ABSTRACT
Background
Comparative studies of carpal tunnel release with ultrasound guidance (CTR-US) vs. mini-open CTR (mOCTR) are limited, prompting development of this randomized trial to compare efficacy and safety of these techniques.
Research design and methods
Patients were randomized (2:1) to CTR-US or mOCTR, treated by experienced hand surgeons (median previous cases: 12 CTR-US; 1000 mOCTR), and followed for 3 months.
Results
Among 149 randomized patients, 122 received CTR-US (n = 94) or mOCTR (n = 28). Mean incision length was 6 ± 2 mm in the wrist (CTR-US) vs. 22 ± 7 mm in the palm (mOCTR) (p < 0.001). Median time to return to daily activities (2 vs. 2 days; p = 0.81) and work (3 vs. 4 days; p = 0.61) were similar. Both groups reported statistically significant and clinically important improvements in Boston Carpal Tunnel Questionnaire Symptom Severity and Functional Status Scales, Numeric Pain Scale, and EuroQoL-5 Dimension 5-Level, with no statistical differences between groups. Freedom from wound sensitivity and pain favored CTR-US (61.1% vs. 17.9%; p < 0.001). Adverse event rates were low in each group (2.1% vs. 3.6%; p = 0.55).
Conclusions
The efficacy and safety of CTR-US were comparable to mOCTR despite less previous surgical experience with CTR-US. The choice of CTR technique should be determined by shared decision-making between patient and physician.
Clinical trial registration
www.clinicaltrials.gov identifier is NCT05405218.
1. Introduction
Carpal tunnel syndrome (CTS) is the most common compressive neuropathy in the upper extremity [Citation1]. Affected individuals may experience numbness, paresthesias, pain, thenar muscle atrophy, and functional deficits such as pinch/grip weakness. The clinical manifestations of CTS are attributed to localized compression of the median nerve underneath the transverse carpal ligament (TCL). Appropriate CTS management options include immobilization, corticosteroid injection, or carpal tunnel release (CTR) [Citation2].
CTR is a common surgical procedure that divides the TCL to reduce pressure on the median nerve and alleviate associated symptoms. CTR techniques used in clinical practice include traditional open CTR (OCTR), mini-open CTR (mOCTR), endoscopic CTR (ECTR), and CTR with ultrasound guidance (CTR-US). Although the long-term safety and effectiveness of different CTR techniques are generally comparable [Citation3,Citation4], patients often express preferences for procedures resulting in smaller incisions [Citation5]. mOCTR is the most common CTR procedure in the United States [Citation6] and is typically performed using a 1–3 cm palmar incision with direct visualization and division of the TCL. In contrast, CTR-US typically uses a <1 cm wrist incision with ultrasound guidance to divide the TCL and visualize the carpal tunnel, median nerve, adjacent nerves and vessels, and associated structures such as tendons. To the authors’ knowledge, two randomized trials have compared CTR-US to mOCTR [Citation4,Citation7], both performed at single centers and with limited sample size and/or follow-up duration. To overcome these limitations, we designed a multicenter randomized controlled trial to compare the efficacy and safety of CTR-US vs. mOCTR in patients with CTS.
2. Methods
2.1. Study design
TUTOR is a multicenter randomized controlled trial comparing the efficacy and safety of CTR-US to mOCTR in patients with CTS. The trial protocol was approved by a central institutional review board (WCG IRB, Puyallup, WA) and was registered prospectively at ClinicalTrials.gov (NCT05405218). Enrolled patients provided informed consent and confirmed willingness to receive CTR-US or mOCTR before study participation. Eligible patients were randomized to receive unilateral CTR-US or mOCTR and remain in follow-up for 1 year. This manuscript presents 3-month outcomes from the trial, the period when most clinical improvement and complications occur after CTR [Citation8,Citation9].
2.2. Participants
Patient screening included a preoperative clinical examination and diagnostic ultrasound of the median nerve, a reliable and accurate tool for CTR diagnosis [Citation10,Citation11]. Key inclusion criteria were a clinical diagnosis of idiopathic CTS, CTS-6 score ≥ 12 [Citation11], median nerve cross-sectional area ≥10 mm2 in the proximal carpal tunnel region (indicating median nerve swelling proximal to the tunnel) [Citation11], absence of CTS in the contralateral hand that interfered with normal daily activities or work, and failure of nonsurgical treatment. Key exclusion criteria were previous surgery on the affected hand or wrist, recent (<6 weeks) corticosteroid injection in the affected hand or wrist, previous CTR in the target hand, recent (<3 months) CTR in the contralateral hand, need for additional operative procedure, and planned surgical or interventional procedure on the contralateral hand or wrist (Supplement ). Patients who met all eligibility criteria were randomized to receive CTR-US or mOCTR.
Table 1. Demographic characteristics of patients treated with CTR-US or mOCTR.
2.3. Randomization and blinding
Patients were randomly assigned to receive unilateral CTR-US or mOCTR in a 2:1 ratio stratified by site using random block sizes. The 2:1 randomization ratio was selected to provide additional safety data for CTR-US. The randomization sequence was developed by a biostatistician and maintained within an electronic data capture system (Viedoc, Philadelphia, PA). Blinding of physicians and patients was impractical due to apparent differences in surgical technique and the postoperative wound location and appearance.
3. Procedures
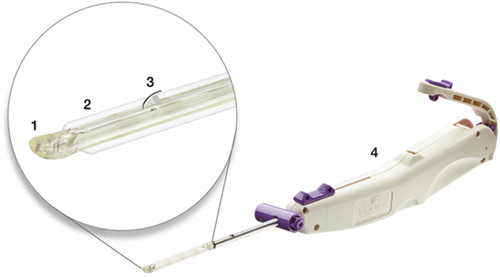
Investigators completed a cadaver-based training program and at least 10 CTR-US procedures before trial participation. The CTR-US technique used an FDA-cleared, single-use, hand-held device (UltraGuideCTR, Sonex Health, Inc., Eagan, MN) inserted into the carpal tunnel through a small incision at the proximal wrist using real-time ultrasound guidance (). Detailed procedural steps with CTR-US have been previously reported [Citation12,Citation13]. Briefly, an ultrasound probe was applied to the volar aspect of the wrist and hand to visualize the relevant anatomy (e.g. TCL, median nerve, ulnar artery), and ultrasound guidance was utilized throughout the procedure. An incision was made proximal to the wrist crease and the device was inserted into the carpal tunnel until the device tip was distal and deep to the TCL as visualized under ultrasound. A pair of balloons in the distal end of the device were inflated to create and maintain space between the cutting portion of the device and the median nerve. A recessed retrograde cutting blade in the device was then activated to transect the TCL distal to proximal. After the blade was recessed and the balloons deflated, TCL transection was verified by probing the ligament under ultrasound visualization. The incisions were closed with Steri-strips, skin glue, and/or sutures according to the surgeon’s preference.
Figure 1. Device used for carpal tunnel release with ultrasound guidance. Key device characteristics include: 1) blunt dissecting rigid polymer tip; 2) two laterally located polymer balloons (inflated in figure); 3) retractable stainless-steel retrograde cutting blade (in active position in figure); 4) handle housing device controls.
Trial investigators were hand surgeons experienced in mOCTR (median experience: 14 years; interquartile range [IQR]: 8–28 years). The mOCTR technique used a standard longitudinal palmar incision along the axis of the ring finger, between the thenar and hypothenar eminences. The incision extended distally to dissect the palmar fascia with a surgical blade for direct visualization of the TCL, median nerve, its branches, and the superficial palmar arterial arch. The underlying TCL was then divided longitudinally along its ulnar aspect. The incisions were closed with sutures. Ultrasound guidance was not used during the mOCTR procedures.
A key element of the trial design was standardizing postoperative patient care instructions across all sites and in both treatment groups. Investigators instructed patients to ‘return to activities and work as tolerated based on pain, function, and wound healing status’ without providing specific guidance on the expected recovery duration or the recommended time to return to activity/work. This was implemented to minimize bias due to the strong association between physician recommendations for postoperative recovery and the time to return to activity [Citation14] and work [Citation15] after CTR.
4. Outcomes
Patient follow-up was performed according to the clinical practice patterns at each site and remote data collection was performed daily for 14 days, at 1 month, and at 3 months post-treatment. A schedule of study assessments is provided in Supplement . Trial data were recorded on electronic case report forms and routinely monitored for accuracy. Main outcomes included time to return to normal daily activities, time to return to work in any capacity among employed patients, Boston Carpal Tunnel Questionnaire Symptom Severity Scale (BCTQ-SSS) and Functional Status Scale (BCTQ-FSS), Numeric Pain Scale, EuroQoL-5 Dimension 5-Level (EQ-5D-5 L), and device- or procedure-related adverse events (AEs). Patients completed return to normal activity and return to work questionnaires daily for the first 14 days. Patients who did not return to normal activity or work in the first 14 days were asked to provide the date of return to these activities at subsequent follow-up intervals. The BCTQ-SSS and BCTQ-FSS questionnaires were scored from 1 to 5, where higher scores indicated a worse outcome [Citation16]. Hand/wrist pain severity was scored from 0 to 10 on a Numeric Pain Scale. Health-related quality of life was assessed with the EQ-5D-5 L, which provided a health utility value for each patient ranging from 0 to 1 where higher scores indicated better quality of life [Citation17]. The minimal clinically important difference (MCID) after CTR is defined as a change from baseline of −1.14 points for BCTQ-SSS [Citation18], −0.74 points for BCTQ-FSS [Citation18], −2 points for Numeric Pain Scale [Citation19], and 0.09 points for EQ-5D-5 L [Citation20]. Device- and procedure-related AEs were documented periprocedurally, during follow-up by site or patient report, or by site review of wound healing images. An independent medical reviewer adjudicated AEs for classification, seriousness, and relationship to the device and/or procedure. An independent data safety and monitoring board provided trial oversight.
Table 2. Clinical characteristics of patients treated with CTR-US or mOCTR.
5. Statistical analysis
A power analysis determined that 102 evaluable patients provided 80% statistical power to detect a standardized difference (Cohen’s d) of 0.60, assuming 2:1 randomization, a two-tailed alpha level of 0.05, and a two-sample equal-variance t-test. Consequently, we planned to enroll at least 120 patients to account for patient attrition. Primary analyses were performed on a modified intention-to-treat population of randomized patients who received their assigned treatment. Baseline characteristics were reported as mean and standard deviation for normally distributed continuous data, median and IQR for non-normally distributed continuous data, and counts and percentages for categorical data. Time to return to normal daily activities and return to work were preferentially reported as the median and IQR, with group comparisons made with the Mann-Whitney U test and Kaplan-Meier methods. Changes in BCTQ-SSS, BCTQ-FSS, Numeric Pain Scale, and EQ-5D-5 L were analyzed using a linear mixed model with treatment group, time, group-by-time interaction, and baseline value included in the model. These results were reported as the baseline-adjusted least squares mean and 95% confidence interval (95% CI). Adverse events were reported as counts, percentages, and exact 95% CIs, with group comparisons made using Fisher’s exact test. All statistical tests were two-sided with no multiplicity adjustments. Results were deemed statistically significant at p < 0.05.
A preplanned, intention-to-treat analysis was performed on all randomized patients. Missing data were replaced by Fully Conditional Specification multiple imputation [Citation21]. The variables in the model were assigned treatment group, age, sex, employment status, CTS-6 score, and baseline values for BCTQ-SSS, BCTQ-FSS, Numeric Pain Scale, and EQ-5D-5 L, with sequential modeling of return to normal activities, return to work, and BCTQ-SSS, BCTQ-FSS, Numeric Pain Scale, and EQ-5D-5 L at 2 weeks, 1 month, and 3 months. The group differences for return to normal activities, return to work, and BCTQ-SSS, BCTQ-FSS, Numeric Pain Scale, and EQ-5D-5 L at 3 months were calculated by pooling the results of 50 imputed datasets using Rubin’s rules [Citation22].
6. Results
Between July 2022 and January 2023, 149 participants from 11 centers were randomly assigned (2:1) to receive CTR-US (n = 101) or mOCTR (n = 48). Investigators were less experienced with CTR-US than mOCTR (median 12 vs. 1000 previous cases per investigator) before treating patients in the study. A list of oversight committee members and participating centers is provided in Supplement .
Table 3. Procedural details in patients treated with CTR-US or mOCTR.
The median time between randomization and treatment was 18 days (IQR: 9–26). After randomization, 7 patients assigned to CTR-US were withdrawn from the study before surgery, most commonly because patients withdrew consent. Of patients assigned to mOCTR, 20 were withdrawn from the study before surgery (13 refused mOCTR). Ultimately, 122 patients were treated in the study, 94 with CTR-US and 28 with mOCTR. Over 3 months post-treatment, 95.7% of the CTR-US group and 100% of the mOCTR group remained in follow-up (Supplement ).
The demographic and clinical characteristics of patients were typical of previous CTR trials [Citation23] and were well balanced between treatment groups. Comparing patients treated with CTR-US vs. mOCTR, the mean age was 56.7 ± 14.4 vs. 56.8 ± 14.2 years, 63.8% vs. 75.0% were female, and 59.6% vs. 67.9% were employed. Employed patients predominantly held desk-based positions, and the distribution of desk-based, light manual, and heavy manual work activities was comparable between groups (). Carpal tunnel syndrome characteristics, BCTQ-SSS, BCTQ-FSS, Numeric Pain Scale, and EQ-5D-5 L scores were similar between groups ().
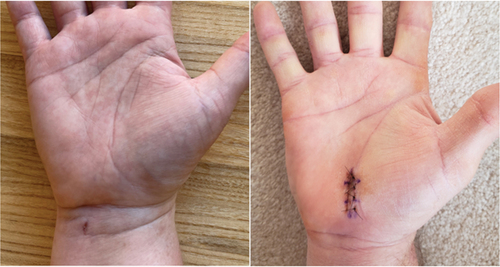
All procedures were completed as planned; no CTR-US cases were converted to OCTR/mOCTR. Most procedures in each group were performed using local anesthesia. The surgical incision in the wrist with CTR-US was significantly shorter than in the palm with mOCTR (6 ± 2 vs. 22 ± 7 mm; p < 0.001). Suture-based wound closure was less common after CTR-US (19% vs. 100% p < 0.001) (). Representative wound healing images after each CTR technique are provided in .
Figure 2. Representative wound healing images in heavy manual laborers 1 week after CTR-US using a wrist incision (left) and mOCTR using a palmar incision (right).
Return to normal activities and work after surgery was rapid in both groups. The median time to return to normal activities was 2 days (IQR: 1–5) with CTR-US and 2 days (IQR: 1–4) with mOCTR (p = 0.81). The mean time to return to normal activities was 5.6 ± 14.8 vs. 6.4 ± 13.5 days. The time to return to normal activities was comparable between treatment groups over the 3-month follow-up period (log-rank p = 0.92) (Supplement ). Among employed patients, the median time to return to work was 3 days (IQR: 1–9) with CTR-US and 4 days (IQR: 1–15) with mOCTR (p = 0.61). Comparing patients with desk-based, light manual, and heavy manual jobs, the median time to return to work was 2, 4, and 9 days with CTR-US and 3.5, 1.5, and 10 days with mOCTR. The mean time to return to work was 8.6 ± 14.3 vs. 17.4 ± 33.6 days. The time to return to work was comparable between treatment groups over the 3-month follow-up period (log-rank p = 0.32) (Supplement ).
Figure 3. BCTQ-SSS and BCTQ-FSS scores over 3 months following CTR-US and mOCTR. Plotted values are baseline-adjusted least squares mean change and 95% confidence interval. (top) at 3 months, the mean change for BCTQ-SSS was − 1.46 for CTR-US and − 1.54 for mOCTR (p = 0.46 between groups). The mean change in each group was statistically significant compared to baseline (both p < 0.001) and exceeded the minimal clinically important difference of a 1.14-point decrease denoted by the green shaded area [Citation18]. (bottom) at 3 months, the mean change for BCTQ-FSS was − 0.83 for CTR-US and − 0.84 for mOCTR (p = 0.92 between groups). The mean change in each group was statistically significant compared to baseline (both p < 0.001) and exceeded the minimal clinically important difference of a 0.74-point decrease denoted by the green shaded area [Citation18].
![Figure 3. BCTQ-SSS and BCTQ-FSS scores over 3 months following CTR-US and mOCTR. Plotted values are baseline-adjusted least squares mean change and 95% confidence interval. (top) at 3 months, the mean change for BCTQ-SSS was − 1.46 for CTR-US and − 1.54 for mOCTR (p = 0.46 between groups). The mean change in each group was statistically significant compared to baseline (both p < 0.001) and exceeded the minimal clinically important difference of a 1.14-point decrease denoted by the green shaded area [Citation18]. (bottom) at 3 months, the mean change for BCTQ-FSS was − 0.83 for CTR-US and − 0.84 for mOCTR (p = 0.92 between groups). The mean change in each group was statistically significant compared to baseline (both p < 0.001) and exceeded the minimal clinically important difference of a 0.74-point decrease denoted by the green shaded area [Citation18].](/cms/asset/77aade34-eab8-4c92-bd25-cc8bbddf6c59/ierd_a_2218548_f0003_oc.jpg)
Symptom severity, functional status, pain severity, and health-related quality of life improved in both groups over the 3-month follow-up period (all changes p < 0.001), all mean changes exceeded the MCID, and there were no statistical differences between the groups. Comparing CTR-US to mOCTR, the least squares mean change was −1.46 vs. −1.54 (p = 0.46) for BCTQ-SSS, where the changes in each group exceeded the MCID of −1.14. Mean BCTQ-SSS values at 3 months were 1.6 and 1.5, respectively. The least squares mean change was −0.83 vs. −0.84 (p = 0.92) for BCTQ-FSS, where the changes in each group exceeded the MCID of −0.74. Mean BCTQ-FSS values at 3 months were 1.4 in both groups (). The least squares mean change was −3.2 vs. −3.8 (p = 0.09) for the Numeric Pain Scale, where the changes in each group exceeded the MCID of −2.0 (). Mean Numeric Pain Scale values at 3 months were 1.3 and 0.7, respectively. The least squares mean change was 0.11 vs. 0.12 (p = 0.90) for EQ-5D-5 L, where the changes in each group exceeded the MCID of 0.09 (). Mean EQ-5D-5 L values at 3 months were 0.89 and 0.88, respectively. Patient-reported wound appearance and satisfaction were comparable between groups, while freedom from wound sensitivity/pain favored the CTR-US group (61.1% vs. 17.9%; p < 0.001) (Supplement ). In the intention-to-treat, as-randomized analysis, there were no statistical differences between treatment groups for baseline patient characteristics, time to return to normal activities, time to return to work, or BCTQ-SSS, BCTQ-FSS, Numeric Pain Scale, and EQ-5D-5 L at 3 months ().
Figure 4. Numeric Pain Scale scores over 3 months following CTR-US and mOCTR. Plotted values are baseline-adjusted least squares mean change and 95% confidence interval. At 3 months, the mean change was − 3.2 for CTR-US and − 3.8 for mOCTR (p = 0.09 between groups). The mean change in each group was statistically significant compared to baseline (both p < 0.001) and exceeded the minimal clinically important difference of a 2-point decrease denoted by the green shaded area [Citation19].
![Figure 4. Numeric Pain Scale scores over 3 months following CTR-US and mOCTR. Plotted values are baseline-adjusted least squares mean change and 95% confidence interval. At 3 months, the mean change was − 3.2 for CTR-US and − 3.8 for mOCTR (p = 0.09 between groups). The mean change in each group was statistically significant compared to baseline (both p < 0.001) and exceeded the minimal clinically important difference of a 2-point decrease denoted by the green shaded area [Citation19].](/cms/asset/fd6034ee-a6a7-45b2-bf1f-4997bf821eba/ierd_a_2218548_f0004_oc.jpg)
Figure 5. EQ-5D-5 L scores over 3 months following CTR-US and mOCTR. Plotted values are baseline-adjusted least squares mean change and 95% confidence interval. At 3 months, the mean change was 0.11 for CTR-US and 0.12 for mOCTR (p = 0.90 between groups). The mean change in each group was statistically significant compared to baseline (both p < 0.001) and exceeded the minimal clinically important difference of a 0.09-point increase denoted by the green shaded area [Citation20].
![Figure 5. EQ-5D-5 L scores over 3 months following CTR-US and mOCTR. Plotted values are baseline-adjusted least squares mean change and 95% confidence interval. At 3 months, the mean change was 0.11 for CTR-US and 0.12 for mOCTR (p = 0.90 between groups). The mean change in each group was statistically significant compared to baseline (both p < 0.001) and exceeded the minimal clinically important difference of a 0.09-point increase denoted by the green shaded area [Citation20].](/cms/asset/3214db03-fdbc-4083-b84e-d97a63b40d94/ierd_a_2218548_f0005_oc.jpg)
Table 4. Results of intention-to-treat, as-randomized analysis.*
No device-related AEs or device malfunctions were reported in the study. Three procedure-related AEs were reported; 2 (2.1%; 95% CI: 0.3–7.5%) in the CTR-US group and 1 (3.6%; 95% CI: 0.1–18.4%) with mOCTR (p = 0.55). One patient treated with CTR-US was diagnosed with a partial third common palmar digital nerve injury after surgery, presenting with resolution of carpal tunnel symptoms but new mid-palmar pain, paresthesias, and increased numbness in the third webspace. Complete TCL release was confirmed on postoperative day 17 with open exploration, revealing an epineurial laceration without axonal disruption on the dorsal aspect of the third common digital nerve, 2 cm distal to its median nerve origin. A nerve wrap was placed and the incision was closed with sutures. At the latest follow-up, carpal tunnel symptoms were absent, thenar and grip strength were normal and symmetrical, and sensation continued to improve with a two-point discrimination of 6 mm for the ulnar middle finger, 8 mm for the radial ring finger, and 4 mm for the remaining fingers. This was the only serious AE reported in the study. Nonserious procedure-related AEs included neurapraxia after CTR-US that was treated with a corticosteroid injection, and wound dehiscence after mOCTR that resolved after placing additional sutures. No infections, cysts/seromas, or tendon, muscle, or vascular complications were reported in either group.
7. Discussion
There were several important findings in this multicenter randomized trial comparing CTR-US to mOCTR, the most common surgical procedure for CTS [Citation6]. First, both treatments resulted in statistically significant and clinically important improvements in symptoms, function, and health-related quality of life, with low complication rates, over 3 months. Second, the efficacy and safety of CTR-US and mOCTR were comparable, despite less experience of the investigators with CTR-US. Finally, enrollment in this trial was complicated by 13 patient refusals to receive mOCTR after randomization. While the intention-to-treat analysis results were comparable to those of the as-treated analyses, these enrollment challenges highlight the importance of patient preference in the choice of CTR technique.
An interesting result of this trial was the rapid return to normal activities (2 days median) and work (4 days median) in the mOCTR group. These are the fastest reported times among any previous OCTR, mOCTR, or ECTR study, and considerably faster than times to return to normal activities and work of 13 days and 23 days, respectively, reported in a meta-analysis of CTR [Citation14]. While we preferentially reported median values, the mean values in both groups were considerably higher and more representative of results reported in previous studies. A design feature of this trial may have also influenced these times in the mOCTR group. Postoperative patient care instructions were standardized in both groups and at all sites, without specific guidance on expected recovery duration or return to activity/work. We implemented these guidelines to minimize bias based on the known relationship between the duration of physician-recommended recovery and time to return to activity [Citation14] and work [Citation15] after CTR. Such instructions are typical after CTR-US; however, postoperative instructions after mOCTR often advise 1–3 weeks of limited activity [Citation14]. A final plausible contributing factor to the rapid recovery in patients treated with mOCTR is that trial investigators were highly experienced surgeons. Consequently, the trial results may reflect benchmark mOCTR outcomes.
Two previous randomized trials have compared CTR-US with mOCTR [Citation4,Citation7]. Capa-Graza et al. [Citation7] followed 40 patients randomized to CTR-US or mOCTR for 3 months post-treatment. The mean time to return to normal activities and work was 4.4 ± 1.5 days with CTR-US and 25.9 ± 5.4 days with mOCTR, Quick Disabilities of the Shoulder and Hand scores over 3 months favored CTR-US, and no complications were reported in either group. In the randomized trial of Rojo-Manuate et al. [Citation4], 92 patients received CTR-US or mOCTR and were followed for 12 months. The mean time to return to normal daily activities and work (4.9 ± 5.4 vs. 25.5 ± 24.3 days) and QDASH scores favored CTR-US, whereas complication rates were comparably low (0% with CTR-US vs. 4% with mOCTR). In the current trial, the time to return to activity and work, the improvement in patient-reported outcomes, and complication rates with CTR-US were comparable to these previous randomized trials and studies of CTR-US performed in real-world settings [Citation12,Citation13]. The primary differences in the current trial were the notably shorter time to return to work with mOCTR and the lack of statistically significant differences in patient-reported outcomes favoring CTR-US. Overall, the clinical results achieved with CTR-US in this study appear consistent among studies, whereas the variability in results after mOCTR deserves further investigation.
The primary strengths of this study were the multicenter randomized trial design, trial oversight provided by an independent data safety and monitoring board, AE adjudication provided by an independent medical reviewer, and implementation of trial design and data analysis elements intended to minimize bias. Nonetheless, several limitations of this study warrant discussion. First, 13 patients randomized to mOCTR refused treatment despite reporting a willingness to be randomized to either treatment group. Although the difference in withdrawal rates reflects a patient preference for CTR-US over mOCTR, this may have biased the study results. This risk was partially addressed by performing an intention-to-treat, as-randomized analysis where the results were comparable to the primary as-treated analysis. Additionally, the statistical power of the trial remained approximately 80% despite the differential dropout rate between groups. However, unmeasured factors such as differences in patient motivation, treatment expectations, or socioeconomic factors between groups may have influenced the study results. Factors that influence patient preferences when electing to undergo CTR warrant further study. A second limitation was that trial investigators were considerably more experienced with mOCTR than CTR-US, which may have biased the results. Third, the study investigated a CTR-US technique that used inflatable balloons to create space and subsequently transect the TCL with a cutting blade. Multiple CTR-US techniques have been described that vary by approach to access the TCL, ability to create space in the carpal tunnel, and method of TCL transection [Citation24]. Consequently, the current results may not be generalizable to other CTR-US techniques. Fourth, performance and expectation bias may have confounded the results due to the unblinded trial design. A final limitation is that the 3-month follow-up results presented here may not be fully indicative of the final trial outcomes at 1 year. While most clinical improvements and complications after CTR are typically observed in the first 3 months after surgery [Citation8,Citation9], longer-term data will be crucial to corroborate these early outcomes. Patients in this trial will continue in follow-up for 1 year, after which the final results will be published.
8. Conclusions
In this multicenter randomized trial, the efficacy and safety of CTR-US performed through a wrist incision were comparable to mOCTR performed through a palmar incision despite less previous surgical experience with CTR-US. Both treatment groups reported rapid return to work and activity, statistically significant and clinically important improvements in symptoms, function, and health-related quality of life, with low complication rates, through 3 months. Overall, the choice of CTR technique should be determined by shared decision-making between the patient and physician.
Declaration of interest
Larry Miller received personal fees from Sonex Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author contributions
Conception and design: Kyle Eberlin, Larry Miller
Data analysis: Larry Miller
Data interpretation: All authors
Drafting of the paper: Kyle Eberlin, Larry Miller
Critical review and revision of the paper: Benjamin Amis, Thomas Berkbigler, Christopher Dy, Mark Fischer, James Gluck, Thomas Kaplan, Thomas McDonald, Alexander Palmer, Paul Perry, Marc Walker, James Watt
Final approval of the version to be published: All authors
Agree to be accountable for all aspects of the work: All authors
Supplemental Material
Download MS Word (241.6 KB)Acknowledgments
We thank the patients and the research staff at each investigative center for participating in this trial.
Data availability statement
Raw data will not be available because the clinical trial remains in progress.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17434440.2023.2218548.
Additional information
Funding
References
- Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282(2):153–158. DOI:10.1001/jama.282.2.153
- Graham B, Peljovich AE, Afra R, et al. The American Academy of orthopaedic surgeons evidence-based clinical practice guideline on: management of carpal tunnel syndrome. J Bone Joint Surg Am. 2016;98(20):1750–1754. DOI:10.2106/JBJS.16.00719
- Shin EK. Endoscopic versus open carpal tunnel release. Curr Rev Musculoskelet Med. 2019;12(4):509–514.
- Rojo-Manaute JM, Capa-Grasa A, Chana-Rodríguez F, et al. Ultra-minimally invasive ultrasound-guided carpal tunnel release: a randomized clinical trial. J Ultrasound Med. 2016;35(6):1149–1157. DOI:10.7863/ultra.15.07001
- Alokozai A, Lindsay SE, Eppler SL, et al. Patient willingness to pay for faster return to work or smaller incisions. Hand (N Y). 2021;16(6):811–817. DOI:10.1177/1558944719890039
- Munns JJ, Awan HM. Trends in carpal tunnel surgery: an online survey of members of the American society for surgery of the hand. J Hand Surg Am. 2015;40(4):767–771 e762.
- Capa-Grasa A, Rojo-Manaute JM, Rodríguez FC, et al. Ultra minimally invasive sonographically guided carpal tunnel release: an external pilot study. Orthop Traumatol Surg Res. 2014;100(3):287–292. DOI:10.1016/j.otsr.2013.11.015
- Tran TA, Williams LM, Bui D, et al. Prospective pilot study comparing pre- and postsurgical CTSAQ and Neuro-QoL Questionnaire with median nerve high-resolution ultrasound cross-sectional areas. J Hand Surg Am. 2018;43(2):e184 181–e184 189. DOI:10.1016/j.jhsa.2017.08.015
- Ozer K, Malay S, Toker S, et al. Minimal clinically important difference of carpal tunnel release in diabetic and nondiabetic patients. Plast Reconstr Surg. 2013;131(6):1279–1285. DOI:10.1097/PRS.0b013e31828bd6ec
- Lin TY, Chang KV, Wu WT, et al. Ultrasonography for the diagnosis of carpal tunnel syndrome: an umbrella review. J Neurol. 2022;269(9):4663–4675. DOI:10.1007/s00415-022-11201-z
- Fowler JR, Cipolli W, Hanson T. A comparison of three diagnostic tests for carpal tunnel syndrome using latent class analysis. J Bone Joint Surg Am. 2015;97(23):1958–1961.
- Bergum RA, Ciota MR. Office-based carpal tunnel release using ultrasound guidance in a community setting: long-term results. Cureus. 2022;14:e27169.
- Fowler JR, Chung KC, Miller LE. Multicenter pragmatic study of carpal tunnel release with ultrasound guidance. Expert Rev Med Devices. 2022;19(3):273–280. DOI:10.1080/17434440.2022.2048816
- Miller LE, Chung KC. Determinants of return to activity and work after carpal tunnel release: a systematic review and meta-analysis. Expert Rev Med Devices. 2023;20:417–425. In press. DOI:10.1080/17434440.2023.2195549.
- Ratzon N, Schejter-Margalit T, Froom P. Time to return to work and surgeons’ recommendations after carpal tunnel release. Occup Med (Lond). 2006;56(1):46–50.
- Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75(11):1585–1592. DOI:10.2106/00004623-199311000-00002
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. DOI:10.1007/s11136-011-9903-x
- Kim JK, Jeon SH. Minimal clinically important differences in the Carpal Tunnel Questionnaire after carpal tunnel release. J Hand Surg Eur Vol. 2013;38(1):75–79.
- Randall DJ, Zhang Y, Li H, et al. Establishing the minimal clinically important difference and substantial clinical benefit for the pain visual analog scale in a postoperative hand surgery population. J Hand Surg Am. 2022;47(7):645–653. DOI:10.1016/j.jhsa.2022.03.009
- Marti C, Hensler S, Herren DB, et al. Measurement properties of the EuroQoL EQ-5D-5L to assess quality of life in patients undergoing carpal tunnel release. J Hand Surg Eur Vol. 2016;41(9):957–962. DOI:10.1177/1753193416659404
- Van Buuren S, Brand JP, Groothuis-Oudshoorn CG, et al. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76(12):1049–1064. DOI:10.1080/10629360600810434
- Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons; 2004.
- Bodavula VK, Burke FD, Dubin NH, et al. A prospective, longitudinal outcome study of patients with carpal tunnel surgery and the relationship of body mass index. Hand (N Y). 2007;2(1):27–33. DOI:10.1007/s11552-006-9019-x
- Petrover D, Hakime A, Silvera J, et al. Ultrasound-guided surgery for carpal tunnel syndrome: a new interventional procedure. Semin Intervent Radiol. 2018;35(4):248–254. DOI:10.1055/s-0038-1673360
