ABSTRACT
Introduction
Abnormal postpartum uterine bleeding occurs commonly after birth and can quickly become an obstetric emergency. With postpartum hemorrhage representing the leading cause of maternal mortality, accounting for 25% of maternal deaths due to obstetric complications, there is a critical need for effective and easy to use treatment options.
Areas covered
This profile describes the Jada System, a novel intrauterine vacuum-induced hemorrhage control device that provides a rapid and effective treatment option for abnormal postpartum uterine bleeding and postpartum hemorrhage. In addition to explaining the mechanism of action of vacuum-induced hemorrhage control and reviewing the device’s safety and effectiveness, this profile elucidates how the Jada System compares to currently available medications and devices for treatment of this obstetric emergency.
Expert opinion
New therapies to address this life-threatening condition are needed to reduce the risk of maternal mortality and severe maternal morbidity. Data demonstrate that the Jada System provides rapid, effective control of abnormal postpartum uterine bleeding and postpartum hemorrhage, while offering reported ease of use and short treatment duration. These results suggest that use of the Jada System in treatment algorithms may improve outcomes.
1. Introduction
Postpartum hemorrhage (PPH) accounts for 25% of maternal deaths related to obstetric causes, rendering it the leading cause of maternal mortality [Citation1]. Postpartum hemorrhage has recently been formally defined as blood loss greater than 1000 ml. However, both ReVITALize and ACOG highlight that a blood loss of 500–999 mL is abnormal and should trigger increased supervision and potential interventions as clinically indicated [Citation2,Citation3]. Large state-wide perinatal quality collaboratives continue to cite blood loss between 500 and 999 mL as abnormal and care teams often initiate treatment in this range to minimize ongoing blood loss [Citation4]. Many factors may contribute to abnormal postpartum uterine bleeding or PPH; however, uterine atony is the most prevalent cause, responsible for 80% of postpartum hemorrhages and occurring in approximately one in 40 births in the United States [Citation5,Citation6]. Obstetric hemorrhage continues to be associated with a large proportion of maternal morbidity and mortality, thus novel treatment options represent a significant unmet need [Citation7].
Uterine atony occurs when the interlacing myometrial muscle fibers of the uterus fail to contract after placental delivery [Citation8]. This uterine contraction is a natural physiological process that controls bleeding by constricting the vasculature of the uterus [Citation6]. If abnormal postpartum uterine bleeding occurs, initial conservative management including uterine massage and administration of therapeutic oxytocin is initiated. If these interventions are determined to be unsuccessful in regaining uterine tone and controlling uterine bleeding, additional treatments are implemented. These include additional conservative methods such as therapeutic doses of uterotonic medications.
If these medications do not adequately control abnormal postpartum uterine bleeding or cannot be used in a patient due to contraindications, additional interventions are employed. For uterine atony, the next available intervention is often the insertion of a uterine balloon tamponade (UBT) device. Uterine balloon tamponade devices apply pressure directly to the vasculature by expanding against the inner walls of the uterus for 12 to 24 hours. If the abnormal postpartum uterine bleeding continues, more aggressive and increasingly invasive procedures may be indicated, including surgical interventions such as arterial ligation, compression sutures and hysterectomy.
Recent efforts focused on reducing the rate of maternal mortality and morbidity related to obstetric causes have been developed and implemented. These efforts include patient safety bundles designed to facilitate earlier identification and treatment of abnormal postpartum uterine bleeding and PPH. As development and implementation of these bundles continue, it is imperative that the literature on current treatment options is evaluated to ensure the safety and effectiveness of these interventions. Furthermore, it is critical to consider new treatments that can be adopted into current systems and clinical decision-making paradigms.
The focus of this review is on the Jada® System (Alydia Health®, Menlo Park, CA, USA), a novel vacuum-induced hemorrhage control (VHC) device that offers a safe, and effective option for treating abnormal postpartum uterine bleeding or PPH. The Jada System is a recently FDA-cleared device that utilizes low-level vacuum to rapidly control abnormal postpartum uterine bleeding and PPH. This review will highlight the mechanism of action, effectiveness, and safety of the device as well as discuss future research directions and opportunities to incorporate this device into PPH management algorithms.
2. Overview of the market
Abnormal postpartum uterine bleeding and PPH occur in approximately one in 40 births in the United States [Citation5,Citation6]. These serious conditions can be life-threatening and require expeditious, effective treatment. The Jada System offers a rapid, conservative treatment for abnormal postpartum uterine bleeding or PPH with the potential to cease hemorrhage. The device may be used in conjunction with uterotonics or as an alternative when uterotonic use is contraindicated in common medical conditions, such as hypertension and asthma. The Jada System applies the same principles as the relied-upon uterotonics with the goal of returning an atonic uterus to a contracted state, thereby effectively treating abnormal postpartum uterine bleeding or PPH.
3. Introduction to the device
3.1. How the device works
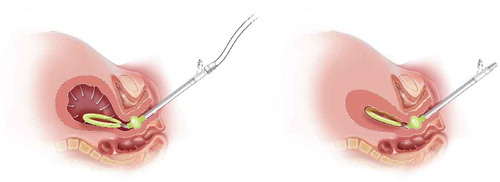
The Jada System is an intrauterine device that utilizes low-level vacuum (i.e. 80 mm Hg ± 10 mm Hg) to encourage the natural forces of uterine contraction to control abnormal postpartum uterine bleeding or PPH after childbirth. The VHC device is made of soft medical-grade silicone and consists of an elliptical intrauterine loop on the distal end of a tube. The intrauterine loop is lined with 20 vacuum pores that are 4 mm in diameter, oriented inward along the loop. A soft shield covers the outside of the loop to protect uterine tissue from the vacuum and is designed to prevent occlusion of the pores located on the inner surface of the loop. These pores enable the application of vacuum to collapse the uterus and evacuate any pooled blood from within the uterus. Between the intrauterine loop and the tube is the cervical seal, which limits vacuum to only the uterine cavity when placed at the external cervical os and filled with sterile fluid. The proximal end of the device tube connects to sterile vacuum tubing with an inline canister and a regulated vacuum source (typically wall suction or a transportable regulated vacuum source; see ).
After vaginal birth, manual sweep of the uterine cavity is performed prior to device placement to remove any clots and ensure there are no retained products of conception. After vaginal or cesarean birth (once the hysterotomy is closed), the device is placed transvaginally. Notably, the device requires 3 cm cervical dilation at a minimum for transcervical placement. Once in place, the cervical seal is filled with 60–120 mL sterile fluid to ensure seal for vacuum. Prior to connecting to the device, the vacuum is set to 80 mm Hg (±10 mm Hg). Once in place and connected, the provider can monitor uterine collapse either through transabdominal fundal palpation after a vaginal birth or after the abdomen is closed post cesarean or through direct observation at cesarean if the abdomen is still open. The evacuated blood is observed and quantified as it passes through the tubing into a graduated canister. The intrauterine device remains in place for 1.5 hours at minimum (24 hours maximum), and the therapy time is up to the discretion of the provider and should consider patient status including vital signs, uterine tone, and cumulative blood loss.
The Jada System offers a unique mechanism of action that utilizes vacuum to stimulate the natural physiological response of postpartum uterine contraction. After most births, constriction of the uterine vasculature occurs when contraction of the interlacing muscle fibers of the myometrium control bleeding after placental delivery [Citation8,Citation9]. In an atonic uterus, vessels are not constricted, and abnormal postpartum uterine bleeding ensues. The Jada System applies low-level intrauterine vacuum to facilitate the uterine contraction to constrict myometrial blood vessels.
3.2. Safety and complications
As a minimally invasive treatment for abnormal postpartum uterine bleeding or PPH, the Jada System poses a low risk for adverse events or complications. The pivotal trial of the Jada System (the PEARLE Study) evaluated device effectiveness and safety, and the results supported the regulatory submission for marketing authorization by the U.S. FDA. In the trial, eight adverse events were reported as possibly related to the device or procedure, all of which were anticipated with the introduction of an intrauterine device. These events included endometritis (n = 4), presumed endometritis (n = 1), disruption of vaginal laceration repair (n = 1), bacterial vaginosis (n = 1), and vaginal candidiasis (n = 1) [Citation10]. Additionally, there were no reports in the trial of uterine perforation or lower genital tract tissue injury in relation to the device or procedure. Inherent characteristics of the Jada System design offer additional safety measures. The soft silicone loop mitigates the potential for tissue trauma during insertion. When vacuum is applied, the uterus is palpated to assess for initial collapse, and fundal checks are performed regularly, allowing for uninterrupted monitoring of contraction. Furthermore, blood evacuation is visible in the tubing and canister which allows for continuous monitoring of initial evacuation and effectiveness of therapy or any ongoing uterine bleeding, should it occur. With immediate visible and palpable feedback during the procedure, decision-making is well informed.
3.3. Contraindications
The Jada System is appropriate to provide control and treatment of abnormal postpartum uterine bleeding or hemorrhage attributable to uterine atony when conservative management is warranted. The Jada System should not be used with the following conditions: ongoing intrauterine pregnancy, untreated uterine rupture, unresolved uterine inversion, current cervical cancer, known uterine anomaly, or current purulent infection of the vagina, cervix, or uterus. Additionally, use should be avoided in patients where the cervix is unable to be dilated to 3 cm, since that minimum dilation is required for safe placement of the intrauterine device. The Jada System has not been evaluated for safety and effectiveness in uteri less than 34 gestational weeks in size, with placenta accreta, or in patients with a diagnosis of coagulopathy. In the setting of obstetric hemorrhage, treatment with the Jada System is not a substitute for aggressive resuscitation, blood transfusion or escalation to surgical management when indicated. When there is a concern for clinical deterioration or ongoing bleeding, prompt reassessment, and more aggressive treatments may be indicated.
4. Clinical profile
4.1. Data from Pre-FDA Clearance studies
Safety and effectiveness of the Jada System have been reported in two studies and an unpublished case series. A First-in-Human (FIH) feasibility study with Ethics Committee oversight was conducted in Indonesia. The purpose of the study was to demonstrate the placement, function, and operation of the Jada System. Ten participants were enrolled between July 2014 and February 2015, with estimated blood loss following vaginal delivery in the range of 500–1000 mL. None of the participants presented with retained placenta, uterine lacerations, uterine scarring, or conditions other than atony-related postpartum hemorrhage. The Jada device was successfully placed in all 10 participants with a vacuum pressure of 70 mm Hg – 90 mm Hg used for treatment. The average time from the placement of the Jada device to removal was 152.0 ± 111.7 minutes (range 60–390 minutes). Bleeding was controlled within 2 min for all 10 participants. None of the participants experienced a device-related adverse event. Evaluation of the primary clinical safety endpoints determined that: (1) no safety issues were observed relative to the placement, insertion, or removal of the Jada device, (2) there was no damage to the uterus, cervix, or vagina, and (3) no uterine inversion or folding events were observed during the Jada procedure [Citation11].
As described in the manufacturer’s Instructions for Use (IFU) [Citation12], a case series conducted outside the United States (Kampala, Uganda) described controlled PPH in all 13 enrolled participants. There were no adverse events in the case series designated as definitely related to the Jada System. However, the IFU notes that three enrolled participants experienced EBL at time of study entry that were beyond inclusion criteria with subsequent death of two of these participants following control of abnormal bleeding due to the lack of blood product to compensate for the severe blood loss.
A pivotal study was conducted to collect evidence to support marketing authorization from the U.S. Food and Drug Administration (FDA). The safety and effectiveness of the Jada System was evaluated in the PEARLE study (Prospective, Single Arm, Pivotal Clinical Trial Designed to Assess the Safety and Effectiveness of the Jada System In Treating Primary Postpartum Hemorrhage ‘PPH’) under an approved Investigational Device Exemption (IDE) from FDA. PEARLE was a prospective, single-arm, multi-center treatment study in which each of the 107 participants enrolled at 12 U.S. investigational sites were treated with the Jada System. The primary effectiveness endpoint was the proportion of participants in whom use of the Jada System controlled abnormal postpartum uterine bleeding or PPH without requiring escalating interventions. The primary endpoint was overwhelmingly met in the Intent-To-Treat (ITT) cohort (N = 106), with 94.3% success (with a lower bound 95% confidence limit of 88.1%). In the Per Protocol cohort, there was 99% success (with a lower bound 95% confidence limit of 94.4%). The study showed rapid cessation of bleeding (median time of 3 minutes), short treatment duration (median time of 3.2 hours), and 98% of the first-time users of the device rated it easy to use [Citation7].
4.2. Alternative devices
Until recently, the available treatment option prior to a surgical approach was a UBT device. UBT’s mechanism of action to control bleeding is prolonged outward pressure against the endometrium and inner myometrium. A recent meta-analysis, including over 4500 participants in 91 studies, reported a UBT success rate of 85.9% [Citation13]. Tamponade treatment with a UBT device generally requires indwelling time of 12–24 h and therefore requires prolonged monitoring. Furthermore, there may be concealed bleeding with the UBT in place. Reported associated risks of the UBT device include device expulsion or displacement, tears to the cervix or vaginal lacerations, uterine perforation, and infection [Citation13–15]. Several balloon tamponade designs exist with Bakri Balloon being the most commonly used. It is a silicone balloon that is inserted and filled with up to 500 cc of sterile saline and left in place for twelve to 24 h. Other tamponade devices include the Ellavi, Ebb balloon, BT-Cath, ESM-UBT, improvised condom catheters and Foley catheters.
A newer version of tamponade undergoing early clinical testing is an adapted trauma dressing. The mini-sponge tamponade device, XSTAT, contains compressed mini-sponges within a mesh pouch and a tubular applicator. The compressed sponges expand while absorbing blood and exerting uniform pressure on bleeding sites. The device’s sponges conform to the shape of the uterine cavity and apply pressure for up to 24 h. Originally developed for combat settings, this device was redesigned with an obstetric applicator designed to be deployed vaginally, with the tip placed transcervically in the lower uterine segment [Citation16].
If the non-surgical methods described fail to control the abnormal postpartum uterine bleeding or PPH, surgical interventions become necessary. Definitive surgical treatment is often a hysterectomy, which presents additional serious risks to the patient including surgical morbidity and irreversible implications to fertility.
4.3. Regulatory status
The Jada System received marketing authorization by the U.S. FDA via the 510(k) process in August 2020. Jada is now marketed in the United States.
5. Conclusion
Postpartum hemorrhage is a leading cause of maternal morbidity and mortality. When medical management is insufficient or contraindicated and treatment escalation is indicated, clinicians will often use an intrauterine device as the next line of therapy. The novel Jada System, an intrauterine vacuum-induced hemorrhage control device, works quickly to collapse and contract the uterus to treat abnormal postpartum uterine bleeding and PPH. This safe and effective device may be most impactful if used early in PPH treatment algorithms.
6. Expert opinion
Efforts targeting timely recognition and prompt intervention will be critical to reduce maternal mortality and severe maternal morbidity associated with PPH. To date we have a finite number of options for treating this life-threatening emergency, many options are not desirable for use in early stages of bleeding, and not all options are safe for all patients. In this context, any new therapy deserves careful and timely consideration for potential incorporation into stage-based management algorithms. Clinical evidence demonstrates that the Jada System is safe with rapid and effective results. The ease of use and short duration of treatment may lead to inclusion of the Jada System earlier in hemorrhage treatment algorithms than where previously available device options were employed. In fact, some have suggested that the Jada System may become the next intervention in patients unresponsive to oxytocin alone [Citation17], and certainly for patients in whom only a limited number of uterotonics are safe due to comorbidities, the Jada System provides a much-needed option for conservative management of uterine atony. As with any new technology, additional controlled studies and comparative research are warranted to help define the optimal role for the Jada System in treating abnormal postpartum uterine bleeding and PPH as we strive to decrease maternal morbidity and improve maternal outcomes.
Article highlights
Postpartum hemorrhage is the leading cause of maternal mortality and severe maternal morbidity, and there is a dire need for safe and effective interventions to improve maternal outcomes.
The Jada System is a novel vacuum-induced hemorrhage control device recently cleared by the FDA to offer rapid, effective treatment of abnormal postpartum uterine bleeding and postpartum hemorrhage.
Clinical data demonstrate that the Jada System is safe, effective, and easy to use, supporting its use as a promising addition to the armamentarium for managing abnormal postpartum uterine bleeding and postpartum hemorrhage.
Declaration of interest
D. Goffman’s institution received support for participation in the pivotal trial of Jada; D. Goffman participated in a PPH education presentation supported by Haymarket Education 3/2021; participated in Cooper Surgical Obstetrical Safety Council; and was a speaker for Laborie on PPH 5/2021. K. Rood’s institution received support for participation in the pivotal trial of Jada; K. Rood participated in a PPH education presentation supported by Haymarket Education 3/2021; and provided consultation to Alydia 10/2020-3/2021. H. Simhan’s institution received support for participation in the pivotal trial of Jada. H. Simhan was the founder of Naima Health, LLC. M. D’Alton’s institution received support for participation in the pivotal trial of Jada. M D’Alton has had a leadership role in ACOG II’s Safe Motherhood Initiative, which has received unrestricted funding from Merck for Mothers and serves on the board of March for Moms. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer is a co-inventor of the PPH Butterfly and received royalties from it. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Gülmezoglu AM, Lawrie TA, Hezelgrave N, et al. Interventions to reduce maternal and newborn morbidity and mortality. In: Black RE, Laxminarayan R, Temmerman M, et al. editors. Reproductive, maternal, newborn, and child health: disease control priorities. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/The World Bank; 2016.
- American College of Obstetricians and Gynecologists, Safe Motherhood Initiative. Obstetric hemorrhage checklist. [cited 2021 Jul 19]. Available from: https://www.acog.org/-/media/project/acog/acogorg/files/forms/districts/smi-ob-hemorrhage-bundle-hemorrhagechecklist.pdf
- California Maternal Quality Care Collaborative. OB hemorrhage toolkit v 2.0. [cited 2021 Jul 19]. Available from: https://www.cmqcc.org/resources-tool-kits/toolkits/ob-hemorrhage-toolkit
- Goffman D, Friedman AM, Sheen JJ, et al. A framework for improving characterization of obstetric hemorrhage using informatics data. Obstet Gynecol. 2019;134:1317–1325.
- Callaghan WM, Kuklina EV, Berg CV. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol. 2010;202:353.e1–6.
- Reale SC, Easter SR, Xu X, et al. Trends in postpartum hemorrhage in the United States from 2010 to2014. Anesth Analg. 2020;130:e119.
- Bingham D, Jones R. Maternal death from obstetric hemorrhage. J Obstet Gynecol Neonatal Nurs. 2012;41:531–539.
- Mulic-Lutvica A, Bekuretsion M, Bakos O, et al. Ultrasonic evaluation of the uterus and uterine cavity after normal, vaginal delivery: ultrasound in puerperium. Ultrasound Obstetrics Gynecol. 2001 November;18(5):491–498.
- Baskett TF. A flux of the reds: evolution of active management of the third stage of labour. J R Soc Med. 2000;93:489–493.
- D’Alton ME, Rood K, Smid MC, et al. Intrauterine vacuum-induced hemorrhage-control device for rapid treatment of postpartum hemorrhage. Obstetrics Gynecol. 2020 November;136(5):882–891.
- Purwosunu Y, Sarkoen W, Arulkumaran S, et al. Control of postpartum hemorrhage using vacuum-induced uterine tamponade. Obstetrics Gynecol. 2016 July;128(1):33–36.
- Jada System Instructions for Use. Alydia health. [ cited 2021 July 19]. Available from: https://www.alydiahealth.com/ifu
- Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020 April;222(4):293.e1–293.e52.
- Grange J, Chatellier M, Chevé M-T, et al. Predictors of failed intrauterine balloon tamponade for persistent postpartum hemorrhage after vaginal delivery. PLOS ONE. 2018 October 26;13(10):e0206663. Edited by David N. Hackney. https://doi.org/10.1371/journal.pone.0206663
- Rani PR. Recent advances in the management of major postpartum haemorrhage - a review. J Clin Diagn Res. 2017. DOI:https://doi.org/10.7860/JCDR/2017/22659.9463
- Rodriguez MI, Jensen JT, Gregory K, et al. A novel tamponade agent for management of postpartum hemorrhage: adaptation of the Xstat mini-sponge applicator for obstetric use. BMC Pregnancy Childbirth. 2017 Jun 13;17(1):187. PMID: 28610569; PMCID: PMC5470216.
- Belfort MA. Postpartum hemorrhage: medical and minimally invasive management. [ cited 2021, Jan 21]. Available from: https://www.uptodate.com/contents/postpartum-hemorrhage-medical-and-minimally-invasive-management