ABSTRACT
Background
Needlestick injuries (NSIs) may potentially expose healthcare professionals (HCPs) to bloodborne pathogens. Safety needles are designed to protect against NSIs. We evaluated whether a new fully passive safety needle could be used safely by HCPs.
Research design and methods
The passive safety needle was tested by physicians, nurses, and pharmacists in subcutaneous or intramuscular injection scenarios in simulation studies (1–3). Data collected included successes, close calls, difficulties, use errors, and failures. In study 4, HCPs rated the device safety (21-item questionnaire).
Results
Overall, 104 participants completed 4772 simulated tasks, including 932 injections. 915 injections (98.18%) were performed successfully and no NSIs (0%) were observed in any of the studies. Studies 1 & 2: 84.15% tasks and 96.06% injections were completed successfully, but use errors occurred, mostly arising from the participants’ mental model. There were no failures in Study 3. In Study 4, >98% of participants responded positively to every question, while all felt that the passive safety feature could eliminate NSIs and would better protect against bloodborne pathogens than other existing devices with active or semi-passive safety mechanisms.
Conclusions
The passive safety needle was used successfully by HCPs, did not lead to any NSIs, and was rated as the safest compared to similar devices.
1. Introduction
Sharps injuries in healthcare workers are one of the most important occupational hazards globally, with more than two million exposures occurring annually [Citation1]. Needlestick injuries (NSIs) are the most common type of sharps injury for healthcare professionals (HCPs), which mostly happen when the sharp point of a needle punctures or cuts skin [Citation2]. Because NSIs can potentially lead to infection with hepatitis B or C or HIV, they should be treated seriously. The exact number of NSIs is underestimated, as many are unreported. Available data suggest that several million HCPs, particularly nurses, are at risk of occupational exposure to different bloodborne pathogens every year because of accidental NSIs [Citation3].
An NSI can occur during the whole injection process, starting from the introduction of a needle, withdrawal from tissue, and needle disposal. A considerable number of NSIs occur after the injection (41%), when a needle is contaminated and the risk of infection is the highest during the handling and disposal of a needle [Citation4]. Global guidelines on proper handling and disposal of used sharps, combined with the introduction of safety-engineered needles, has led to a reduction in NSIs [Citation2]. The Occupational Safety and Health Administration (OSHA) has recommended that safety-engineered devices be considered whenever possible, as engineering controls isolate users from the hazard and have been shown to reduce sharps injuries [Citation5,Citation6]. Safety needles with a sharps injury prevention feature are devices designed with an active or passive component or attachment that should protect a user from accidental NSIs. Despite these features, NSIs still occur when using a safety needle [Citation2,Citation7–9]. The EPINet report indicated that 57.5% of injuries in the US occurred during the use of the device, while disposable syringes were, along with suture needles, the devices responsible for the most NSIs (20.7% and 21% respectively) [Citation10]. In a study in the Netherlands in 2018 [Citation7], for example, the rate of NSIs prior to implementation of safety needle devices was 1.9 per 100 healthcare workers. After the safety needle devices had been introduced, the incidence of NSIs increased to 2.2 per 100 healthcare workers. The most commonly-reported causes for NSIs were difficulties in operating the safety device and improper disposal of needles. Thus, it is crucial to assess not only the safety feature of the device, but also its usage in terms of its ability to reduce the risk of accidental NSIs.
We performed four studies using a fully passive safety needle (DropSafe Sicura, manufactured by Pikdare S.p.A. and distributed by Pikdare S.p. A., HTL-Strefa, Inc., and HTL-STREFA S.A. companies of the MTD Group) designed to ensure safety before, during, and after injection. These studies were in accordance with the relevant Human Factors and Usability Engineering regulations in the US, as well as with the European Union MDR and MHRA regulations, and represent a standard approach during the development and registration of such a device. It possesses an integrated, automatic sharps protection feature () that continuously protects the cannula and inactivates the device, thus preventing repeated use and helping to eliminate NSIs. We aimed to determine whether HCPs could correctly and safely use the new device with minimal or no training in a simulated environment following standard injection procedures. We also collected feedback about whether HCPs felt that medical devices with safety features were important, and how they rated the new device compared to legacy safety devices used in their daily clinical practice.
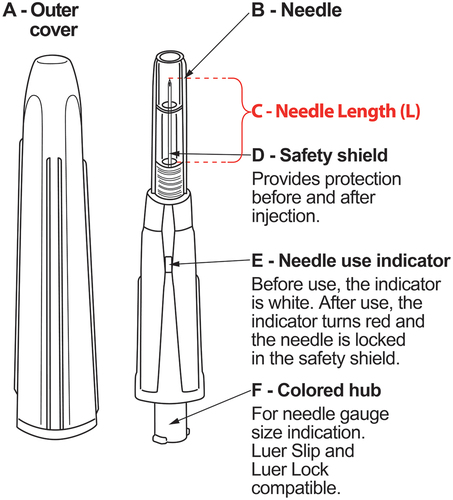
Figure 1. Passive safety needle, available in eight different needle gauges (0.3–1.3 mm) and lengths (13, 16, or 25 mm). A-Outer cover; B-Needle; C-Entire needle length penetrating the skin (L); D-Safety shield; E-Needle use indicator; F-Colored needle hub. This passive system means that the device does not require any activity of the user and is activated automatically during the movement of the needle slider when the needle is inserted into the tissue. The needle is packed in a cover that functions as an additional protection against accidental activation and blocking of the needle. When a user removes the cover, the needle is contained within the safety shield. When administering the injection, the safety shield retracts while the needle inserts into the skin. After injection, the needle automatically locks within the safety shield, preventing further use. When the safety mechanism is activated, a red indicator appears.
2. Methods
2.1. Study designs
Three non-clinical simulation studies were performed to assess the usability of the passive safety needle. The fourth study aimed to evaluate the perception and preference of the users. Before each test began, all participants agreed to take part in the study and read and signed the informed consent form. As there was no significant risk to participants, no ethics review was required.
The aims of Studies 1&2 were to: 1) obtain evidence that the user interface of the new device could be used safely and ensure that product range was not vulnerable to potentially harmful use errors that could cause damage or compromise medical care to the patient or user; 2) confirm it was possible to perform all recommended techniques for subcutaneous and intramuscular injections. In Study 3, the primary objective was to demonstrate that the injury prevention feature of the new device was effective in preventing NSIs, and accordingly determine the true failure rate of devices. The secondary objective was to collect users’ feedback regarding their interactions with the new device related to the prevention of sharps injuries and general features of the device.
In Study 4, a survey was conducted after Study 2 to estimate: a) perceptions of the new device in relation to its safety aspects; b) perceptions of the safety of the new device itself and in comparison to similar products on the market (a hinge cap active safety needle, a retractable safety needle, and a retractable safety syringe); c) preference of the new device for its passive safety feature to eliminate NSIs and protection against bloodborne pathogens; d) perceptions and preference of the new device for its safety features to eliminate NSIs and protection against bloodborne pathogens compared to similar products on the market.
2.2. Study groups
All participants were HCPs and represented the three most significant and popular groups of intended users of the new device – nurses, pharmacists, and physicians. The distribution of the participants differed between the groups to reflect the frequency of performing injections in daily practice. The largest group contained nurses, then pharmacists, and the smallest group contained physicians. All of them had experience in performing injections with safety pen needles, passive systems with a retractable syringe, and/or passive catheters, thereby preventing learning curve artifacts. No training was required for participants in Studies 1&2, apart from familiarization with the product prior to the study session, as per a real-life scenario. Training in the correct technique was provided in Study 3 (10 min during the test session, before simulated use). Participants in Study 4 self-trained before test simulation and the subsequent questionnaire. All participants were recruited and managed by a clinical research organization (Thay Medical, Dorset, England, for Studies 1,2&4; Emergo, Massachusetts, U.S.A., for Study 3). Participants in Studies 1&2 received payments to compensate for their time and travel expenses.
2.3. Device
The passive safety needle is a single use, sterile hypodermic needle with an integrated passive sharps protection feature (). The device is used in combination with a (pre-)filled syringe for subcutaneous and intramuscular injection. The device is Luer-Slip and Luer-Lock compatible, but cannot be used for drawing up drugs from a vial/ampule. It is characterized by an automatic activation of a sharps protection safety mechanism that permanently protects the cannula of the device, thus preventing accidental NSIs and rendering the device unusable after a single use. More specifically, before being used the needle use indicator is white (, device part E). During the introduction of the needle into the injection site the safety shield ( device part D) that covers the needle, retracts automatically into the device. When the safety shield is fully retracted, the entire needle length has penetrated into the tissue. After the delivery of the drug is completed and the needle is withdrawn, the safety mechanism will activate automatically, and the needle use indicator will turn red.
2.4. Procedures
All studies took place at several sites in the U.S.A.: Study 1 in San Antonio, Texas (21 Oct to 3 November 2021); Study 2 in San Antonio, Texas and Clovis, California (25 Feb to 9 March 2022); Study 3 in Concord, Massachusetts (10 sessions, 24–25 Feb, 2022) and Chicago, Illinois (10 sessions, 1–2 Mar, 2022); Study 4 in San Antonio, Texas and Clovis, California (25 Feb to 9 March 2022).
All tasks with the safety needle took place in simulated environments, designed to be equivalent to a real hospital environment, patient exam room, etc. (e.g. Supplementary Figure). Simulated injection sites were suitable for either subcutaneous or intramuscular injections.
2.4.1. Usability and safety studies (Studies 1–3)
The study procedure followed the same process in Studies 1&2: 1) introduction and participant paperwork; 2) simulated use assessment; 3) root cause analysis; 4) final interview and closing off. In Study 3, the procedure was as follows: 1) confirmed informed consent, introduced the test session, conducted the background interview; 2) training; 3) simulated use testing; 4) final interview and closing off. In all three studies the moderator conducted the interview sessions with the participants in the interview room. Sessions in all studies were also recorded. Each study session lasted approximately 90 (Study 1), 60 (Study 2), or 45 (Study 3) minutes.
In Studies 1&2, participants were assigned to perform simulated injections within the following ‘normal use’ scenarios. Study 1: a) subcutaneous injection with 13 mm needle (30 G, 90 degrees with a skin fold); b) subcutaneous injection with 13 mm needle (30 G, 90 degrees without a skin fold); c) subcutaneous injection with 13 mm needle (30 G, 45 degrees with a skin fold); d) subcutaneous injection with 13 mm needle (30 G, 45 degrees without a skin fold); e) subcutaneous injection with 16 mm needle (25 G, 90 degrees with a skin fold); f) subcutaneous injection with 16 mm needle (25 G, 90 degrees without a skin fold) g) subcutaneous injection with 16 mm needle (25 G, 45 degrees with a skin fold); h) subcutaneous injection with 16 mm needle (25 G, 45 degrees without a skin fold); i) intramuscular injection with 25 mm needle (25 G, 90 degrees without a skin fold). Study 2: a) subcutaneous injection with 13 mm needle (30 G, 45 degrees with a skin fold); b) intramuscular injection with 25 mm needle (25 G, 90 degrees without a skin fold); c) subcutaneous injection with 16 mm needle (25 G, 45 degrees with a skin fold). Each task was categorized as a safety critical (i.e. a user task which, if performed incorrectly or not performed at all, would or could cause serious harm to the patient or user, where harm was defined to include compromised medical care), essential, or non-critical task and corresponded to a potential harm and a severity of that harm, where applicable. In Study 3, each participant performed 25 simulated injections at 45 degrees without a skin fold, using one selected version of the device (30 G, 13 mm) during their simulation session.
2.4.2. Perception and preference study (Study 4)
The study procedure in Study 4 was as follows: 1) introduction and participant paperwork (pretest); 2) simulated injections (test); 3) perception and preference study (posttest). All HCPs performed simulated injections according to four scenarios: a) subcutaneous injection with a 13 mm needle (45 degrees with a skin fold); b) intramuscular injection with a 25 mm needle (90 degrees without a skin fold); c) subcutaneous injection with a 16 mm needle (45 degrees with a skin fold); d) knowledge task assessment. Once all scenarios had been completed, the participants took part in the survey.
2.5. Data collection
2.5.1. Usability and safety studies (Studies 1–3)
During each simulation session in Studies 1&2, the moderator used a printed copy of the script to hand-annotate successes, close calls, difficulties, and use errors (definitions in ) with their frequency and corresponding root cause. This data was also collected by the observer directly in the spreadsheet, and was later verified against the moderator data and checked against the recorded videos, if necessary. A final interview with targeted questions from the moderator was conducted after completion of all scenarios to assess whether the tasks had been comprehended. All scenarios and instruction materials were addressed in the form of a questionnaire, which included open answers and answers where the participant used a Likert scale from 1 (strongly disagree) to 5 (strongly agree) to express their opinion.
Table 1. Results from two usability and safety studies of the passive safety needle in nurses, pharmacists, and physicians (studies 1&2).
In Study 3, the moderator documented key observations on paper, and reconciled the data and reviewed the video recordings, if needed. The primary objective was to demonstrate that the sharps injury prevention features of the new device were fully effective in preventing NSIs, and thus to determine the true failure rate of device. This objective was measured by calculating the number of sharps injury prevention feature failures, where failure was defined as one or all of the following outcomes during a particular simulated injection: 1) failure to activate the sharps injury prevention feature; 2) needlestick injury; 3) needle contact after administering the dose resulting in a needlestick. The secondary objective was to collect participants’ feedback regarding their interactions with the new device related to the sharps injury prevention feature, such as their overall impressions of the device’s safety and usability, opinions on the extent of the learning curve, ability to detect activation of the safety feature, and general acceptability of the device (based on the answers to a questionnaire, which included data on how much participants agreed or disagreed with a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree).
2.5.2. Perception and preference study (Study 4)
The questionnaire was based on a Likert scale design, a universal method where each question was rated on a scale of 1 (strongly disagree) to 4 (strongly agree), and included 21 ranking statement- and imagery-based questions about the passive safety needle (Supplementary Table). The data from each session was collected directly on a paper questionnaire. Any adverse incidents that were experienced were also recorded.
3. Results
3.1. Usability and safety studies (Studies 1–3)
A total of 104 participants completed 4772 tasks (): Study 1, n = 30 participants, n = 1140 tasks; Study 2, n = 54 participants, n = 3132 tasks; Study 3, n = 20 participants, n = 500 tasks. Tasks related to the whole injection process, from reading the instructions to disposal of the needle, were completed successfully 73.25%, 88.12%, and 100% of the time in the three studies, respectively. However, some use errors, difficulties, and close calls deemed safety critical were made in Studies 1&2, as outlined in . A part of the assessed tasks in the three studies, concerned successfully performing the injection itself. In total 932 injections were performed with 915 of them being concluded successfully (98.18%),
Table 2. Characteristics of participants in a usability and safety study of the passive safety needle in nurses, pharmacists, and physicians (Study 3).
Table 3. Most problematic errors, difficulties, and close calls encountered that were deemed safety critical* in the usability and safety studies of the passive safety needle in nurses, pharmacists, and physicians (studies 1&2).
Study 1. All participants completed all of their 38 different tasks. Out of the 270 planned injections, 263 (97.4%) were successfully performed by the participants. Tasks were associated with use errors in 23.95% (related to integrity verification, checking the expiry date, priming the needle, and peeling the blister pack of the needle halfway using an aseptic technique), close calls in 0.96% (mostly related to reading the instructions for use (IFU) before use and selecting the correct needle size and syringe for the medication being prescribed), and difficulties in 1.84% (particularly when using the 13 mm needle). The number of use errors was high, but was fairly evenly distributed amongst all 30 participants; only three participants completed all of their tasks with ≤ 5 use errors. The average frequency indicated that physicians (11.8) experienced more use errors than nurses (9) and pharmacists (9), but it should be noted that the physician sample size (n = 5) was lower than the other groups. Even though 273 errors occurred in this study, the majority of them did not affect the successful completion of the simulated injections. The mental model (i.e. the participant’s preexisting perception of the injection process) was deemed to be the biggest issue (n = 107 errors) followed by simulation (n = 41 errors, which included stress, the nature of the tasks, and the simulated use environment). Trust (n = 22) and design-related (n = 10) use errors were more uncommon, the latter mainly associated with the IFU materials (which were unclear about the inability of using the device with a non-prefilled syringe) and the ‘priming’ task.
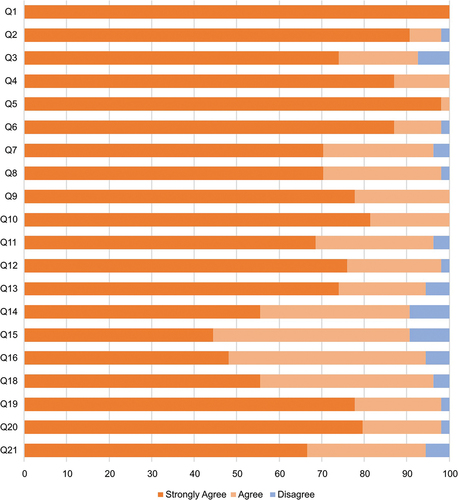
Overall, the device was regarded as ‘good’ or ‘very good’ by the majority of the participants (>67%) for each individual scenario tested (). Furthermore, the participants responded favorably to the usability of the safety needle device, with 70–97% answering ‘good’ or ‘very good’ to all specific questions ().
Figure 2. Evaluation of usability of the passive safety needle across all participants in a) Study 1 and b) Study 3. The values above the columns are number of patients in Study 1 and average rating in Study 3. * in Study 3: 17 right-handed evaluators and one ambidextrous evaluator (who performed all 25 injections with their right hand) responded to statement no. 10; two left-handed evaluators responded to statement no. 11.
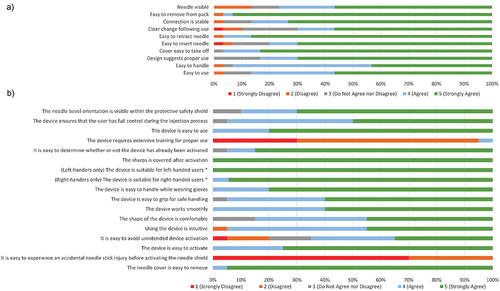
Table 4. Overall evaluation of the passive safety needle by the participants (Study 1).
Study 2. All participants completed all of their 58 different user tasks (). Out of the 162 planned injections, 152 (93.8%) were successfully performed by the participants. Tasks were associated with use errors in 9.8% (difficulties as per Study 1, but also included reading the IFU), close calls in 1.02% (such as reading the IFU and filling the syringe using a blunt fill needle), and difficulties in 1.05% (particularly when trying to hold the correct angle). Once again, use errors were fairly evenly distributed between groups (data not shown). Awareness of the simulated environment contributed to most use errors, while the mental model accounted for a large number of use errors and most close calls.
Study 3. All observations met the criteria for success. No failures were recorded in any group, i.e. no NSIs, no contact with the needle after simulated injection, and no failure of the sharps injury prevention feature. Furthermore, all 500 simulated injections were performed successfully (100%). There were four unanticipated events not considered a failure of the sharps injury prevention feature: two deviations from the planned injection method (one needle shield activated before injection, one participant did not wear gloves); one device malfunction (the shield locked in place before injection, but still prevented the needle from being exposed); one test artifact (two occurrences in one participant where the needle was pressed through the injection pad at an angle that caused the needle to bend; the participant performed an additional two injections to compensate). The impressions of the participants with respect to the safety and usability of the device, opinions on the extent of the learning curve, ability to detect activation of the safety feature, and general acceptability of the device are shown in . All of these factors were rated favorably in 85–100% of participants, but slightly less favorably for the statement, ‘It is easy to avoid unintended device activation’ (where 65% ‘agreed’ or ‘strongly agreed’).
3.2. Perception and preference study (Study 4)
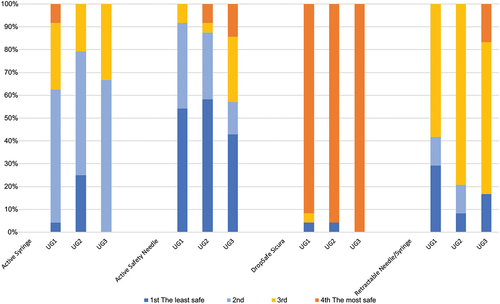
In total, 54 HCPs who took part in Study 2 were included in Study 4 (24 nurses, 24 pharmacists, 6 physicians). Of these, 55.6% had >10 years of experience at giving injections and 83.3% had >2 years of experience using both passive and active safety needle devices. More than 98% of participants responded positively to every question (), with none ‘strongly disagreeing’ with any; there were no significant differences in responses between groups. Most participants (94.44%, n = 51) thought that the passive safety needle was the safest option compared to an active syringe, active safety needle, or retractable needle/syringe ().
4. Discussion
Needlestick injuries are a common hazard in everyday clinical practice for HCPs [Citation3], usually caused by unsafe practices [Citation2]. In the US, for example, most NSIs occurred in nurses (39.4%) and doctors (30.9%), usually in the operating room/recovery (37.4%) or the patient ward/room (30.1%) [Citation10]. The NSIs happened most often during use of the item (57.5%), between steps of a multi-step procedure (10.2%), or any other task occurring after use and before disposal (11.0%) [Citation10]. It is important to ensure that safety needle devices can be used safely and effectively by the HCPs who regularly use them in daily clinical practice (i.e. nurses, pharmacists, and physicians).
We evaluated the performance of a new device in 104 HCPs who completed 4772 simulated tasks. In Studies 1&2, 84.15% (3595/4272) of tasks were completed successfully (performed according to product’s intended use) and the experience of using all three different needle sizes in various types of injections was evaluated positively by > 98% of HCPs in the perception and preference study. In particular, no NSIs occurred in any of these studies.
However, use errors, difficulties, and close calls did occur (). Use errors deemed safety critical, but unlikely to result to a NSI, included reading the IFU, washing hands, selecting appropriate protective equipment before use, verifying the integrity of the device or blister pack before use, checking the expiry date, peeling the blister pack of the needle halfway using an aseptic technique, and priming the needle. Checking the integrity and expiry date were both linked to a very clear and strong mentally-formulated model driven by current practice. For example, the expiry date was normally checked by other staff members or never checked because their institution did not have chance to reach the end of the expiry date of their used needles; ‘trust’ was commonly mentioned as a reason for not checking the integrity. Close calls were mostly related to reading the IFU before use, selecting the correct needle size and syringe for the medication being prescribed, and filling the syringe with the drug using a blunt fill needle. Difficulties encountered included attaching the device to a Luer slip/lock syringe, filling the syringe using a blunt fill needle, and holding the device at the correct angle at the injection site while administering the drug (particularly the 13 mm needle, which requires much more push force for successful administration). In Study 3, there were no failures, i.e. no NSIs, no contact with the needle after simulated injection, and no failure of the sharps injury prevention feature.
Our results indicate that the passive safety needle can be used safely and effectively and is unlikely to result in user errors that could lead to NSIs and they support other studies that demonstrate that passive safety needles are associated with the lowest rate of NSIs and are 10 times less likely to be connected with NSIs [Citation11,Citation12]. This is crucial because although some studies have found that safety needles can reduce NSIs [Citation11–14], this is not always the case [Citation7–9]. For example, in one analysis of 100,000 NSIs exposures, 18% were related to the use of safety needles [Citation8]. Most of these exposures occurred during the procedure, including disposal, and 92% involved a device with a manual activation mechanism. Other studies have demonstrated that passive safety needle devices are associated with the lowest rate of NSIs [Citation11], and are 10 times less likely to be connected with an NSIs incident [Citation12].
When examining the factors leading to NSIs when safety needle devices are used, it was found that the majority could be prevented by proper use and safe disposal of the needles, but also by providing feedback to manufacturers with respect to product design and usability [Citation7]. In our perception and preference survey (Study 4), participants first became thoroughly familiar with the new device while performing subcutaneous and intramuscular injections using different techniques and different needle size versions of the device. Based on this experience, more than 98% of the HCPs responded positively to every question and all had a high estimation of the safety of the passive technology used in the new device, particularly in comparison to similar products present on the market (a hinge cap active safety needle, retractable safety needle, and retractable safety syringe). All HCPs felt that the passive safety feature of the needle could eliminate NSIs and would better protect against bloodborne pathogens compared to the other products, and that this passive safety needle was the safest medical device. This is accordance with the hierarchy of controls recommended by organizations such as the OSHA, where improvements in engineering controls (such as those that remove the manual component of safety mechanisms, for example) can protect users from the hazard [Citation6]. The perspective from different and experienced users of safety needles obtained in our study with respect to the safety and usability of the passive safety needle is important to help other HCPs decide whether to use an active (hinge-cap or similar), semi-passive (retractable syringe), or fully passive safety device to reduce the risk of NSIs. It should be noted, however, that proper adherence to NSIs safety guidelines is always essential [Citation2,Citation14].
The inclusion of different types of users experienced in performing injections with safety needles and the simulation setting that avoids harm to patients and monitored by a moderator to prevent any risk to the users are strengths of these studies. Users were able to provide feedback regarding any aspect of the new device that caused problems. However, the simulation setting itself was a weakness because the awareness that participants were not injecting patients sometimes had an effect on their performance (and contributed to most use errors in Study 2). Furthermore, one should consider that the users used a new device, which was not similar to other routinely used, resulting to users needing some time to familiarize themselves with it. The participant’s preexisting perception of the injection process (their mental model, based on their own experience and workplace habits/procedures) also had an influence on the success of the tasks. In Study 1, for example, participants were aware that needle priming was not necessary when using a prefilled syringe, but some thought that priming would be necessary because of the size of the safety needle device compared to a conventional needle (it was also thought that priming might activate the locking mechanism of the needle or that it was quite difficult to see the drop coming out of the needle through the safety shield). In Study 2, all participants had experience in using needles and assumed the safety needle device would be similar to other passive safety needles currently on the market. The mental model was also associated with most close calls, as participants often assumed that the working principle of the safety needle device was the same or very similar to other needles, leading them to apply the experience they had gained working in their current practices. It is clearly important to verify whether the new passive safety needle can reduce or even prevent NSIs in a real-world setting, where the bias of simulation is likely to be eliminated because the mind-set of the user will be different. The mental model may also represent a learning curve that must be overcome to ensure that safety needles are used correctly according to their specific IFU. Our results indicate that the IFU for this new safety needle require adjustments to ensure that all instructions are clear and precise.
5. Conclusions
These presented studies provide a realistic perspective of how the passive safety needle could be safely and effectively used in combination with a (pre-)filled syringe for subcutaneous and intramuscular injections in real life. The new device was used successfully by most participants in usability and safety studies with no NSIs. Minor errors of use were observed but none resulted in NSIs in any of the studied groups. In the perception and preference study, all participants rated the device as the safest compared to similar devices and potentially useful in NSI prevention.
Declaration of interests
A Serafin was an HTL-STREFA S.A. employee at the time of the study. A Ryk and W Fendler have received a financial sponsorship by PIKDARE S.p.A to support the study design and data analysis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
A Serafin and W Fendler were involved in the conception and design of the study. A Serafin, A Ryk and W Fendler were involved in the analysis and interpretation of the data, the drafting and critical revision of the manuscript as well as the final approval of the version submitted for publication. All the authors agree to be accountable for all aspects of the presented work.
Supplemental Material
Download Zip (1.4 MB)Acknowledgments
The authors would like to thank Deborah Nock for her assistance in preparation of the draft manuscript.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/17434440.2023.2254680
Additional information
Funding
References
- Bouya S, Balouchi A, Rafiemanesh H, et al. Global prevalence and device related causes of needle stick injuries among health care workers: a systematic review and meta-analysis. Ann Glob Health. 2020;86(1):35. doi: 10.5334/aogh.2698
- King KC, Strony RN. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;1 Jan 2022
- Alfulayw KH, Al-Otaibi ST, Alqahtani HA. Factors associated with needlestick injuries among healthcare workers: implications for prevention. BMC Health Serv Res. 2021;21(1):1074. doi: 10.1186/s12913-021-07110-y
- Cooke CE, Stephens JM. Clinical, economic, and humanistic burden of needlestick injuries in healthcare workers. Med Devices (Auckl). 2017;10:225–235. doi: 10.2147/MDER.S140846
- Occupational Safety and Health Administration. Needlestick safety and prevention act [cited 15 Apr 2023; available at https://www.govinfo.gov/content/pkg/BILLS-106hr5178enr/pdf/BILLS-106hr5178enr.pdf.
- Occupational Safety and Health Administration. Recommended practices for safety and Health programs. Identifying hazard control options: the hierarchy of controls [cited 15 Apr 2023; available at https://www.osha.gov/safety-management/hazard-prevention.
- Schuurmans J, Lutgens SP, Groen L, et al. Do safety engineered devices reduce needlestick injuries? J Hosp Infect. 2018 Sep;100(1):99–104. doi: 10.1016/j.jhin.2018.04.026
- Ottino MC, Argentero A, Argentero PA, et al. Needlestick prevention devices: data from hospital surveillance in Piedmont, Italy—comprehensive analysis on needlestick injuries between healthcare workers after the introduction of safety devices. BMJ Open. 2019;9(11):e030576. doi: 10.1136/bmjopen-2019-030576
- Lavoie MC, Verbeek JH, Pahwa M. Devices for preventing percutaneous exposure injuries caused by needles in healthcare personnel. Cochrane Database Syst Rev. 2014;3: CD009740. doi: 10.1002/14651858.CD009740.pub2
- International Safety Center. U.S. Epinet sharps injury and blood and body fluid exposure surveillance research group. Sharps Injury Data Report For 2021; 41 Hospitals Contributing Data; 1684 Total Injuries [cited 2 Jun 2023 available at: https://internationalsafetycenter.org/exposure-reports/.
- Tosini W, Ciotti C, Goyer F, et al. Needlestick injury rates according to different types of safety-engineered devices: results of a French multicenter study. Infect Control Hosp Epidemiol. 2010;31(4):402–407. doi: 10.1086/651301
- Jackson AP, Almerol LA, Campbell J, et al. Needlestick injuries: the role of safety-engineered devices in prevention. Br J Nurs. 2020;29(14):S22–S30. doi: 10.12968/bjon.2020.29.14.S22
- Lamontagne F, Abiteboul D, Lolom I, et al. Role of safety-engineered devices in preventing needlestick injuries in 32 French hospitals. Infect Control Hosp Epidemiol. 2007 Jan;28(1):18–23. doi: 10.1086/510814
- Cullen BL, Genasi F, Symington I, et al. Potential for reported needlestick injury prevention among healthcare workers through safety device usage and improvement of guideline adherence: expert panel assessment. J Hosp Infect. 2006;63(4):445–451. doi: 10.1016/j.jhin.2006.04.008