifications occur only when an arrhythmia is detected. Scheduled downloads occur when the patient follows a series of instructions to send all of the episodes from the home monitor or phone app to a central server to be adjudicated by a physician. While alert notifications create less transmission, problems with connectivity can cause a truncation of the data collected. On the other hand, scheduled downloads can create a heavy burden on the adjudicator due to unnecessary data acquisition. We suggest using a hybrid strategy in obtaining transmissions as alert notifications and scheduled downloads and tailor it to a patient’s own clinical situation. Ideally, SCRM technology will continue to improve as to minimize false-positive detections and reduced data deluge through creating improved detection algorithms as well as educating providers on how to troubleshoot devices and customize settings.
One future consideration pertains to the growing options of wearable devices for monitoring of AF. The Apple Heart Study recently published data suggesting the Apple Watch was a feasible method to detect silent AF [Citation38]. Technology such as Kardia, Apple Watch, FitBit all offer a less invasive option for rhythm monitoring; however, they have significant limitations when compared to SCRMs. While they do offer prolonged monitoring when compared to Holter monitors, these wearable devices provide only intermittent monitoring as opposed to SCRM’s that provide continuous, prolonged rhythm monitoring. While these wearable devices may have a role in outpatient rhythm monitoring in the future, as of now this technology is relatively new and the clinical accuracy of these devices is yet to be confirmed.
Article highlights
SCRM is the most reliable strategy for prolonged ambulatory rhythm monitoring and is indicated for surveillance of known arrhythmias such as atrial fibrillation (AF) or identification of infrequent arrhythmias such as tachyarrhythmia and brady-arrythmias in patients with a risk of sudden cardiac death.
Typically, the device is implanted in an oblique angle in the left parasternal area. Angle of implantation does not correlate with the amplitude of R waves.
An oblique angle in female patients is associated with increased risk of premature device explantation.
Proper patient education regarding proximity to the home monitor and availability of adequate internet service is critical for successful data transmission.
Custom programming at the time of device implantation should incorporate extension of arrhythmia episode duration to clinically acceptable maximum and turning off clinically irrelevant features.
Custom programming of SCRMs decreases incidence of false positive (FP) episodes and resource utilization for data adjudication.
Alert notifications should be turned on for clinically actionable arrhythmias only, specific to indication of SCRM implantation.
In the event of a high incidence of false-positive transmissions, targeted SCRM reprogramming can be done to minimize these transmissions.
After the clinical indication of SCRM is met, the device can be reprogrammed from diagnostic to surveillance mode to minimize data deluge.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Bisignani A, De Bonis S, Mancuso L, et al. Implantable loop recorder in clinical practice. J Arrhythm. 2019 (1880-4276 (Print));35:25–32.
- Lee R, Mittal S. Utility and limitations of long-term monitoring of atrial fibrillation using an implantable loop recorder. Heart Rhythm. 2018;15(2):287–295.
- Afzal MR, Mease J, Koppert T, et al. Incidence of false-positive transmissions during remote rhythm monitoring with implantable loop recorders. Heart Rhythm. 2020;17(1):75–80.
- Afzal MR, Nadkarni A, Niemet L, et al. Resource use and economic implications of remote monitoring with subcutaneous cardiac rhythm monitors. Clin Electrophysiol. 2021. DOI:10.1016/j.jacep.2020.10.014.
- Afzal MR, Casmer A, Buck B, et al. Incidence and risk factors for early explantation of subcutaneous cardiac rhythm monitors. Clin Electrophysiol. 2020;6(14):1858–1860.
- Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883–1948.
- Drak-Hernández Y, Toquero-Ramos J, Fernández JM, et al. Effectiveness and safety of remote monitoring of patients with an implantable loop recorder. Revista Española De Cardiología (English Edition). 2013;66(12):943–948.
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study: comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol. 2007;49(19):1951–1956.
- Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486.
- Oudeman M, Tjon A, Huijgen J, et al. A new approach to determine the results of minimally invasive pulmonary vein isolation using a continuous loop monitor: preliminary results. Eur J Cardiothorac Surg. 2015;48(6):855–860.
- Dekker LR, Pokushalov E, Sanders P, et al. Continuous cardiac monitoring around atrial fibrillation ablation: insights on clinical classifications and end points. Pacing Clin Electrophysiol. 2016;39(8):805–813.
- Steinberg JS, Shah Y, Bhatt A, et al. Focal impulse and rotor modulation: acute procedural observations and extended clinical follow-up. Heart Rhythm. 2017;14(2):192–197.
- Kapa S, Epstein AE, Callans DJ, et al. Assessing arrhythmia burden after catheter ablation of atrial fibrillation using an implantable loop recorder: the ABACUS study. J Cardiovasc Electrophysiol. 2013;24(8):875–881.
- Korada SKC, Buck B, Koppert T, et al. Implantable loop recorder insertion angle is not associated with sensed R-wave amplitude. Clin Electrophysiol. 2020;6(9):1185–1186.
- Lauschke J, Busch M, Haverkamp W, et al. New implantable cardiac monitor with three-lead ECG and active noise detection. Herz. 2017;42(6):585–592.
- Steffel J, Wright DJ, Schäfer H, et al. Insertion of miniaturized cardiac monitors outside the catheter operating room: experience and practical advice. Ep Europace. 2017;19(10):1624–1629.
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Ep Europace. 2018;20(1):e1–e160.
- Pürerfellner H, Sanders P, Pokushalov E, et al. Miniaturized Reveal LINQ insertable cardiac monitoring system: first-in-human experience. Heart Rhythm. 2015;12(6):1113–1119.
- Pürerfellner H, Sanders P, Sarkar S, et al. Adapting detection sensitivity based on evidence of irregular sinus arrhythmia to improve atrial fibrillation detection in insertable cardiac monitors. EP Europace. 2018;20(FI_3):f321–f8.
- Verma A, Champagne J, Sapp J, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med. 2013;173(2):149–156.
- Hindricks G, Pokushalov E, Urban L, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010;3(2):141–147.
- Yoon JG, Fares M, Hoyt W, et al. Diagnostic accuracy and safety of confirm Rx™ insertable cardiac monitor in pediatric patients. Pediatr Cardiol. 2021 Jan;42(1):142-147.
- Ciconte G, Saviano M, Giannelli L, et al. Atrial fibrillation detection using a novel three-vector cardiac implantable monitor: the atrial fibrillation detect study. EP Europace. 2017;19(7):1101–1108.
- Sanders P, Pürerfellner H, Pokushalov E, et al. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: results from the Reveal LINQ Usability Study. Heart Rhythm. 2016;13(7):1425–1430.
- Mariani J, Lovibond S, Gould P, et al. The BIO|CONCEPT.BIOMONITOR III study: sensing performance and Home Monitoring transmission success of a new miniaturized implantable cardiac monitor. Asia Pacific Heart Rhythm Society Scientific Session Bangkok; Thailand; 2019.
- Richards M, Perschbacher D, Saha S. A novel algorithm improves detection of arrythmias with regular R-R intervals. San Francisco, CA: Heart Rhythm Society; 2019.
- Maines M, Zorzi A, Tomasi G, et al. Clinical impact, safety, and accuracy of the remotely monitored implantable loop recorder Medtronic Reveal LINQTM. Ep Europace. 2018;20(6):1050–1057.
- Solbiati M, Costantino G, Casazza G, et al. Implantable loop recorder versus conventional diagnostic workup for unexplained recurrent syncope. Cochrane Database Syst Rev. 2016;(4). DOI:10.1002/14651858.CD011637.pub2
- Podoleanu C, DaCosta A, Defaye P, et al. Early use of an implantable loop recorder in syncope evaluation: a randomized study in the context of the French healthcare system (FRESH study). Arch Cardiovasc Dis. 2014;107(10):546–552.
- Farwell DJ, Freemantle N, Sulke N. The clinical impact of implantable loop recorders in patients with syncope. Eur Heart J. 2006;27(3):351–356.
- Providência R, Candeias R, Morais C, et al. Financial impact of adopting implantable loop recorder diagnostic for unexplained syncope compared with conventional diagnostic pathway in Portugal. BMC Cardiovasc Disord. 2014;14(1):1–11.
- Davis S, Westby M, Pitcher D, et al. Implantable loop recorders are cost-effective when used to investigate transient loss of consciousness which is either suspected to be arrhythmic or remains unexplained. Europace. 2012;14(3):402–409.
- Implantable loop recorder for unexplained syncope—Assessment Report. In: Medical Services Advisory Committee DoHaA, editor. Australia: Australian Government; 2003.
- Zimetbaum PJ, Kim KY, Josephson ME, et al. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med. 1998;128(11):890–895.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132.
- Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70(5):e39–e110.
- Brignole M, Vardas P, Hoffman E, et al. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace. 2009;11(5):671–687.
- Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917.