Abstract
Titanium dioxide white pigment consists of particles of various sizes, from which a fraction is in the nano range (<100 nm). It is applied in food as additive E 171 as well as in other products, such as food supplements and toothpaste. Here, we assessed whether a human health risk can be expected from oral ingestion of these titanium dioxide nanoparticles (TiO2 NPs), based on currently available information. Human health risks were assessed using two different approaches: Approach 1, based on intake, i.e. external doses, and Approach 2, based on internal organ concentrations using a kinetic model in order to account for accumulation over time (the preferred approach). Results showed that with Approach 1, a human health risk is not expected for effects in liver and spleen, but a human health risk cannot be excluded for effects on the ovaries. When based on organ concentrations by including the toxicokinetics of TiO2 NPs (Approach 2), a potential risk for liver, ovaries and testes is found. This difference between the two approaches shows the importance of including toxicokinetic information. The currently estimated risk can be influenced by factors such as absorption, form of TiO2, particle fraction, particle size and physico-chemical properties in relation to toxicity, among others. Analysis of actual particle concentrations in human organs, as well as organ concentrations and effects in liver and the reproductive system after chronic exposure to well-characterized TiO2 (NPs) in animals are recommended to refine this assessment.
Introduction
Titanium dioxide white pigment (TiO2) is a commonly used pigment in food (additive known as E 171), but it is also applied in paints, coatings, pharmaceuticals, and cosmetics, including toothpaste (Shi et al., Citation2013). The white color is best achieved with particles of 200-300 nm. However, during the production of such particles, also particles of <100 nm (i.e. nanoparticles, or NPs) are produced (TDMA, Citation2013). The presence of NPs in E 171 and in several food products has been reported (e.g. Peters et al., Citation2014; Weir et al., Citation2012).
TiO2 exists in different crystal structures: anatase, rutile and brookite, or a mixture of these. The US FDA has approved the use of TiO2 in food in 1966 by allowing levels up to 1% in food (FDA, Citation2015). The use of TiO2 in anatase form has been accepted as a food additive in the EU for decades as well at quantum satis (i.e. as much as necessary) for a selected list of products (Food additives database). Since 2004, also rutile TiO2 is allowed (EFSA, Citation2004; Directive 2008/128/EC). Since 1969, the establishment of an acceptable daily intake (ADI) for TiO2 has been considered unnecessary by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, Citation1970). JECFA justified this conclusion based on the insolubility, low absorption, absence of accumulation in tissues and absence of toxic effects of TiO2. Recent studies with TiO2 NPs, however, show that these particles can have toxic effects (e.g. reviews of Iavicoli et al., Citation2011,Citation2012; Shi et al., Citation2013) and repeated exposure to these particles may result in significant tissue accumulation at a higher age (Geraets et al., Citation2014).
With actual oral intake of TiO2 NPs and recent indications of tissue accumulation and toxic effects from these NPs, it is relevant to assess whether the exposure to the NPs present in the pigment grade TiO2 can lead to health risks. A first assessment in this direction has been performed by Bachler et al. (Citation2015), who estimated the oral intake of TiO2 NPs for the German population, calculated the resulting steady state organ concentrations, and found that these were lower than the concentrations that have been reported to cause effects in vitro. We currently aim to refine this assessment by (1) inclusion of higher quality data on NP content of E 171, (2) inclusion of more data on TiO2 levels in products, (3) inclusion of newer and higher quality toxicokinetic data to estimate tissue distribution and accumulation over time, (4) use of in vivo hazard data, selected from an extensive literature review; and (5) inclusion of effect concentrations in reproductive organs, based on recent hazard findings.
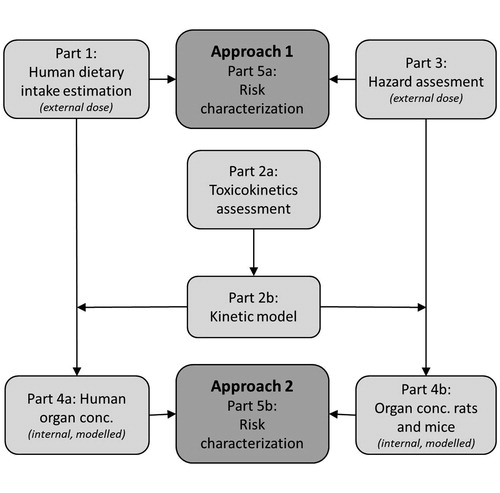
Rompelberg et al. (Citation2016) estimated the oral intake of total TiO2 and TiO2 NPs from food, toothpaste and supplements for the Dutch population. Here, the available information on hazard and toxicokinetics was gathered to assess whether this intake could lead to human health risks. For nanomaterials, it was concluded by the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) that in principle the traditional approach of risk assessment as used for chemicals would be applicable i.e. using the subsequent information blocks on exposure assessment, hazard identification, hazard characterization, and finally the risk characterization (SCENIHR, Citation2009). In addition to this traditional approach (Approach 1, based on external doses), a risk assessment using a kinetic model (Approach 2) was performed, where the risk characterization was based on internal concentrations and life-long exposure and tissue accumulation were taken into account in a quantitative way. A risk assessment that considers internal dose is relevant especially for (nano)materials that show potential for tissue accumulation, as illustrated for synthetic amorphous silica (SAS) (van Kesteren et al., Citation2015). A scheme of this dual risk assessment process is presented in . The different parts in risk assessment, described in for both Approach 1 and 2, are described in more detail below. Finally, assumptions in the risk assessment and data gaps are discussed in order to come to a balanced view on the potential health risks of TiO2 NPs in food. Generally, TiO2 NP data were preferentially used, but where relevant, data on titanium white pigment was also included.
Figure 1. Scheme of the risk assessment process. In approach 1, the external human dietary intake (Part 1, described by Rompelberg et al., Citation2016) and the lowest NOAEL from toxicity studies (Part 3) are compared for the risk characterization (Part 5a). For approach 2, the external intake estimation and the NOAEL are extrapolated to internal doses (Parts 4a and 4b) using the toxicokinetic assessment and a kinetic model as illustrated in Parts 2a and 2b, resulting in a risk characterization based on internal concentrations (Part 5b).
Part 1: human dietary intake
The dietary intake has been estimated by Rompelberg et al. (Citation2016) for TiO2 added during the production process and for TiO2 NPs therein. The intake was based on intake via food, supplements and toothpaste, according to the Dutch National Food Consumption Survey (DNFCS), and on the average measured total Ti or TiO2 particle levels in such products. Subsequently, the variation in amounts and products consumed in the population was taken into account by considering the P95 of the TiO2 NPs intake, i.e. 95% of the population has an intake equal to or lower than the P95. The P95 of the TiO2 NPs intake of each age group was estimated to be as follows:
4.2 μg/kg bw/d for children of 2–6 years old,
1.6 μg/kg bw/d for ages 7–69 years, and
0.74 μg/kg bw/d for humans of 70 years and older.
For the risk assessment, children of 0–1 years old were assumed to have no intake of TiO2 and children of 1–2 years old were assumed to have the same intake as those of 2–6 years old.
Part 2: toxicokinetics assessment
Toxicokinetics study for TiO2 NPs
The best publicly available toxicokinetic data for modeling at the start of this work were the data of Geraets et al. (Citation2014). The data available to and used by Bachler et al. (Citation2015) for calibrating their PBPK model (the data of Xie et al., (Citation2011)) was measured by labeling the TiO2 NPs with iodine. Bachler et al. (Citation2015) discussed that it seems that this label was detached from a large part of the NPs at a certain point in time. This instability of the label may provide unreliability in the data of Xie et al. (Citation2011) which is not present in the data of Geraets et al. (Citation2014).
Geraets et al. (Citation2014) administered male and female Wistar rats with different forms of TiO2 NPs (see Supplementary Material, Table S1) by the intravenous (i.v.) route and/or oral route on five consecutive days followed by a recovery period up to day 90. Tissues were collected and analyzed for the element Ti at day 6, 14, 30 and 90 (i.v.) or only at day 6 (oral). The fraction absorbed after oral ingestion was estimated to be maximally 0.02%. After i.v. exposure, Ti distributed rapidly from the systemic circulation to various tissues. The liver was found to be the main target tissue followed by spleen and lung. Elimination was found to be low (54-86% of the administered dose remained in the organs after 90 days recovery), thus leading to accumulation of Ti in various organs after repeated dosing. Such an accumulation would not be expected based on the data of Xie et al. (Citation2011) and was not included in the PBPK model of Bachler et al. (Citation2015).
In some studies it is debated whether TiO2 NPs are absorbed after oral exposure, as an increase in organ levels is not always found (Cho et al., Citation2013). Other studies, however, suggest that there is absorption based on the dose-dependent increase of Ti in various organs in oral studies with NPs and visual detection of (TiO2) particles in these organs (Tassinari et al., Citation2014). In the Supplementary Material (section 2), it is shown how the weight of evidence points to the presence of absorption, albeit at very low levels.
Kinetic model
A kinetic model () was constructed to enable the calculation of target doses, defined as concentration of TiO2 NPs in the various organs where effects were found in the key studies (see Part 3). The model was based on the data obtained after i.v. administration of TiO2 NPs (Geraets et al., Citation2014), given that these contained less non-detects and had a better signal-to-noise ratio than the oral data reported in the same study. For fitting the model to the data, blood (directly receiving the i.v. applied dose and being the means of the systemic transport to the various tissues), liver and spleen (where the highest levels of TiO2 NPs were found) were considered as separate compartments in the model. Other organs were combined in a “rest” compartment. The number of sampling time points in the study did not allow for a more complicated model without loss of predictive power. The following aspects were included:
the kinetics of tissue distribution is mainly determined by the kinetics of uptake by macrophages and internalization by other tissue cells and to a lesser extent by the NP transport by blood flow or by diffusion into tissue. This is similar to the approach applied by Bachler et al. (Citation2015) in their model;
like for other NPs (Lankveld et al., Citation2010), the kinetics of uptake into tissues of TiO2 is fast compared to the kinetics of translocation and elimination during the terminal phase;
TiO2 elimination occurs from the liver only (a decrease of liver levels was observed);
there is some translocation from the liver to the spleen (an increase of spleen levels was observed long after the last dose).
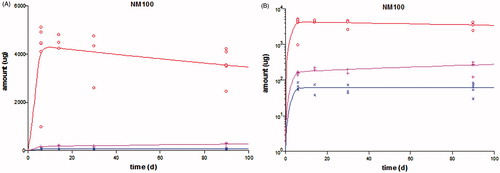
The model was fitted to the entire rat experimental i.v. dataset containing a liver, a spleen and a “rest” compartment, for each of the different forms of TiO2 (anatase: NM100, NM102; rutile: NM103 and NM104; numbering according to JRC Repository, see Supplementary Material, Table S1 for details about the physico-chemical properties). The different kinetic constants obtained for these different forms of NPs are found in the Supplementary Material (Table S5), as well as the model script. An example of the fit to the data for one form of TiO2 is found in . Figures with the model fit to the data of the other particles types are found in the Supplementary Material (Figure S1). Interestingly, the different NPs showed different kinetic behavior: the rutile NPs in this study reached up to a factor of four higher levels in the spleen than the anatase NPs. Nevertheless, data on other forms of TiO2 are needed to make any general conclusions on the relationship between physicochemical properties of the NPs and kinetic behavior. After calibration of the model parameters by fitting the model to the data, several modifications were made to the model.
Figure 2. Scheme of the kinetic model for TiO2 NPs. The fraction Floss accounts for an apparent difference in the total amount of TiO2 recovered in blood and the sampled tissues and the total amount of TiO2 NPs administrated, which can be explained by a difference in nominal and actually administered dose, or loss of the NPs in e.g. tissues that were not sampled. P.O. = per oral, I.V. = intravenous, Fabs= fraction absorbed, kabs = kinetic rate of absorption, the kp’s are kinetic rates from the blood to the liver (l), spleen (s), gonads (g) or rest (r), or from liver to spleen (ls).
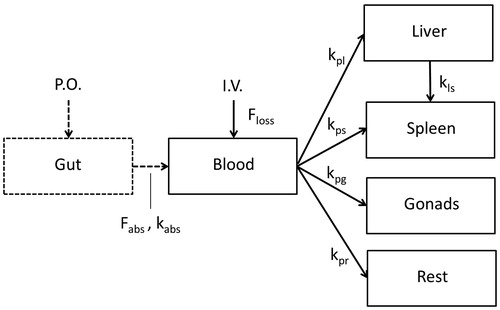
Figure 3. Result of model fit (line) to the measured kinetic data (symbols) in rats after 5 consecutive days of i.v. dosing of NM100 and subsequent follow-up until day 90 (data from Geraets et al. (Citation2014)). The amount of the element Ti found in the various organs is plotted on normal linear scale (A) or logarithmic scale (B). The red symbols and lines are the data and model fit in liver, the pink those in spleen, and the blue those in the “rest”.
Firstly, an additional “gut” compartment for modeling oral absorption was included for predictions from orally applied doses, assuming 0.02% absorption (Fabs) (see Supplementary Material section 2 for substantiation of this value). For kabs, a value of 67 d−1 (per one quarter of an hour) was applied, which was based on expert judgement. The model appeared to be quite insensitive to this parameter (see Supplementary Material, Figures S3 and S4). This model is based on the assumption that the distribution of TiO2 particles after oral absorption is the same as after i.v. dosing. A model with an oral distribution, where the blood stream from the intestines first passes the liver before any other organ, showed no significant differences in organ concentrations. This may be due to the low uptake rate constant in liver, in comparison with the intestines. However, toxicokinetic behavior may also be influenced by the presence and composition of a protein corona. This, in turn, is dependent on the surrounding environment of a NP, which is different for oral and i.v. exposure. Indeed, adherence and uptake by enterocytes was found to be affected (in different directions) by the presence of food proteins and intestinal fluid on the surface of NPs (Sinnecker et al., Citation2014). This may lead to some inaccuracy in our kinetic model for oral exposures. Nevertheless, to the best of our knowledge, our approach is currently the best approximation given that there are no suitable oral kinetic data to fit a model.
Secondly, a parameter for growth was added to the rat model, based on the data of Luecke et al. (Citation2007), to allow organ level calculations in young animals and children.
Thirdly, in the hazard assessment part, key studies showed toxic effects in ovaries and testes after low oral doses of TiO2-NP (see Part 3). Therefore, these organs were added to the model in a separate gonad compartment. Fitting all parameters of the extended model to the data was problematic, as the values for the gonads differed by several orders of magnitude to those for the other organs. Therefore, the kinetic constants for the gonads were determined by fitting the extended model to the data, with the parameters for the other compartments fixed (determined in the earlier fit with the simpler model). This had no consequences for the rest of the model, as the transfer rate to ovaries or testes is negligible compared to those to liver, spleen and rest compartment (see Supplementary Material, table S6). The model was also divided in a female version and a male version, using the physiological data of Luecke et al. (Citation2007). It should be noted that the Ti concentration data for gonads are limited because the females were only analyzed during two time points (day 6 and 90), while the males were analyzed on all four time points (Geraets et al., Citation2014). The levels in the testes were just on or above the limit of detection (LOD) at the first time point (day 6) and decreased to below the LOD at day 90 or earlier. The limited ovary data indicate no elimination. Testes were assumed to have no elimination either and this worst-case assumption was taken due to the limited data, i.e., the data did not allow to observe a clear decrease, and an increase was not observed either.
Finally, the kinetic model was used to calculate the organ levels of TiO2 in the selected rat toxicity studies, using the kinetic constants of the most similar type of NP (by expert judgement, see Part 4). To calculate the human organ burdens from the human intake, the rat model was converted to a human model using the physiological data of Luecke et al. (Citation2007), and kinetic parameters of the rat which were scaled allometrically to those of humans (transfer, translocation and elimination rates). As the relative testes weight of humans is about a factor 20 lower than that of the rat, an additional correction factor of 0.05 was applied to the kinetic uptake constant for the testes of the human. Since some toxicity studies were performed with mice, a mouse model was also made by applying the physiological data of Luecke et al. (Citation2007) and allometric scaling of the kinetic parameters. The relative testes weight of the mouse did not differ much from that of the rat, so no additional correction factor was used for the mice studies.
A sensitivity analysis for the kinetic model can be found in the Supplementary Material (Figures S3 and S4). The highest relative sensitivity was 1, for the fraction absorbed, meaning the % change in the organ concentration was equal to the % change in the fraction absorbed. This was according to theoretical expectation. Additionally, the model prediction for the liver concentrations in the oral study described by Geraets et al. (Citation2014) was compared to the measured liver concentrations in that study (see Supplementary Material, Figure S5). The predicted concentrations were higher than the measured concentrations by a factor 1.5 to > 4. This is not unexpected, as the value for fraction absorbed, which is applied in the model, is a worst-case estimation.
Part 3: hazard assessment: key toxicity data of TiO2 (NPs)
Hazard data of TiO2 and TiO2 NPs were compiled and key toxicity data selected for further risk assessment. A literature research was performed for data on the toxic effects of TiO2, both in bulk and in NP form. Important sources were the review of Iavicoli et al. (Citation2012), the SCCS opinion on TiO2 in nano form (SCCS, Citation2013), the public IUCLID dossier , the OECD SIAR, the data collected by the OECD WPMN , and a Scopus literature search. Only in vivo oral studies were considered, as this risk assessment focuses on the risks via food and the derivation of risks from in vitro studies is still under development. I.v. toxicity studies were evaluated to serve as supportive qualitative information, i.e. on type and possible mechanisms of induced effects. The studies of the Medical College of Soochow University (Suzhou, China) were not considered, as some were retracted by the journal (Gui et al., Citation2015; Zhao et al., Citation2015) and others showed the same 5% standard deviation for all measured values, i.e. application of the same inappropriate statistical methods.
From the available studies (full list available in the Supplementary Material, Table S7), key studies were selected using the following criteria:
Repeated exposures, preferably chronic studies;
Adequate characterization of the TiO2 (NPs);
Lowest value of the No Observed Adverse Effect Level (NOAEL) or of the Lowest Observed Adverse Effect Level (LOAEL) among similar studies on the same endpoints;
Preferably including hazard analysis of liver and spleen, as distribution of Ti to these tissues is highest;
Coverage of additional endpoints/tissues/organs;
Coverage of both rutile and anatase TiO2, non-coated.
No single study fulfilled all these criteria, therefore four key studies and one major supportive study were selected (see ).
Table 1. Details of four key studies followed by one supportive study for the risk assessment of TiO2 NPs in food.
The study with the longest exposure period was selected as the first key study. This is the 2-year oral carcinogenicity study of the NCI, which concludes that no adverse effects or carcinogenicity were found up to a dose of 2500 mg/kg bw/d of pigment TiO2 (NCI, Citation1979). The annexes of the report, however, do show some dose-related non-neoplastic effects (see for details) that indicate a provisional LOAEL of 1250 mg/kg bw/d. Because the TiO2 material was not well characterized in this study, there is no information on the presence of NPs in the tested substance. Therefore, other studies with well-characterized NPs were added to the selection of key studies.
The studies applying the longest exposure of all studies with characterized and uncoated TiO2 NPs, were those of Chen et al. (Citation2015), Wang et al. (Citation2013) and the 28-d study in Warheit et al. (Citation2015a). Chen et al. (Citation2015) focused on cardiac toxicity and inflammatory response in initially young rats (3 weeks old at the start of the study) after 30 and 90 days of exposure. They report decreases of lactate dehydrogenase (LDH), hydroxybutyrate dehydrogenase (HBDH), and triglycerides and increase of white blood cells and granulocytes at 50 mg/kg bw/d after 90 days. Warheit et al. (Citation2015a) reported no effects after a daily dose of 24 000 mg/kg bw/d; the only dose tested in their study following OECD TG 407. Wang et al. (Citation2013) investigated effects in liver and spleen in adult male rats (8 weeks old at the start of the study) as well as young rats (3 weeks old at the start of the study) after 30 days of exposure. No effects were found in spleen for both age groups at any dose, but adverse effects on the liver were observed and differed between the age groups. In young rats, liver edema and corresponding decreased serum levels of the enzyme aspartate aminotransferase (AST) were observed at 50 and 200 mg/kg bw/d, and increased serum levels of total bilirubin (TBIL) at 200 mg/kg bw/d. The alterations in these serum enzyme parameters indicate liver damage in the young animals after oral TiO2 exposure. In adult rats, only infiltration of inflammatory cells was seen at 10 and 50 mg/kg bw/d (and not at 200 mg/kg bw/d) and also increased serum levels of TBIL were seen at 200 mg/kg bw/d. More information on these markers for liver damage can be found in the Supplementary Material (section 4).
The infiltration of inflammatory cells in livers of adult rats was considered to indicate only a slight liver damage. In addition, small foci of inflammatory cells can be regularly observed as background histopathology in most rat strains. In comparison, more serious liver damage was observed in the young rats, mainly based on edema and the alterations in serum liver enzymes/proteins. TBIL alterations can be caused by increased bilirubin production (e.g. by internal hemorrhage), decreased liver metabolism, or obstruction of the bile ducts. The observed increase in TBIL in the study of Wang et al. (Citation2013) supports the other signs of liver damage and could also match the hyperplasia of bile ducts seen in the NCI study (1979) at 1250 mg/kg bw/d and higher.
The study of Chen et al. (Citation2015), evaluating the effects of TiO2 NPs on the cardiovascular system after oral administration, was not used in the risk assessment, because the distribution to the liver is >500-fold higher than the distribution to the heart (Geraets et al., Citation2014), and the NOAELs of Chen et al. (Citation2015) and Wang et al. (Citation2013) were comparable.
Shukla et al. (Citation2014) detected liver effects in adult mice at lower doses than in the adult rats of Wang et al. (Citation2013), after 14 days of exposure. This study is used as a supportive study. They observed that ALT and alkaline phosphatase (ALP) increased at 10 mg/kg bw/d and higher dosage, indicating possible bile duct obstruction or infiltrative diseases of the liver. Notably, no change in TBIL was observed, which could be expected to accompany the increased ALP levels. Further, the relative liver weight increased significantly at 100 mg/kg bw/d. DNA damage in liver cells was shown by an increase in the Olive tail moment (OTM) in the comet assay at 10 mg/kg bw/d and higher.
Other studies with TiO2 NPs also report effects on markers indicating liver damage (Orazizadeh et al., Citation2014), which were observed in combination with clear histopathological effects (Wang et al., Citation2007; Abdel Azim et al., Citation2015), albeit at higher doses. All collected studies with well-characterized TiO2 NPs in which effects on liver were investigated, reported effects in the liver, except the guideline study described by Warheit et al (Citation2015a). The latter was performed with a single, exceptionally high dose of TiO2 pigment, which may, for example, have resulted in lower relative absorption, reduced peristaltic movements, etc. Furthermore, this study was not performed on young rats, whereas Wang et al. (Citation2013) mostly found effects in young rats. The study of Wang et al. (Citation2013) was therefore selected as the second key study for hazard identification. Mechanistically, the effects on the liver can be explained by the induction of oxidative stress induced by TiO2 NPs, which can lead to DNA damage and inflammatory responses, and eventually cell death.
Based on our evaluation of the key study of Wang et al. (Citation2013), supported by other studies, a provisional Point of Departure (PoD) for effects on liver (liver edema and changes in markers for liver damage) with a NOAEL of 10 mg/kg bw/d was selected. Given that no effects were found on the spleen, a provisional PoD of 200 mg/kg bw/d was selected and considered a NOAEL, derived from the study with TiO2 NPs that had the longest exposure time (Wang et al., Citation2013).
Wang et al. (Citation2013) and Shukla et al. (Citation2014) did not study the reproductive system, while Jia et al. (Citation2014) found effects on the male reproductive system of mice at a dose comparable to that where liver effects were found. Jia et al. (Citation2014) used a slightly longer exposure time (42 days). At 50 mg/kg bw/d and higher, increased sperm abnormalities, decreased serum testosterone and an increase in vacuoles in the seminiferous tubules were observed. At the highest dose (250 mg/kg bw/d), a decreased body weight and decreased layers of spermatogenic cells were seen. These effects in the male reproductive system are supported by an in vitro study showing uptake of TiO2 NPs in Leydig cells and cytotoxicity and anti-proliferative effects of TiOs NPs on these cells (Komatsu et al., Citation2008).
Effects on the endocrine and reproductive system were also seen in the rat study of Tassinari et al. (Citation2014). They did not observe any effect on the male reproductive system, however, they applied lower doses (1 and 2 mg/kg bw/d) and a shorter exposure time (5 days) than Jia et al. (Citation2014). In contrast to the observation of Tassinari et al. (Citation2014) of an increase in serum testosterone in the males, Jia et al. (Citation2014) reported a decrease in serum testosterone. High local levels of testosterone in the seminiferous tubules are necessary to maintain the process of spermatogenesis, therefore a decrease in testosterone levels better matches the increase in sperm abnormalities and spermatogenic layers. While Jia et al. (Citation2014) only looked at the male reproductive organs, Tassinari et al. (Citation2014) also studied females and investigated the thyroid and adrenals in both sexes. They found histopathological effects and serum hormone level changes at 1 mg/kg bw/d. It is remarkable that such effects are seen at such low doses after 5 days exposure only, however the occurrence of endocrine effects is supported by the study of Jia et al. (Citation2014) and the effects seen in offspring after subcutaneous dosing by Takeda et al. (Citation2009).
In contrast, Warheit et al. (Citation2015a) showed no testes weight changes in a 28-d guideline study with TiO2 pigment and reported that no microscopic pathology was observed in the testes. Females were not included in this study. As discussed for liver effects, the study of Warheit et al. (Citation2015a) was performed with an exceptionally high dose and not in young animals as applied by Jia et al. (Citation2014). Taken together, the studies of Jia et al. (Citation2014) and Tassinari et al. (Citation2014) were selected as additional key studies. Due to the limited and contrasting data, we used a provisional PoD of 10 mg/kg bw/d (Jia et al., Citation2014) for effects on the male reproductive system and a provisional PoD of 1 mg/kg bw/d, based on a LOAEL for the thyroid and female reproductive system.
Other recent oral reprotoxicity guideline studies performed with rabbits observed no effects after exposure to six different well-characterized TiO2 materials, including pigments and fully nano-sized TiO2 (Warheit et al., Citation2015b). These studies focused on developmental toxicity, and do not cover the reproductive organ toxicity effects reported by Jia et al. (Citation2014) and Tassinari et al. (Citation2014). Developmental toxicity studies, such as the ones of Warheit et al. (Citation2015b), do not analyze hormone levels and organ histopathology, for example, and only dose female rats that are already pregnant from gestation day 5 onwards. Effects of a substance on the ability to become pregnant are thus not investigated in these studies. It is therefore considered that the negative guideline studies do not overrule the positive studies of Jia et al. (Citation2014) and Tassinari et al. (Citation2014).
Part 4: determination of internal concentrations
Organ concentrations of TiO2 NPs were calculated for the rats and mice in the key studies and for the Dutch consumer using the estimation for oral intake (Rompelberg et al., Citation2016) and the kinetic model described in Part 2. For each calculation, the kinetic constants of the particle type corresponding best with the tested or consumed particle type were applied, based on expert judgement. First, the crystal form was matched, followed by the primary particle size (details provided in the Supplementary Material, Table S8). For the human intake, the varying intake over the three different age groups (see description in Part 1 and Rompelberg et al. (Citation2016)) was given as input.
Because of this varying intake over age groups, growth of the children, elimination from the liver and accumulation in the spleen, the organ levels were found to increase in a non-linear manner with age, and spleen levels even increasing at >70 years, as can be seen in Figure S2 in the Supplementary Material. For the risk assessment, the organ levels for three different ages were taken: 20, 40, and 80 years old. This enabled an assessment at which age a human health risk might occur. In the animal studies, the organ levels were predicted for the age at which the effects were observed, i.e. at the end of the study.
Part 5: risk characterization
Based on the current information on intake, toxicokinetics, and toxicity, a risk characterization of TiO2 NPs from oral intake was performed. As indicated previously, the risk characterization was performed in two ways: by the traditional approach, based on external exposure (human) and dose (animals) levels inducing no adverse effects (NOAEL) or an adverse effect (LOAEL) (Approach 1), and by an approach based on internal dose levels by using toxicokinetic modeling that considers the specific organ concentration inducing a toxic effect and accounts for tissue accumulation over time (Approach 2).
Approach 1: traditional approach (Part 5a in )
For this approach, a lifelong daily intake (averaged over the age groups) is necessary to compare to the daily dose in the animal studies. The lifelong daily intake of TiO2 NPs for the P95 of the population was calculated to be 1.7 μg/kg bw/d (Rompelberg et al., Citation2016), based on the intakes given in Part 1. The average life expectancy applied for the Dutch population is 80 years, as reported by the Dutch Central Bureau of Statistics (CBS) for 2012 (CBS, Citation2014). This external human exposure level was compared to the lowest NOAELs or LOAELs found in the key toxicity studies, resulting in margins of exposure (). Acceptable margins of external exposure (MoEe) were calculated from the relevant safety factors applicable in each case (ECHA, Citation2012). Safety factors were taken from REACH guidance by lack of specific guidance on these factors for food additives. These MoEe do not include any additional uncertainty related to the limited knowledge on the NPs at stake, for example related to the dosimetry and differences in TiO2 properties in food and in toxicity studies. These acceptable MoEes presented here have not been subject to international review and the derived risk estimations should therefore only be used for the purpose of this evaluation.
Table 2. Derived and acceptable margins of external exposure (MoEe) for the different effect levels found in the key studies, based on external doses. MoEes lower than the estimated acceptable MoEe are indicated in bold.
For three of the four key studies, the MoEes from the provisional NOAELs or LOAELs were above the estimated acceptable MoEes, indicating an unlikely safety concern. Only the MoEe for the study of Tassinari et al. (Citation2014) was lower than the estimated acceptable MoEe, mainly caused by the safety factors that had to be applied to these study results. Due to the extrapolation from a 5-day study to lifelong exposure, a high safety factor had to be applied.
Taking the P95 for the intake for every age group to calculate a lifelong daily intake of TiO2 NPs for the P95 of the population is a worst-case approach. It assumes that the dietary ingestion of TiO2 for a person would be in the same high 5% group (or lower 95%) at every age during his or her life and thus that a person would choose the brands with highest TiO2 levels and consume these in large quantities during his or her entire life, which is an unlikely scenario. If a risk appears for this worst-case exposure scenario, it is relevant to also assess the risk with the average lifetime intake of TiO2 NPs based on the mean intakes per age group, instead of the P95 intakes. The average lifetime intake of TiO2 NPs based on the mean intakes per age group is 0.62 μg/kg bw/d (Rompelberg et al., Citation2016). For the effects on ovary found in Tassinari et al. (Citation2014), the MoEe is then 1613, which is still below the estimated acceptable MoEe.
Thus, based on external exposures, a risk for effects on the liver, spleen and testes is unlikely, but a risk for effects on the ovaries cannot be excluded. The latter is based on extrapolation from a very short term study (5 days) to chronic exposure, therefore confirmation of these results in longer term studies is recommended.
Approach 2: internal dose approach (Part 5b in )
For the risk characterization according to Approach 2, the estimated internal doses obtained from Part 4 were compared; the organ concentrations at which effects were found in the animal studies versus the organ concentrations in humans estimated from their consumption of TiO2-containing food. The resulting margins of internal exposure (MoEi, ) were compared to the estimated acceptable MoEis. Considered safety factors included remaining interspecies differences, intraspecies differences, study duration and the difference between a NOAEL and LOAEL, but did not include allometric scaling within the interspecies differences, as this was already included in the model calculations. As previously mentioned, these evaluated acceptable MoEis have not been subject to international review and therefore should be used for the sole purpose of this evaluation. The precise values of all calculated MoEis can be found in the Supplementary Material (Table S9).
Figure 4. Margins of internal exposure (MoEis) between the organ concentrations resulting from food intake of the human population (P95) at 20 years (bars without pattern), 40 years (striped bars) and 80 years (bars with block pattern) and the organ concentrations at which effects were found in these organs in the animal studies referenced. The calculated MoEis for the study of Tassinari et al. (Citation2014) are below 1 and therefore not visible on the scale of the graph. The black lines show the estimated acceptable MoEi for that study, correcting for inter- and intraspecies differences, study duration and use of a LOAEL instead of a NOAEL, where applicable. Green bars indicate MoEis above the estimated acceptable MoEi (i.e. no risk anticipated), while red bars indicate MoEis lower than the estimated acceptable MoEi (i.e. a risk is possible).
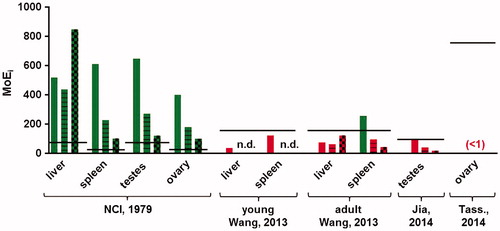
The MoEis decrease with age for all organs except the liver, which reflects the accumulation of TiO2 in the spleen and gonads, and the decrease in liver concentration after age 70 due to the reduced intake and continuous elimination from the liver. further shows that when the MoEis are based on the NCI 2-year carcinogenicity study with uncharacterized TiO2, (which is assumed to be pigment-grade; NCI, Citation1979), they are higher than the estimated acceptable MoEi; indicative of an unlikely risk. However, the NP content of the tested material in this study is unknown. For all three studies with TiO2 NPs, the MoEis were lower than the estimated acceptable MoEi, indicative of potential risks for effects on the liver, testes, and ovary. Most data for spleen also indicate MoEis lower than the estimated acceptable ones, but one should keep in mind that the NOAEL applied here was the highest dose tested, thus higher doses may also have shown no effects. Based on the available information it is not possible to draw a conclusion on a risk for effects on this organ.
It should be noted that although the calculated MoEis for all three studies with TiO2 NPs were lower than the estimated acceptable MoEi, the expected levels in human organs are still lower than the organ levels that are related to effects in animals. Only the expected levels in the human ovaries are higher than those related to effects in this organ by Tassinari et al. (Citation2014), but as explained above, the extrapolation from this study contains a large uncertainty.
Discussion, conclusions and recommendations
The aim of this assessment was to determine whether a human health risk might be expected from the ingestion of TiO2 NPs from food, food supplements and toothpaste. Therefore, a quantitative risk assessment was performed based on both external doses and internal concentrations using the information presently available. This evaluation is relevant given the current widespread occurrence of TiO2 NPs in food products and the resulting daily exposure. Furthermore, the present risk assessment identifies specific information gaps, which can be used to gather specific additional information to improve the risk assessment. The uncertainties and assumptions in this risk assessment are discussed, followed by reflections on the risk assessment outcome.
Uncertainties and assumptions
The uncertainties on the human intake calculation can be found in Rompelberg et al. (Citation2016). Some uncertainties in the risk assessment are covered by safety factors (uncertainty about the interspecies, intraspecies, study duration and LOAEL to NOAEL extrapolation). However, there are additional uncertainties, which are discussed below.
Toxicokinetics:
The kinetic model was based on the data of Geraets et al. (Citation2014), which was found to be an improvement upon the dataset of Xie et al. (Citation2011), because of the use of an apparently unstable iodine-label by the latter. The recent datasets of Disdier et al. (Citation2015) en Elgrabli et al. (Citation2015) show roughly comparable TiO2 NP levels in liver and spleen after one or two days with consideration of the dose applied and observed variation among animals (see Table S2 in the Supplementary Information). Lung levels after one or two days varied among the three papers, while kidney levels of Geraets et al. (Citation2014) and Elgrabli et al. (Citation2015) were comparable at this timepoint. Elimination from the organs varied considerably between the three papers, with different patterns per organ. For the liver, Disdier et al. (Citation2015) found a much longer elimination time than Geraets et al. (Citation2014) for their particles of 21 nm consisting of 75% anatase and 25% rutile (liver half-life of 265 d vs. 95 d), while Elgrabli et al. (Citation2015) found a much shorter elimination time than Geraets et al. (Citation2014) for their anatase particles of <25 nm (half-life of 12.6 d in the liver). Overall, the TiO2 NP estimations used in this assessment fall within the range reported in the current literature.
Because the kinetic model was only partly mechanism-based due to the current limited knowledge on the behavior of NPs, the model might not be fully predictive for situations other than the study the model was based on and fitted to.
The oral absorption of 0.02% determined in Geraets et al. (Citation2014) was based on a limited number of data, as the detection limit posed problems with measuring the Ti organ levels after such low absorption. The weight of evidence in the Supplementary Material shows that this is the best available estimate, and that it is in line with observations in other studies. The sensitivity analysis shows the oral absorption is a critical factor in the estimation of the internal concentration as organ concentrations are directly proportional to the fraction absorbed. Additional quantitative confirmation of the oral absorption is needed, even though testing at human relevant oral doses is challenging due to the analytical detection limits.
As previously mentioned, the data used for the kinetic model in this risk assessment were i.v. data. The protein corona maybe different in case of oral exposure, which may result in a different tissue distribution of TiO2 NPs between oral and i.v. exposure.
The kinetic constants for ovaries and testes were important parameters for the risk assessment, but were based on a limited data set. These kinetic constants might be improved by additional kinetic data.
Allometric scaling is a default approach for scaling of metabolic processes between animal species, yet the appropriateness for nanomaterials is unclear.
It is unknown whether the doses used in the toxicity studies, which are all much higher than human intakes (>250-fold) and are given as a daily bolus dose in most studies instead of consumption and thus gradual uptake during the day, are absorbed differently than the amounts ingested by humans. SiO2 NPs have been reported to gelate at higher doses in the gut (van der Zande et al., Citation2014), resulting in lower oral absorption at a high dose than at lower doses. We are not aware of studies indicating whether TiO2 NPs can gelate as well in the gut, although it is common knowledge that NP aggregation increases with concentration. Therefore, absorption in animals studies, which generally use high doses, might be under predicted if gelation occurs in the gut. The implication of this phenomenon is that higher absorption might occur at lower doses and with exposure through food rather than gavage, resulting in lower NOAELs than predicted with animal studies, and an under prediction of the risk.
Hazard identification and characterization:
There are currently no high-quality studies available with chronic exposure to well-characterized, uncoated TiO2 NPs, including analysis of the relevant endpoints. There are indications that reproduction organs may be affected, but this has not yet been thoroughly investigated. Moreover, data on effects on the gut are lacking, while it is hypothesized that particles such as TiO2 NPs could possibly break immune-tolerance by effects in the gut (Powell et al., Citation2010). The NOAELs used in this risk assessment could be both an under- and an overestimation of the true overall NOAEL. Considering the body of evidence, the NOAEL for effects on the liver and spleen are expected to be more accurate than those for effects on testes and ovaries.
The key studies have only tested the anatase form of TiO2, and this was assumed to be representative for exposure via food ingestion by the Dutch population. As the anatase form has been accepted as food pigment for a longer period of time than the rutile form (see Introduction), it seems plausible that it is the most commonly used form in food products.
Rutile TiO2 seems to be less toxic, as seen in in vitro cytotoxicity assays (Sayes et al., Citation2006), and in vivo studies (Warheit et al., Citation2007), thus in that respect the inclusion of only anatase studies can lead to an overestimation of the health risk. However, the toxicokinetic behavior of rutile particles results in slightly higher levels in liver and much higher levels (factor 3-4) in spleen. The inclusion of only toxicity studies with anatase TiO2 could therefore lead to underestimation or overestimation of the health risk.
In the calculations based on the NCI study and human intakes, which encompass pigment TiO2, it was assumed that only the fraction <100 nm exerts any toxic effect. This is of course an artificial, rigid boundary; a gradient in the occurrence of toxic effects with decreasing particle size is more likely to occur. If also particles greater than 100 nm contribute to the effects, the risk would be underestimated. In addition, as the hazard information comes from studies often testing only one particular size within the nano-range, the risk may be over- or underestimated.
It is still unclear whether and how particle size and other physicochemical properties influence the toxic effects of TiO2. From the postulated mechanism of action, it would be expected that a smaller size would provide more surface per mass unit to produce radicals and thus oxidative stress. For some NPs indeed a smaller size has been associated with an increase in toxicity, e.g. nanosilver (Park et al., Citation2011).
The short exposure time in the Tassinari et al. (Citation2014) study results in a rather large uncertainty in the extrapolation to chronic exposure. It is unknown whether the effects seen after this short duration will also occur after longer duration, when the animals have had the chance to adapt to the exposure.
The selected PoDs were not agreed upon internationally and hence named “provisional”. Likewise, the applied safety factors were not agreed upon internationally, although they originate from a European guidance document (i.e. REACH guidance).
Reflection on risk assessment outcome
The conclusion of the risk assessment of TiO2 NPs based on external doses (Approach 1) would be that a human health risk is not expected for effects in liver and spleen, but a human health risk cannot be excluded for effects on the ovaries (based on very limited information).
When based on organ concentrations by including the toxicokinetics of TiO2 NPs (Approach 2), the outcome of the risk assessment indicates a potential risk for liver, spleen, ovaries and testes. For ovaries, the estimated organ concentrations in humans are higher than the estimated organs concentrations causing adverse effects in animals, though it should be noted that the robustness of the assessment for ovaries is limited and less than for liver. Based on toxicity in testes (with limited data) and liver, a human health risk appears to be possible, as the MoEi is below the estimated acceptable MoEi. For the spleen, the MoEi was lower than the estimated acceptable MoEi, but no conclusion can be drawn on risk, as the MoEi could only be based on a top dose that showed no effects. In other words, the PoD of effects on spleen could well be higher than tested for, and thus the MoEi could well be larger than currently calculated.
We have considered the risk assessment outcome when it would have been based on the intake estimations of Sprong et al. (Citation2016). They used the same DNFCS, but in combination with use levels of TiO2 as provided by industry. Based on their P95 intake estimations, a lifetime average P95 intake of TiO2 NPs is 8.7 μg/kg bw/d, which is a factor 5.1 higher than the value derived from Rompelberg et al. (Citation2016). This higher intake would have given lower MoEes in Approach 1, but would not have changed the conclusion on risk. For Approach 2, the MoEis for the NCI study could become lower than the estimated acceptable MoEi for spleen (at 80 years), testes (at 40 years) and ovaries (at 80 years). However, this also does not change the original conclusion.
This difference between the two risk assessment approaches shows the importance of including toxicokinetic information. Such information is often not considered in risk assessment. Especially in the case of accumulating substances, such as TiO2 NPs, the lifelong exposure of humans in comparison to certain study durations can make a difference in whether organ levels can reach effect levels. Here, the toxicokinetic information has been used to build a kinetic model in order to extrapolate to organ concentrations in humans as a function of lifelong exposure.
In Approach 2, using the toxicokinetic information, there was a difference in the outcome of the risk characterizations using the hazard data of the NCI study and those based on the studies with characterized NPs: the former showed an unlikely risk, the latter a possibility of risk. It should be noted that all these studies show some toxic effects. We have verified whether assumptions applied in the calculations may explain this difference. When focusing on the liver, the calculated liver concentrations at the effect level differ by a factor seven between the NCI study and the study of Wang et al. (Citation2013). This difference in internal concentration may well be explained by animal variation, a lower absorption at the very high doses up to 2500 mg/kg bw/d in the NCI study, as seen for silica (van der Zande et al., Citation2014), or a smaller fraction of NPs in the tested titanium white in the NCI study. Peters et al. (Citation2014) found a range of 0.012–0.31% NPs (by weight) in seven different brands of E 171 and for the NCI study the highest measured fraction of 0.31% was assumed as a worst-case scenario. If the fraction in the NCI study had been 0.012%, the calculated liver concentration at the effect level would have been 26-fold lower, becoming lower than that of the study of Wang et al. (Citation2013). The MoEi for liver effects according to the NCI study at 40 years of age would then, for example, be lower than the acceptable MoEi. Thus, the fact that the true fraction of NPs is not known and the assumption made for this fraction greatly influence the risk characterization based on this study. The NCI study and its different risk characterization outcome in comparison with the other studies should therefore be interpreted with caution.
Bachler et al. (Citation2015) also performed a risk assessment for TiO2 NPs in food, drugs and toothpaste based on internal concentrations, using a PBPK model. They compared the organ concentrations expected in human consumers to the effect concentrations in in vitro studies and concluded that the risk for the German population was small. This can be mainly attributed to the difference in hazard data. Bachler et al. (Citation2015) considered in vitro studies, including one with liver cells where the effect level did exceed the estimated liver concentration. The similar, estimated liver concentration of our assessment and that of Bachler et al. (Citation2015) did give a MoEi that was lower than the estimated acceptable margin with one of our key in vivo studies. Due to their longer duration, the data of these in vivo studies are preferable over those of in vitro assays used by Bachler et al. (Citation2015). In addition, the reproductive organs were not included in the assessment of Bachler et al. (Citation2015). We consider that our assessment, based on in vivo studies, taking chronic exposure and accumulation into account and including the reproductive organs, improves upon the risk assessment by Bachler et al. (Citation2015).
Conclusion and recommendations
Based on the currently available information, we conclude that a health risk from the ingestion of TiO2 NPs via food, supplements and toothpaste is possible, directed towards effects in liver and maybe the reproductive organs. For liver, the margin between estimated concentrations in humans after chronic exposure and the concentration corresponding to liver damage in animal studies ranged between 36 and 122 (depending on age), for the most critical study, where over 150 would be considered as safe. Even though there is a margin, health risks cannot be excluded as greater margins are desired to allow for factors such as inter- and intraspecies differences and study duration.
Here, we provide an approach that includes toxicokinetic information in the risk assessment evaluation. Refinement of this approach can be attained with information on the extent to which physicochemical properties of TiO2 NPs affect their kinetic behavior and hazard. In addition, more information on the organ concentrations in humans are needed, as well as information on the effects in liver and reproductive organs and on fertility after longer (chronic) exposure including realistic doses. Confirmation by additional studies is therefore necessary.
Declaration of interest
The authors report no conflicts of interest.
This work was funded by The Netherlands Food and Consumer Product Safety Authority and we are grateful for their contribution to discussions.
Supplementary material available online
Supplementary Materials
Download Zip (249.5 KB)Acknowledgements
The advice of Bas Bokkers, Christiaan Delmaar, Aldert Piersma, Andre Muller, Petra van Kesteren, Cathy Rompelberg (all of RIVM) is appreciated.
Notes
2The International Uniform Chemical Information Database (IUCLID) is the database managed by the European Chemicals Agency (ECHA), which hosts all dossiers of chemical substances registered under the REACH framework. The public version includes all toxicological information and is accessible at http://echa.europa.eu/information-on-chemicals/registered-substances.
References
- Azim SA, Darwish HA, Rizk MZ, Ali SA, Kadry MO. 2015. Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: possible role of some antioxidants. Exp Toxicol Pathol 67:305–14
- Bachler G, von Goetz N, Hungerbuhler K. 2015. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology 9:373–80
- CBS (Central Bureau for Statistics, the Netherlands). 2014. Levensverwachting; geslacht en leeftijd, vanaf 1950 (per jaar) [Life expectancy; gender and age, from 1950 (per year)] [Online]. Available at: http://statline.cbs.nl/StatWeb/publication/?VW=T&DM=SLNL&PA=37360NED&D1=3&D2=a&D3=0&D4=20-l&HD=130212-0832&HDR=T,G2,G1&STB=G3. Accessed on 12 July 2015
- Chen Z, Wang Y, Zhuo L, Chen S, Zhao L, Luan X, et al. 2015. Effect of titanium dioxide nanoparticles on the cardiovascular system after oral administration. Toxicol Lett 239:123–30
- Cho WS, Kang BC, Lee JK, Jeong J, Che JH, Seok SH. 2013. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part Fibre Toxicol 10:9
- Disdier C, Devoy J, Cosnefroy A, Chalansonnet M, Herlin-Boime N, Brun E, et al. 2015. Tissue biodistribution of intravenously administrated titanium dioxide nanoparticles revealed blood-brain barrier clearance and brain inflammation in rat. Part Fibre Toxicol 12:27
- ECHA (European Chemicals Agency) 2012. Guidance on information requirements and chemical safety assessment. Chapter R.8: Characterization of dose [concentration]-response for human health. Version 2.1. Helsinki: ECHA [Online]. Available at: http://echa.europa.eu/documents/10162/13632/information_requirements_r8_en.pdf. Accessed on 12 July 2015
- EFSA (European Food Safety Authority). 2004. Opinion of the Scientific Panel on Food Additives, Flavorings, Processing Aids and materials in Contact with Food on a request from the Commission related to the safety in use of rutile titanium dioxide as an alternative to the presently permitted anatase form. Question No. EFSA-Q-2004-103. EFSA J. 163:1–12
- Elgrabli D, Beaudouin R, Jbilou N, Floriani M, Pery A, Rogerieux F, Lacroix G. 2015. Biodistribution and Clearance of TiO2 Nanoparticles in Rats after Intravenous Injection. PLoS One 10:e0124490
- FDA (Food and Drug Agency). 2015. Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices [Online]. Available at : http://www.fda.gov/forindustry/coloradditives/coloradditiveinventories/ucm115641.htm. Accessed on 12 July 2015
- Geraets L, Oomen AG, Krystek P, Jacobsen NR, Wallin H, Laurentie M, et al. 2014. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part Fibre Toxicol 11:30
- Gui S, Sang X, Zheng L, Ze Y, Zhao X, Sheng L, et al. 2015. Retraction note: intragastric exposure to titanium dioxide nanoparticles induced nephrotoxicity in mice, assessed by physiological and gene expression modifications. Part Fibre Toxicol 12:22
- Iavicoli I, Leso V, Fontana L, Bergamaschi A. 2011. Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur Rev Med Pharmacol Sci 15:481–508
- Iavicoli I, Leso V, Bergamaschi A. 2012. Toxicological effects of titanium dioxide nanoparticles: a review of in vivo studies. J Nanomaterials 2012:1–36
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). 1970. Specification for the identity and purity of food additives and their toxicological evaluation. Some food colors, emulsifiers, stabilizers, anticaking agents, and certain other substances. Thirteenth Report of the Joint FAO/WHO Expert Committee on Food Additives, Rome, 27 May- 4 June 1969. World Health Organization technical report series no. 445, FAO nutrition meetings report series no. 46. Geneva: FAO and WHO
- Jia F, Sun Z, Yan X, Zhou B, Wang J. 2014. Effect of pubertal nano-TiO2 exposure on testosterone synthesis and spermatogenesis in mice. Arch Toxicol 88:781–8
- Komatsu T, Tabata M, Kubo-Irie M, Shimizu T, Suzuki K, Nihei Y, Takeda K. 2008. The effects of nanoparticles on mouse testis Leydig cells in vitro. Toxicol in Vitro 22:1825–31
- Lankveld DP, Oomen AG, Krystek P, Neigh A, Troost-De Jong A, Noorlander CW, et al. 2010. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 31:8350–61
- Luecke RH, Pearce BA, Wosilait WD, Slikker W, Young JF. 2007. Postnatal Growth Considerations for PBPK Modeling. J Toxicol Environ Health Part A 70:1027–37
- NCI (National Cancer Institute). 1979. Bioassay of titanium dioxide for possible carcinogenicity. Carcinogenesis Technical Report Series No 97. Bethesda (Maryland): U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health
- Orazizadeh M, Fakhredini F, Mansouri E, Khorsandi L. 2014. Effect of glycyrrhizic acid on titanium dioxide nanoparticles-induced hepatotoxicity in rats. Chem Biol Interact 220:214–21
- Park MVDZ, Neigh AM, Vermeulen JP, De La Fonteyne LJJ, Verharen HW, Briedé JJ, et al. 2011. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32:9810–17
- Peters RJ, Van Bemmel G, Herrera-Rivera Z, Helsper HP, Marvin HJ, Weigel S, et al. 2014. Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles. J Agric Food Chem 62:6285–93
- Powell JJ, Faria N, Thomas-Mckay E, Pele LC. 2010. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun 34:J226–33
- Rompelberg C, Heringa MB, van Donkersgoed G, Drijvers J, Roos A, Westenbrink S, Peters R, van Bemmel G, Brand W, Oomen AG. 2016. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology. [Epub ahead of print]. doi: 10.1080/17435390.2016.1222457
- Sayes CM, Wahi R, Kurian PA, Liu Y, West JL, Ausman KD, et al. 2006. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci 92:174–85
- SCCS (Scientific Committee on Consumer Safety). 2013. Opinion on titanium dioxide (nano form). COLIPA no. S75. Report no. SCCS/1516/13. Revision of 22 April 2014 [Online]. Available at: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_136.pdf. Accessed on 12 July 2015
- SCENIHR. 2009. Opinion. Risk assessment of products of nanotechnologies. Scientific Committee on Emerging and Newly Identified Health Risks, European Commission, DG SANCO, Brussels, Belgium [Online]. Available at: http://ec.europa.eu/health/archive/ph_risk/committees/04_scenihr/docs/scenihr_o_023.pdf. Accessed on 07 September 2015
- Shi H, Magaye R, Castranova V, Zhao J. 2013. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fiber Toxicol 10:33
- Shukla RK, Kumar A, Vallabani NV, Pandey AK, Dhawan A. 2014. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine (Lond) 9:1423–34
- Sinnecker H, Ramaker K, Frey A. 2014. Coating with luminal gut-constituents alters adherence of nanoparticles to intestinal epithelial cells. Beilstein J Nanotechnol 5:2308–15
- Sprong C, Bakker M, Niekerk M, Vennemann M. 2016. Exposure assessment of the food additive titanium dioxide (E 171) based on use levels provided by the industry. Bilthoven, the Netherlands: RIVM report 2015-0195
- Takeda K, Suzuki K-I, Ishihara A, Kubo-Irie M, Fujimoto R, Tabata M, et al. 2009. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J Health Sci 55:95–102
- Tassinari R, Cubadda F, Moracci G, Aureli F, D'amato M, Valeri M, et al. 2014. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology 8:654–62
- TDMA (Titanium Dioxide Manufacturers Association). 2013. About titanium dioxide [Online]. Available at: http://www.tdma.info/files/pdf/about_tio2/About_TiO2__Brochure__-_July_2013.pdf. Accessed on 12 July 2015
- Van Der Zande M, Vandebriel RJ, Groot MJ, Kramer E, Herrera Rivera ZE, Rasmussen K, et al. 2014. Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Part Fibre Toxicol 11:8
- Van Kesteren PC, Cubadda F, Bouwmeester H, Van Eijkeren JC, Dekkers S, De Jong WH, Oomen AG. 2015. Novel insights into the risk assessment of the nanomaterial synthetic amorphous silica, additive E551, in food. Nanotoxicology 9:442–52
- Wang J, Zhou G, Chen C, Yu H, Wang T, Ma Y, et al. 2007. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168:176–85
- Wang Y, Chen Z, Ba T, Pu J, Chen T, Song Y, et al. 2013. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small 9:1742–52
- Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM, et al. 2007. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 230:90–104
- Warheit DB, Brown SC, Donner EM. 2015a. Acute and subchronic oral toxicity studies in rats with nanoscale and pigment grade titanium dioxide particles. Food Chem Toxicol 84:208–24
- Warheit DB, Boatman R, Brown SC. 2015b. Developmental toxicity studies with 6 forms of titanium dioxide test materials (3 pigment-different grade & 3 nanoscale) demonstrate an absence of effects in orally-exposed rats. Reg Toxicol Pharmacol 73:887–96
- Weir A, Westerhoff P, Fabricius L, Hristovski K, Von Goetz N. 2012. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46:2242–50
- Xie G, Wang C, Sun J, Zhong G. 2011. Tissue distribution and excretion of intravenously administered titanium dioxide nanoparticles. Toxicol Lett 205:55–61
- Zhao X, Sheng L, Wang L, Hong J, Yu X, Sang X, et al. 2015. Retraction note: mechanisms of nanosized titanium dioxide-induced testicular oxidative stress and apoptosis in male mice. Part Fiber Toxicol 12:23
