ABSTRACT
Background
Telemonitoring during the perioperative trajectory may improve patient outcomes and self-management. The aim of this study is to assess the feasibility of and patient’s experiences with telemonitoring before and after major abdominal surgery to inform future study design.
Methods
Patients planned for elective major abdominal surgery wore a sensor and answered well-being questions on a tablet daily for at least 2 weeks preoperatively up to 30-days postoperatively. Feasibility was assessed by participation and completion rate, compliance per day, weekly satisfaction scores, and reasons for nonscheduled contact.
Results
Twenty-three patients were included (participation rate of 54.5%) with a completion rate of 69.6%. Median compliance with the wearable sensor and well-being questions was respectively: 94.7% and 83.3% preoperatively at home; 100% and 66.7% postoperatively in-hospital; and 95.4% and 85.8% postoperatively at home. Median weekly satisfaction scores for both wearing the sensor and well-being questions were 5 (IQR, 4–5). Contact moments were related to absence of sensor data and technological issues (76.0%) or patient discomfort and insecurity (24.0%).
Conclusions
In this study, telemonitoring showed high satisfaction and compliance during the perioperative trajectory. Future trial design regarding the effectiveness of telemonitoring requires embedding in clinical practice and support for patients, relatives, and healthcare personnel.
1. Introduction
In recent years, interest in telemonitoring before or, mainly, after major abdominal surgery has increased with the aim to improve postoperative outcomes or support self-management of patients. For these purposes, several wearable sensors and (mobile) applications are available to monitor physical and mental parameters at home or in-hospital [Citation1–3]. Despite this, perioperative telemonitoring is currently still barely used in clinical practice. Evidence for the effectiveness of perioperative telemonitoring in major abdominal surgery is scarce, probably due to limitations of the used technology or methodology in these studies [Citation4,Citation5].
In development and evaluation of telemedicine services, it is important that the evaluation method chosen matches the state of the technology development [Citation6]. Most studies on perioperative telemonitoring are still in the feasibility phase: telemonitoring is hereby used as a standalone service and endpoints focus on its feasibility and usability [Citation6]. To work toward consequent study designs for effectiveness, not only the technology development is of importance but also how the telemonitoring will be implemented and used by its stakeholders. For example, compliance of patients to treatment is related to clinical outcomes in chronic diseases [Citation7]. However, actual use and patient’s experiences are hardly evaluated for perioperative telemonitoring services [Citation8,Citation9] but only by future intention-to-use of patients, which does not match actual usage behavior [Citation10,Citation11]. Additionally, evaluation afterward causes non-response due to patient dropout [Citation12,Citation13] or recall bias for patients.
Therefore, the aim of this study is to assess the feasibility of and patient’s experiences with telemonitoring during the perioperative trajectory of patients undergoing major abdominal surgery. Patients wore a wearable sensor and answered questions using a mobile app daily for at least 2 weeks before surgery up to 30-days after surgery both at home and in-hospital. This study addresses benefits and barriers and intends to provide recommendations on trial design of studies that may also focus on clinical- and cost-effectiveness of perioperative telemonitoring.
2. Patients and methods
2.1. Design
A single-center prospective observational cohort pilot study was performed at the University Medical Center Groningen (UMCG) between January 2020 and January 2021. The protocol was approved by the Ethical Committee of the UMCG (PROMISE-study, research register number #201900432), and this study was executed in accordance with the STROBE guidelines [Citation14] and the Declaration of Helsinki. The aim was to include 20 patients, and this number was supplemented if patients dropped out before surgery. Clinical data from study measurements are reported separately [not published yet].
2.2. Participants
Patients of 18 years or older were included if they were planned for elective open abdominal surgery; were expected to be at least 2 weeks on the waiting list; and had access to WiFi at home. Patients were excluded if they were mentally incapable of participation; could not mobilize without aids; or were unable to wear wearable sensors. Patients were informed about this study after they were planned for open abdominal surgery at the outpatient clinic and were asked for informed consent one week later.
2.3. Outcome measures
Outcome measures for feasibility were participation rate, completion rate, compliance, weekly satisfaction, and number of and reasons for contact moments between researcher and patient or representative.
Patient’s experiences comprised overall satisfaction, intention-to-use and intention–to-recommend-to-others, and positive and negative experiences, and future expectations of perioperative telemonitoring.
2.4. Study protocol
illustrates the study protocol in relation to the perioperative trajectory. After a patient gave informed consent, the researcher visited the patient at home with the telemonitoring devices and instructions. From this moment patients received telemonitoring until 30 days after surgery or earlier if a patient decided to or needed to stop (e.g. due to severe postoperative complications). Telemonitoring consisted of wearable sensor measurements and daily questions about well-being (‘experience sampling’), as explained below.
Figure 1. The study protocol in relation to the perioperative trajectory, including the elements of telemonitoring: (a) a wearable sensor (Everion®, Biovotion AG, Zürich, Switzerland), and (b) experience sampling (Activity Coach, Roessingh Research and Development, Enschede, The Netherlands [Citation15]).
![Figure 1. The study protocol in relation to the perioperative trajectory, including the elements of telemonitoring: (a) a wearable sensor (Everion®, Biovotion AG, Zürich, Switzerland), and (b) experience sampling (Activity Coach, Roessingh Research and Development, Enschede, The Netherlands [Citation15]).](/cms/asset/0474c22b-a86a-4f02-aa07-8527f419f6d8/ierd_a_2108703_f0001_oc.jpg)
Patients wore the Everion® biosensor (Biovotion, now Biofourmis AG, Zürich, Switzerland), a CE class IIa-certified wearable sensor for monitoring vital signs and physical activity, on their upper arm, as depicted in ). Patients were instructed to wear the sensor during the day and charge it during the night, and nurses also received these instructions at the surgical ward. The Healthy Chronos app and platform (Healthy Chronos, Alphen a/d Rijn, the Netherlands) were used to transfer data from the wearable sensor to a tablet (Samsung Galaxy Tab A 10.1 2019) through Bluetooth, and to the database using WiFi. Without an active WiFi connection, measured data were saved locally at the sensor for up to 5 days. Patients and researchers (MH, RM) checked whether data was transmitted to the Healthy Chronos app and platform on a daily basis. If data from the wearable sensor was not transmitted to the platform for 2 days or more, the researcher contacted the patients to solve potential technical problems.
Experience sampling was performed through a mobile app (Activity Coach) on the same tablet, running on the RRD-eHealth platform (Roessingh Research and Development, Enschede, The Netherlands [Citation15]). Twice a day, at one random moment between 9:00 AM and 1:00 PM and at 8:00 PM, patients received a notification to answer short questions about well-being (pain, fear, nausea, and fatigue) on a 0–10 Visual Analogue Scale (VAS), as illustrated in ). Patients could also choose to receive notifications via SMS and answer the questions via the internet browser on their smartphone. Researchers did not check data transmission for experience sampling during the study period and did not remind patients to respond nor were nurses at the surgical ward instructed to do so.
Patients were asked to answer the Customer Satisfaction Score on a 5-point Likert scale (very unsatisfied to very satisfied) for wearing the sensor and experience sampling in the app weekly. Researchers contacted the patient as part of the study protocol before hospital admission to remind the patient to bring the devices to the hospital, and at 30 days after surgery to confirm study completion and to make an appointment to return the devices. At return of the devices, one of the researchers conducted a structured final evaluation questionnaire on paper or digitally including both closed and open questions regarding patient’s experiences with the telemonitoring. The final evaluation questionnaire consisted of the following questions: (1) overall satisfaction (scale 0 to 10); (2) intention-to-use and intention-to-recommend-to-others according to the System Usability Scale (strongly agree to strongly disagree); and (3) open questions to describe at least one positive and one negative experience, important factors for use, and expectations of telemonitoring.
Patients could contact the researchers by telephone during office hours in case of study-related questions or difficulties. The surgical procedure, clinical diagnostics, or treatment were not affected by this study, and were performed in accordance with standard of care. Patients and healthcare personnel did not have insight into the study data and did not receive feedback based on the monitoring.
2.5. Data collection
Telemonitoring data was retrospectively retracted from the Healthy Chronos platform and RRD-eHealth database. Data was processed and analyzed in Matlab R2021b (MathWorks, Inc., Natick, MA, USA). Days within the protocol included the day after delivery of devices (start telemonitoring) until 30 days after surgery or earlier patient drop-out (end of telemonitoring). The day of surgery and days of admittance at the intensive care unit during the study period were outside protocol. Specific periods were documented as outside protocol if the sensor was worn by one of the researchers for testing, or if the study was paused, i.e. due to significantly delayed surgery or prolonged technical problems.
Patient and surgery characteristics, contact moments between researcher and patient or representative, and answers to the final evaluation questionnaire were stored in REDCap version 10.0.23 (Vanderbilt University, Nashville, TN, USA). Contact moments that were part of the study protocol (e.g. contact before hospital admission or at 30 days after surgery) were excluded from data analysis.
2.6. Data analysis
Participation rate was calculated as the percentage of patients included in the study of all patients who received the information letter. Completion rate was the percentage of patients who received telemonitoring until 30 days after surgery of all included patients.
Compliance with wearing the sensor and experience sampling was calculated as the percentage of days with data of all days within the protocol per perioperative phase: preoperative at home; postoperative in-hospital; and postoperative at home. No minimum was set for the number of measured data points or hours per day with data to calculate compliance. The median number of days within the protocol with interquartile ranges (IQR) and the median number and IQR of hours per day with data for all patients were also shown. For experience sampling, the median number of responses in both the morning and at 8.00 PM and IQR were calculated as well.
Weekly satisfaction scores for wearing the wearable sensor and experience sampling in the app scores were calculated and shown in boxplots with median values, IQR, ranges, and outliers per week before and after surgery for all patients. Mean weekly scores were added to this figure to emphasize extreme values. Median weekly satisfaction scores and IQR were also computed for the total preoperative and postoperative period.
Contact moments were described per perioperative phase and in total. This included the total number of contact moments, number of patients with extra contact moments, and median number of contact moments with IQR per patient. Besides, type of contact (i.e. outgoing or ingoing, call or visit), and reasons for contact were described.
As for patient’s experiences, median overall satisfaction with IQR was calculated for both wearing the sensor and experience sampling. Intention-to-use and intention–to-recommend-to-others were presented as percentages per score (strongly agree to strongly disagree). Positive and negative experiences and future expectations were described in order of how often it has been mentioned.
3. Results
3.1. Study participation
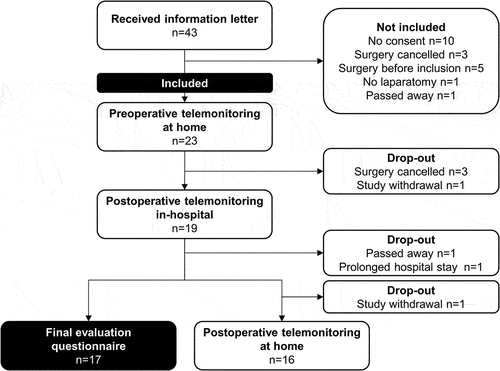
Twenty-three patients planned for elective open abdominal surgery were included in this study; all were monitored preoperatively at home, 19/23 postoperatively in-hospital (82.6%) and 16/23 postoperatively at home (69.6%). The study flow diagram is shown in and patient characteristics are shown in . The participation rate of patients that received the information letter was 54.5%. Reasons not being included in the study are shown in . Reasons for 10/43 patients (23.3%, two reasons in two patients) not willing to participate were: expected extra mental burden (n = 8), insecurity about technology use (n = 1), and not willing to wear a sensor on the arm during this period (n = 3).
Table 1. Characteristics of included patients (n = 23).
Nineteen patients underwent surgery. For these patients, the median number of days from inclusion to surgery was 21.6 with an IQR of 13.8–41.2 days, and median length of hospital stay was 10.2 days (IQR 6.2–13.0 days). In total, 16 patients received telemonitoring until 30 days after surgery, resulting in a completion rate of 69.6%. Reasons for study drop-out were cancelation of surgery (n = 3), complicated postoperative course (n = 2), and study withdrawal (n = 2). Withdrawal was related to recurrent technical problems (i.e. no data transfer) in combination with surgery-related mental burden, because either surgery was repeatedly postponed due to COVID-19 (n = 1), or being at home after hospital discharge was experienced as difficult (n = 1).
3.2. Compliance
Compliance with wearing the wearable sensor and experience sampling per perioperative phase is shown in . Of the three perioperative phases, compliance with wearing the sensor was highest at the surgical ward with a median of 100% of days. At home, compliance during the preoperative and postoperative phase was similarly high with median compliance around 92%. Experience sampling at home was performed in 83.3% and 87.5% of preoperative and postoperative days, respectively. Compliance with experience sampling was lowest at the surgical ward (median 72.7% of days with data). The median number of responses was slightly higher in the morning compared to 8:00 PM postoperatively.
Table 2. Compliance with wearing the wearable sensor and experience sampling in the three phases of the perioperative trajectory (in median and interquartile ranges, IQR).
3.3. Weekly satisfaction
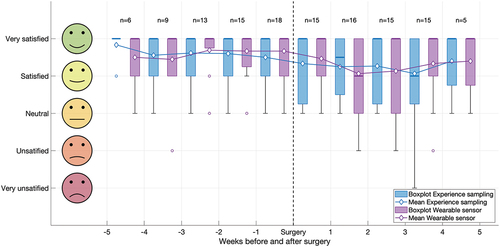
shows boxplots and means of the weekly satisfaction scores for wearing the sensor and experience sampling in the app. On average, patients were very satisfied about wearing the sensor (median 5 [IQR, 4–5] on a 5-point Likert scale) and answering questions in the app (median 5 [IQR, 4–5]), both preoperatively and postoperatively.
Figure 3. Boxplot and mean satisfaction per week before and after surgery from the Customer Satisfaction Score for wearing the sensor (purple) and experience sampling in the app (blue). Boxplots show the median values (bold lines), interquartile ranges (limits of boxes), ranges (whiskers), and outliers (circles).
3.4. Contact moments
Concerning the contact moments outside the protocol, the number, type, and reason are shown per perioperative phase in . Of all 96 contact moments, 61.5% occurred during the preoperative phase of which 33.9% during the first week of inclusion. Preoperative contact moments occurred in 16/23 patients (69.6%), whereas 6/16 patients (37.5%) still needed extra contact moments postoperative at home. Most contact moments were outgoing calls from the researchers (45.8%) when data was not transferred to the database (53.1%). Other main reasons for contact in any phase were: technical problems (in total 22.9%), such as empty batteries or connectivity problems; discomfort of the patient (12.5%), such as wrong size of the bracelet; and insecurity of the patient (11.5%), such as removal of an app from the tablet by accident. The latter mainly occurred during the preoperative period. Of first contact moments due to no data transfer or technical problems, 59.1% were solved or did not require further action. A subsequent call or visit (at home or in combination with another appointment in-hospital) was needed in 13.6% and 12.1% of contact moments respectively, and 7.6% led to a (temporary) protocol stop. Possible solutions were reset of the HealthyChronos app or charging the sensor. Contact because of no data transfer or technical problems occurred for 59.5% in the first 9 patients who initially used an earlier version of the app. Of all contact moments, 75.8% comprised contact with the patient, 14.7% with a family member, and 9.5% with nurses.
Table 3. Number of contact moments outside the protocol, type of contact, and reason of contact per perioperative phase and in total. Percentages are calculated with respect to the total number of contact moments per phase.
3.5. Patient experiences
Seventeen patients (73.9%) completed the final evaluation questionnaire, as illustrated in . Median overall satisfaction was 9.0 (IQR, 8.0–10.0) for both wearing the sensor and experience sampling. As for the intention-to-use, 16 patients (94.1%) would definitely use telemonitoring, and one patient (5.9%) would probably use it. Fifteen patients (88.2%) would also definitely recommend it to others, one (5.9%) probably, and one (5.9%) probably not.
Positive experiences mentioned by 16 patients included the low effort (n = 11), contributing to the improvement of care (n = 5), not noticing wearing the sensor (n = 3), and receiving messages (n = 1). Negative experiences of 12 patients comprised questions being short and monotonous (n = 3), skin irritation of wearable sensor (n = 3), no possibility to explain answers (n = 3), connectivity problems (n = 3), easy to forget to take the sensor from the charger in the morning (n = 2), vibrations of the sensor (n = 1), being too restricted (n = 1), too much burden (n = 1), and having no insight in data (n = 1).
Patients considered the following factors important for telemonitoring being used in future perioperative care: being monitored (n = 9), self-monitoring (n = 5), monitoring blood pressure (n = 3), sleep (n = 1) and eating pattern (n = 1), monitoring and feedback on physical activity to improve condition after surgery (n = 2), involvement nurses (n = 2), being able to choose time for experience sampling or wear sensor (n = 2), and receiving a reminder to wear the sensor (n = 1).
Expectations about future use comprised feeling of security at home (n = 5), integrating personal measurements (n = 2), decreasing workload nurses (n = 1), less hospital visits (n = 1), easier contact with healthcare personnel (n = 1), monitoring with a chip (n = 1), and more patient-centered monitoring (n = 1).
4. Discussion
4.1. Main findings
This study assessed the feasibility and patient’s experiences of telemonitoring at home and in-hospital before and after major abdominal surgery. Overall, telemonitoring as used in this pilot study showed to be feasible from patient’s perspective. Patients had high compliance with wearing the sensor and experience sampling throughout the complete perioperative phase. Weekly and overall satisfaction, intention-to-use and intention-to-recommend-to-others were high.
Compliance with wearing the sensor was highest in-hospital compared to the pre- and postoperative phase at home. Other studies at the surgical ward merely used wearable patches, for which compliance is less relevant [Citation16–19]. One potential barrier for postoperative telemonitoring could be that patients have increased physical and mental burden after surgery. For example, Jonker et al. [Citation20] observed that patients are less compliant with telemonitoring at home when they experienced postoperative complications. High in-hospital compliance with the sensor may be due to additional nurse involvement, easier on-site support by researchers, or possibly better WiFi connectivity in-hospital. In a recent study, the feasibility of the Everion biosensor was investigated in 20 pediatric patients undergoing chemotherapy for cancer [Citation21,Citation22]. Participants were instructed to wear the Everion as often as possible for 14 days. They showed that no (heart rate) data were available for 35% of hours. Reasons for this were forgetting to wear the sensor and demotivating technical problems [Citation21], which were also apparent in our results. Their compliance may be lower than ours due to differences in patient population and used methods to compute compliance. To increase compliance with wearing the sensor at home, it is advisable to remind patients to wear the sensor, or to notify when the sensor needs to be charged or is fully charged. The use of a sensor with prolonged battery life may also limit moments in which patients forgot to wear the sensor in the morning.
Our results show that experience sampling had slightly lower compliance compared to wearing the sensor, especially in-hospital. To our best knowledge, literature about monitoring patients’ well-being with a mobile app in-hospital is lacking. Other published studies regarding symptom monitoring in patients after major abdominal surgery started inclusions after discharge [Citation23–25], where a median daily adherence of 95% (range 32–100%) has been reported [Citation24]. Apps for preoperative use in patients undergoing major surgery have been hardly described yet. Recently, Van der Velde et al. [Citation26] evaluated the usability of an app to improve preoperative health and risk behavior before major elective surgery. Although they reported that 73% of patients activated the app, they did not describe actual use. Experience sampling took more effort for patients, because they needed to actively respond. At home, patients were called if sensor data were not transferred for two consecutive days, while experience sampling responses were not monitored during the study period. Patients might have forgotten to answer the questions when they were not at home or preoccupied at that moment. At the surgical ward, patients were even more likely to forget or did not want to wear the sensor and answer daily questions, potentially because of their postsurgical state-of-mind. Nurses were only instructed to place the sensor on the upper arm of the patient in the morning and were not asked to stimulate experience sampling to limit bias in compliance. Nurse involvement may also have a positive effect on compliance with experience sampling in-hospital. Moreover, experience sampling might be well suitable for in-hospital telemonitoring when combined with well-being assessments by nurses in usual care. Integration of telemonitoring in clinical practice is important for compliance and should be taken into account in future trial design.
Weekly and overall satisfaction for both wearing the sensor and experience sampling, intention-to-use and intention-to-recommend-to-others were high. Huis in ‘t Veld et al. [Citation27] showed no relationship between satisfaction and compliance in their study regarding a teletreatment app for chronic pain, although they found a trend between compliance and clinical benefit. However, according to the Technology Acceptance Model, the intention to accept technology is based on ease of use and perceived usefulness [Citation27,Citation28]. Patients also mentioned that telemonitoring will be most useful during the postoperative period at home to improve monitoring and increase their feeling of security. They also found self-monitoring important in this respect. In a recent study on a general ward, 67% of abdominal surgery patients (n = 27) felt safer when monitored with a wearable patch and 89% would like to keep wearing the patch at home after surgery [Citation29]. The high satisfaction we found might be related to the fact that patients experienced the telemonitoring as low effort, although we did not evaluate this. Future studies investigating the feasibility of telemonitoring services should also focus on perceived usefulness in patients to increase their acceptance of telemonitoring, and therefore potentially increase compliance and clinical benefit.
4.2. Strengths and limitations
A strength of this study is that we included a diverse patient population with varying age, comorbidities, diagnoses, and surgical interventions. This study provides a comprehensive picture of the feasibility of and patient’s experiences with perioperative telemonitoring in patients undergoing major abdominal surgery. A limitation concerns the relatively small sample size. Another limitation is the observational pilot study design in which patients did not have any benefit from telemonitoring. On the other hand, this study design enabled us to overcome connectivity issues and learn from patients’ experiences for the first time. Another limitation is that it is difficult to distinguish compliance and technical problems, because compliance with wearing the sensor was measured as percentage of days with data available, and data might have also been unavailable due to technical problems while a patient did wear the sensor. Regardless of our reports of contact moments in case of technical problems, this may have led to an underestimation of actual compliance.
4.3. Facilitators and barriers: recommendations for future trial design
Our results show that important facilitators were the low effort and ease to wear the sensor. Another potential facilitator for acceptance and use of telemonitoring services we identified may be the ability to integrate patient-specific preferences and to adjust the telemonitoring service to a patient’s sleep-rhythm and habits. Patients were notified for experience sampling twice a day. Compliance with experience sampling might be increased if patients could choose the moment of notification (time-based monitoring of patient-reported outcome measures), or receive a reminder. Contrary to our expectations and our aim to minimize the potential burden of the questionnaires, several patients mentioned that the questions were too short and monotonous. Besides, patients appreciate the opportunity to explain answers about their well-being. This should be taken into account in future studies using experience sampling.
Main barriers for participation in and completion of the study were surgery-related mental burden and recurrent technical issues. Most contact moments occurred preoperatively and were mostly technology related. An important facilitator for successful implementation of telemonitoring services in clinical practice will therefore be the technical support (e.g. training, education and helpdesk or available coordinator) provided to patients and relatives and healthcare personnel. We provided information about the study preferentially face-to-face and visited the patients at home to deliver the devices and install WiFi on the tablet. This requires a substantial time investment of healthcare personnel. Feasibility for healthcare personnel has not been evaluated in this study and should be taken into account and assessed. Implementation of telemonitoring should actually lead to time savings, for example by fewer hospital visits or length of hospital stay of patients, also for healthcare personnel. In future trial design for studies evaluating the effectiveness of telemonitoring, an optimized care pathway is needed to maximize its effect instead of only adding telemonitoring to standard of care.
A general barrier for the implementation of telemonitoring is the current readiness of technology [Citation5,Citation30]. Recent validation studies with the same sensor showed that data availability decreased during physical activity and validity for the measured vital signs was poor to moderate [Citation31,Citation32]. Koenig et al. also showed problems with data quality assignment, data quality during physical activity, and connectivity of the Everion biosensor [Citation21]. It is preferable to have continuous connection to transfer sensor data to the database, for example by using 4G or 5G monitoring instead of Bluetooth and WiFi. Although we did not evaluate this, WiFi speed at patients’ homes may also be relevant for optimal connectivity. Currently, most available wearable sensors still depend on Bluetooth or WiFi for data transfer [Citation2]. Battery performance of wearable sensors should be improved to limit charging periods and enable continuous monitoring of multiple parameters, such as vital signs, for at least several days.
Another barrier is that telemonitoring studies often are limited by selection bias [Citation33,Citation34]. However, not all patients included in our study had (extensive) experience with technology or a high educational level. In that case, the role of family is important for success. For example, a recent study showed that patients who considered telemonitoring less useful had a lower educational level and were more frequently living on their own [Citation20]. For future studies, we recommend involving relatives in telemonitoring at an early stage, especially at home.
5. Conclusions
In this small prospective study, telemonitoring before and after major abdominal surgery was feasible from a patient’s perspective. Compliance was high with technical support and nurse involvement during in-hospital stay. Compliance may be further increased with patients’ input about the preferred moments for notifications and with reminders to wear a sensor or improved sensor technology. Surgery-related mental burden and technical issues were the main barriers for patient participation. For future trial design regarding the effectiveness of telemonitoring, optimal implementation in clinical practice is required, and training and support for patients, relatives, and healthcare personnel is highly recommended.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author contributions
All authors were involved in conception and design of this study, and interpretation of data. MH carried out the data acquisition and data analyses and drafted the manuscript. RM carried out the data acquisition and contributed to the data analyses. RS, MT and JV critically revised the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work.
Additional information
Funding
References
- Michard F. Smartphones and e-tablets in perioperative medicine. Korean J Anesthesiol. 2017;70(5):493–499.
- Joshi M, Ashrafian H, Aufegger L, et al. Wearable sensors to improve detection of patient deterioration. Expert Rev Med Devices. 2019;16(2):145–154.
- Soon S, Svavarsdottir H, Downey C, et al. Wearable devices for remote vital signs monitoring in the outpatient setting: an overview of the field. BMJ Innov. 2020;6(2):55.
- Leenen JPL, Leerentveld C, van Dijk JD, et al. Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: systematic review. J Med Internet Res. 2020;22(6):e18636.
- Haveman ME, Jonker LT, Hermens HJ, et al. Effectiveness of current perioperative telemonitoring on postoperative outcome in patients undergoing major abdominal surgery: a systematic review of controlled trials. J Telemed Telecare 2021:1357633X2110477 DOI:10.1177/1357633X211047710.
- Jansen-Kosterink S, Vollenbroek-Hutten M, Hermens H. A renewed framework for the evaluation of telemedicine. Eighth Int. Conf. eHealth, Telemedicine and Social Medicine April 24-28, 2016. Venice, Italy. Med. eTELEMED; 2016.
- Cramer JA, Benedict A, Muszbek N, et al. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76–87.
- Jonker LT, Haveman ME, de Bock GH, et al. Feasibility of perioperative ehealth interventions for older surgical patients: a systematic review [Internet]. J Am Med Dir Assoc Elsevier Inc. 2020:1–8. DOI:10.1016/j.jamda.2020.05.035.
- Patel B Thind A . Usability of mobile health apps for postoperative care: systematic review. JMIR Perioperative Medicine. 2020;3(2): e19099 .
- Jansen-Kosterink S. The added value of telemedicine services for physical rehabilitation [Internet]; 2014. [cited 2022 Aug 8]. Available from: https://ris.utwente.nl/ws/portalfiles/portal/6030962/Thesis_Jansen-Kosterink.pdf
- Scott AR, Alore EA, Naik AD, et al. Mixed-methods analysis of factors impacting use of a postoperative mhealth app. JMIR mHealth uHealth. 2017;5(2):e11.
- Da La Cruz Monroy MFI, Mosahebi A. The use of smartphone applications (apps) for enhancing communication with surgical patients: a systematic review of the literature. Surg Innov. 2019;26(2):244–259.
- Keng CJS, Goriawala A, Rashid S, et al. Home to stay: an integrated monitoring system using a mobile app to support patients at home following colorectal surgery. J Patient Exp. 2020;7(6):1241–1246.
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457.
- op den AH, Tabak M, Perianu MM, et al. Development and evaluation of a sensor-based system for remote monitoring and treatment of chronic diseases - the continuous care & coaching platform. Proc. 6th Int. Symp. eHealth Serv. Technol. EHST 2012. SCITEPRESS. Science and Technology Publications; 2012. p. 19–27.
- Downey C, Randell R, Brown J, et al. Continuous versus intermittent vital signs monitoring using a wearable, wireless patch in patients admitted to surgical wards: pilot cluster randomized controlled trial. J Med Internet Res. 2018;20(12):1–10.
- Skraastad EJ, Borchgrevink PC, Nilsen TIL, et al. Postoperative quality and safety using Efficacy Safety Score (ESS) and a wireless patient monitoring system at the ward: a randomised controlled study. Acta Anaesthesiol Scand. 2020;64(3):301–308.
- Weenk M, Bredie SJ, Koeneman M, et al. Continuous monitoring of vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res. 2020;22(6):e15471.
- Breteler MJM, Huizinga E, van Loon K, et al. Reliability of wireless monitoring using a wearable patch sensor in high-risk surgical patients at a step-down unit in the Netherlands: a clinical validation study. BMJ Open. 2018;8(2):e020162.
- Jonker LT, Plas M, de Bock GH, et al. Remote home monitoring of older surgical cancer patients: perspective on study implementation and feasibility. Ann Surg Oncol. 2021;28(1):67–78.
- Koenig C, Ammann RA, Kuehni CE, et al. Continuous recording of vital signs with a wearable device in pediatric patients undergoing chemotherapy for cancer—an operational feasibility study. Support Care Cancer. 2021;29(9):5283. • Of interest, since it evaluated the feasibility of the Everion biosensor in a more difficult patient population.
- Haemmerli M, Ammann RA, Roessler J, et al. Vital signs in pediatric oncology patients assessed by continuous recording with a wearable device, NCT04134429. Sci Data. 2022;9(1):89.
- Heuser J, Maeda A, Yang L, et al. Impact of a mobile app to support home recovery of patients undergoing bariatric surgery. J Surg Res. 2021;261:179–184.
- Gustavell T, Sundberg K, Segersvärd R, et al. Decreased symptom burden following surgery due to support from an interactive app for symptom management for patients with pancreatic and periampullary cancer. Acta Oncol (Madr). 2019;58(9):1307–1314.
- Zand A, Nguyen A, Stokes Z, et al. Patient experiences and outcomes of a telehealth clinical care pathway for postoperative inflammatory bowel disease patients. Telemed J E Health. 2019;26(7) :889–897.
- van der Velde M, Valkenet K, Geleijn E, et al. Usability and preliminary effectiveness of a preoperative mHealth app for people undergoing major surgery: pilot randomized controlled trial. JMIR mHealth uHealth. 2021;9(1):1–15.
- HuisIn’t Veld RMHA, Kosterink SM, Barbe T, et al. Relation between patient satisfaction, compliance and the clinical benefit of a teletreatment application for chronic pain. J Telemed Telecare. 2010;16(6):322–328.
- Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Q Manag Inf Syst. 1989;13(3):319–339
- Leenen JPL, Dijkman EM, Van Dijk JD, et al. Feasibility of continuous monitoring of vital signs in surgical patients on a general ward: an observational cohort study. BMJ Open. 2021;11(2):e042735.
- Dechant H, Tohme WG, Mun SK, et al. Health systems evaluation of telemedicine: a staged approach. Telemed J. 1996;2(4):303–312.
- Haveman ME, van Rossum MC, Vaseur RME, et al. Continuous monitoring of vital signs with wearable sensors during daily life activities: validation study. JMIR Form Res. 2022;6(1):e30863.
- Haveman ME, vanMelzen R, Schuurmann RCL, et al. Continuous monitoring of vital signs with the Everion biosensor on the surgical ward: a clinical validation study. Expert Rev Med Devices. 2021;18(Sup1):145–152.
- Vonk Noordegraaf A, Anema JR, Van Mechelen W, et al. A personalised eHealth programme reduces the duration until return to work after gynaecological surgery: results of a multicentre randomised trial. BJOG An Int J Obstet Gynaecol. 2014;121(9):1127–1135.
- Bouwsma EVA, Huirne JAF, Van De Ven PM, et al. Effectiveness of an internet-based perioperative care programme to enhance postoperative recovery in gynaecological patients: cluster controlled trial with randomised stepped-wedge implementation. BMJ Open. 2018;8(1):1–10.