Abstract
The pulmonary delivery of nanoparticles (NPs) is a promising approach in nanomedicine. For the efficient and safe use of inhalable NPs, understanding of NP interference with lung surfactant metabolism is needed. Lung surfactant is predominantly a phospholipid substance, synthesized in alveolar type II cells (ATII), where it is packed in special organelles, lamellar bodies (LBs). In vitro and in vivo studies have reported NPs impact on surfactant homeostasis, but this phenomenon has not yet been sufficiently examined. We showed that in ATII-like A549 human lung cancer cells, silica-coated superparamagnetic iron oxide NPs (SiO2-SPIONs), which have a high potential in medicine, caused an increased cellular amount of acid organelles and phospholipids. In SiO2-SPION treated cells, we observed elevated cellular quantity of multivesicular bodies (MVBs), organelles involved in LB biogenesis. In spite of the results indicating increased surfactant production, the cellular quantity of LBs was surprisingly diminished and the majority of the remaining LBs were filled with SiO2-SPIONs. Additionally, LBs were detected inside abundant autophagic vacuoles (AVs) and obviously destined for degradation. We also observed time- and dose-dependent changes in mRNA expression for proteins involved in lipid metabolism. Our results demonstrate that non-cytotoxic concentrations of SiO2-SPIONs interfere with surfactant metabolism and LB biogenesis, leading to disturbed ability to reduce hypophase surface tension. To ensure the safe use of NPs for pulmonary delivery, we propose that potential NP interference with LB biogenesis is obligatorily taken into account.
Introduction
The intrapulmonary administration of nanoparticles (NPs) is an attractive approach in nanomedicine (Mansour et al., Citation2009; Sadhukha et al., Citation2013). In medical applications, superparamagnetic iron oxide nanoparticles (SPIONs) have been reported to be a good choice (Sadhukha et al., Citation2013). SPIONs are often coated with a thin layer of amorphous silica in order to reduce iron dissolution, increase surface porosity and offer a good platform for surface functionalization (Baber et al., Citation2011).
In the lungs, NPs have versatile interactions with pulmonary cells that can lead to various adverse effects (Arick et al., Citation2015). In the alveoli, NPs come in contact with the surfactant film, resulting in the coating of NPs with surfactant, and thereafter with alveolar macrophages and the epithelium. The surfactant is crucial for reducing the surface tension in alveoli. NPs that react with the surfactant alter its biophysical properties leading to impaired lung functioning (Dwivedi et al., Citation2014). Alveolar macrophages are removing NPs by phagocytosis from the alveolar space. Since many NPs used for medical applications are engineered to avoid alveolar macrophage clearance (Patel et al., Citation2015), their interactions with squamous alveolar type I cells (ATI) and cuboidal alveolar type II cells (ATII) are even more likely to occur. ATII cells are responsible for the synthesis of lung surfactant and its secretion into alveolar lumen.
Lung surfactant is composed mainly of lipids (∼90%; majority are phospholipids) that are in ATII cells stored in specialized lysosome-related organelles with acid interior, called lamellar bodies (LBs). They are secreted into alveoli by regulated exocytosis, leading to the formation of surfactant film in the air–water interface. Adverse effects of inhaling particles are commonly accompanied by the increased phospholipid content in lung alveoli – phospholipidosis (Ma et al., Citation2011). Pulmonary phospholipidosis can be a result of the increased production and secretion of lung surfactant by the ATII cells. An increased production of lung surfactant was suggested as a protective mechanism to reduce particle induced toxicity (Ratoi et al., Citation2014), but the exact role of increased surfactant production is still not clear. ATII cells were reported to internalize NPs to a much greater extent than the bigger particles (Griese & Reinhardt, Citation1998), and they do so presumably by using pathways for surfactant recycling (Wang & Petersen, Citation2013).
A large portion (up to 95%) of surfactant from alveoli is recycled by ATII cells using surfactant re-internalization into LBs and re-secretion (Andreeva et al., Citation2007). Here, endocytosis by ATII cells is a crucial part of the surfactant turnover, resulting in the formation of specialized endosomes, called multivesicular bodies (MVBs). They are filled with numerous, phospholipid rich, small intraluminal vesicles (ILVs), which by incorporation into the growing whorled membranes of LBs, deliver a substantial part of phospholipids into the LBs (Weaver et al., Citation2002). Wang & Petersen (Citation2013) found that A549 human lung cancer cells, which are frequently used as an in vitro model of the ATII cells, internalize lipid-coated Au NPs in MVBs and LBs. They observed that NPs promote LB formation, and since LBs serve to secrete surfactant, it was suggested this should be employed as a mechanism to remove NPs from the cell. NPs have also been found in LBs in several other studies (Escamilla-Rivera et al., Citation2016; Esquivel-Gaon et al., Citation2015; Moret et al., Citation2015; Stearns et al., Citation2001; Singh et al. Citation2007), but the consequences of this internalization are currently not clear. A few authors have reported an increased cellular quantity of LBs in A549 cells due to NP exposure (Davoren et al., Citation2007; Simon-Deckers et al., Citation2008; Wang & Petersen, Citation2013), and they suggested this be employed as a mechanism of cell defense. In contrast, Moret et al. (Citation2015) reported that A549 cells exposed to polyethylene glycol-coated organically-modified silica (PEG-ORMOSIL) NPs have decreased the cellular quantity of LBs and lowered the amount of surfactant proteins A and C (SP-A, SP-C), indicating an interference of NPs with LB functioning. However, it has not been investigated how NPs interfere with the biosynthesis of surfactant lipids and LB biogenesis.
The majority of surfactant lipids are phosphatidylcholines (PC) (∼80%) that are essential for the maintenance of low surface tension in alveoli (Arick et al., Citation2015). In addition to the recycling of surfactant described above, an important portion of the PC arises from the synthesis in ATII cells. PC is synthesized either by the cytidine diphosphocholine (CDP-choline) de novo pathway (Kennedy pathway), where CTP: phosphocholine cytidylyltransferase (PCYT) is the rate-limiting enzyme, or by the remodeling of lyso-PC to PC within the Land’s cycle by the lysophosphatidylcholine acyltransferase family of enzymes (LPCAT) (Agassandian & Mallampalli, Citation2013). Surfactant phospholipid de novo synthesis and remodeling pathways rely in particular on the availability of free fatty acids, which depends on their uptake from extracellular sources, de novo synthesis within the cell, and on the lipolytic release from lipid droplets (Currie et al., Citation2013; Penno et al., Citation2013). In PC synthesis, free fatty acids are esterified to glycerol-3-phosphate, giving rise to lysophosphatidic acid, phosphatidic acid and diacylglycerol. Diacylglycerol either enters the Kennedy pathway or is further acylated to triacylglycerol that is stored within cytosolic lipid droplets (Prentki & Madiraju, Citation2008).
After the synthesis, the surfactant PC is transported to LBs, where PC is incorporated into whorled membranes (Weaver et al., Citation2002). The exact mechanism of newly synthesized PC delivery to the LBs is currently not clear, but PC is presumably transported from the endoplasmic reticulum to the LBs by phospholipid transfer protein (PLTP), followed by the uptake into vesicles by the ATP-binding cassette transporter 3 protein (ABCA3) (Agassandian & Mallampalli, Citation2013), which is localized on the limiting membrane of LBs.
The aim of our work was to study the effects of silica-coated SPIONs (SiO2-SPIONs) at non-cytotoxic concentrations on ATII-like A549 human lung cancer cells, with the emphasis on the lipid surfactant metabolism and LB biogenesis. Based on the fact that LBs are extensively associated with the endocytic pathway and on the data found in the literature that NPs can enter ATII cells in the process of surfactant recycling (Wang & Petersen, Citation2013), we hypothesize that a substantial amount of SiO2-SPIONs will be endocytosed in A549 cells, what may interfere with the lipid surfactant metabolism and LB biogenesis.
Methods
Chemicals
Cell culture media, primers for qPCR and all other chemicals used in our experiments were from Sigma-Aldrich (Steinheim, Germany), unless stated otherwise.
Nanoparticle synthesis, preparation and characterization of particle suspensions
The NPs of the iron oxide maghemite (γ-Fe2O3) were synthesized using co-precipitation from aqueous solutions as described in the Supplementary Material. The SPIONs were coated with an approximately 5 nm-thick layer of silica. The synthesized NPs were characterized using X-ray powder diffractometry (XRD) (Bruker AXS Endeavor, Billerica, MA) and TEM (JEOL 2100, Tokyo, Japan). The suspensions of NPs were monitored with electro-kinetic measurements of the zeta-potential (Brookhaven Instruments Corporation, ZetaPALS, Holtsville, NY). The hydrodynamic diameter distributions of the NPs in their aqueous suspensions were obtained using dynamic light scattering (DLS) (ANALYSETTE12 DynaSizer, Idar-Oberstein, Germany).
Cell culture
A549 cells were cultured in Dulbecco’s modified Eagle’s medium, supplemented with 4 mM L-glutamine and 5% (v/v) fetal bovine serum (FBS). Cells were grown at 37 °C in a humidified atmosphere with 5% CO2. Prior to experimental work, A549 cells were confirmed to be mycoplasma negative using the MycoAlert™ Kit (Lonza, Basel, Switzerland).
Cytotoxicity assay
MTT cytotoxicity assay was performed to select non-cytotoxic concentrations to be used in further experiments. The cytotoxicity of uncoated-SPIONs and SiO2-SPIONs was evaluated using a modified protocol of Mosmann (Citation1983). Briefly, 7000 A549 cells/well were seeded in 96-well plates. After a 24 h incubation, the cells were treated with uncoated-SPIONs (5–100 μg/mL) and SiO2-SPIONs (1–100 μg/mL). After a 3, 24 and 48 h exposure, the 0.5 mg/mL MTT reagent was added and all other procedures were as described in cited protocol. The absorbance of reduced MTT was measured by microplate reader (BioTek, Cytation 3, Bad Friedrichshall, Germany). All cytotoxicity experiments were performed at least twice in five replica wells.
Estimation of acid organelle quantity and phospholipid rich organelle quantity
The quantity of acid organelles in A549 cells after 48 h treatment with uncoated-SPIONs (5–100 μg/mL) and SiO2-SPIONs (1–100 μg/mL) has been estimated by the normalization of results from Neutral red uptake assay that evaluates cellular quantity of acid organelles with the results of Coomassie Blue assay that evaluates the amount of cellular proteins (which is proportional to the cell number) (Mukherjee et al., Citation2012; Repetto et al., Citation2008). All experiments were performed at least twice in five replica wells. The quantity of phospholipid rich organelles in A549 cells after 48 h treatment with uncoated-SPIONs and SiO2-SPIONs (5–50 μg/mL) has been estimated by the HCS LipidTOX™ Green phospholipidosis detection reagent (Life Technologies, Carlsbad, CA), according to the manufacturer’s recommendations. As a positive control for the induction of phospholipidosis, we used 30 μM propranolol. For each treatment condition, four independent repetitions have been performed. Detailed descriptions of the methodology are provided in the Supplementary Material.
Phosphatidylcholine (PC) measurement
The amount of PC in A549 cells treated with uncoated-SPIONs and SiO2-SPIONs (5–50 μg/mL) for 48 h, was measured by LabAssay™ Phospholipid Kit (Wako Pure Chemical Industries Ltd., Osaka, Japan), according to the manufacturer’s protocol. As a positive control for the induction of phospholipidosis, we used 30 μM propranolol. For each treatment condition, at least six independent repetitions were performed. Detailed procedure is described in the Supplementary Material.
Measurements of cytosolic lipid droplets
For measuring the intracellular amount of lipid droplets in A549 cells treated with SiO2-SPIONs (20 and 50 μg/mL) for 48 h, we used Nile red staining and flow cytometry as described previously (Pucer et al., Citation2013). As a positive control for the induction of intracellular lipid droplet formation, we used 100 μM oleic acid (OA). For each treatment condition, four repetitions have been done. Precise procedure is described in the Supplementary Material.
Transmission electron microscopy (TEM)
A semi-quantitative TEM analysis of organelles associated with LB biogenesis (LBs, MVBs and AVs) has been done on A549 cells treated with SiO2-SPIONs (5–50 μg/mL) for 48 h. As a positive control for the induction of phospholipidosis, we used 30 μM propranolol. Analysis has been performed for at least 30 cells at a particular concentration of NPs as described in the Supplementary Material.
Isolation of mRNA, reverse transcription and qPCR
A549 cells (4.5 × 104 cells/cm2) were seeded in 6-well plates. After a 24 h incubation, allowing the cells to adhere, the cells were exposed to SiO2-SPIONs (5–50 μg/mL) for 6, 24 or 48 h. After the treatment, the cells were washed with DPBS and trypsinized. For RNA isolation, we used the High Pure RNA Isolation Kit (Roche, Mannheim, Germany), according to the manufacturer’s protocol. The concentration of isolated RNA was measured spectrophotometrically (NanoDrop 2000c, Thermo Scientific, Waltham, MA). A reverse transcription was performed using the High Capacity cDNA Reverse Transcripton Kits (Applied Biosystems, Carlsbad, CA) following the manufacturer’s protocol. cDNA samples were stored at −20 °C. A qPCR relative gene expression analysis was performed as described previously (Pucer et al., Citation2013) on a StepOnePlus Real-Time by StepOnePlus Real-Time PCR system (Applied Biosystems, Darmstadt, Germany) using the FastStart Universal SYBR Green Master (Rox) from Roche (Mannheim, Germany) and the primers listed in Table S1. DNA topoisomerase 1 (TOP1) and splicing factor 3 A subunit 1 (SF3A1) were used as reference (housekeeping) genes.
Surface tension measurement of the hypophase
The ability of A549 cells to form surfactant film, that reduces hypophase surface tension, was studied by the drop spreading method, according to the protocol of Blank et al. (Citation2006). A549 cells on the PET membrane inserts were treated for 48 h with uncoated-SPIONs (20–50 μg/mL), SiO2-SPIONs (5–50 μg/mL) and 30 μM propranolol. Afterwards, medium was removed from the upper well while the lower well remained filled with cell medium. Confluent cell culture was exposed to air for either 1 h (before the formation of the surfactant film) or for 24 h (when surfactant film should reduce surface tension), followed by surface tension measurement. For each treatment condition, at least four independent repetitions were performed. Precise procedure is described in the Supplementary Material.
Statistical analysis
GraphPad Prism software (GraphPad Software, San Diego, CA) was used for graph production and for the statistical analysis using Student’s t-test or one-way ANOVA with Bonferroni’s post test for multiple comparisons. p values lower than 0.05 were considered statistically significant.
Results
Particle characteristics
The X-ray diffraction (XRD) of the precipitated nanocrystals showed a single spinel phase, whereas the chemical analyses showed that less than 3% of the iron was present in the oxidation state 2+, confirming that the iron oxide nanocrystals were composed of maghemite (γ-Fe2O3). Their average NP sizes, evaluated by TEM, were determined to be 12.4 ± 2.4 and 19.3 ± 2.0 nm for uncoated-SPIONs and SiO2-SPIONs, respectively. The amorphous layer of silica appears to be homogeneous and of a fairly constant thickness, close to 5 nm (SiO2-SPIONs) (). The silica shell shows a relatively acidic character, because its structure is terminated with negatively charged –OH surface groups for the whole range of considered pH values, which gives SiO2-SPIONs more negative zeta potential in comparison to uncoated-SPIONs (Kralj et al., Citation2012). In the cell culture medium, SiO2-SPIONs formed a more stable suspension and smaller aggregates compared to uncoated-SPIONs ().
Figure 1. Transmission electron micrographs of SPIONs. (A, B) uncoated-SPIONs and (C, D) SiO2-SPIONs.
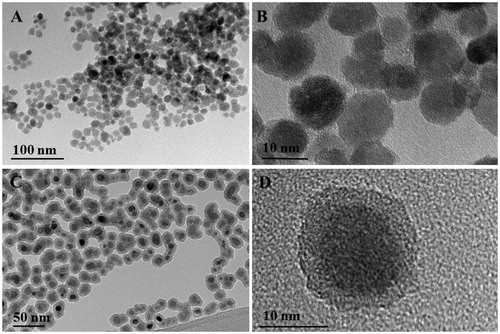
Table 1. Characteristics of SPIONs.
Cytotoxicity of uncoated-SPIONs and SiO2-SPIONs
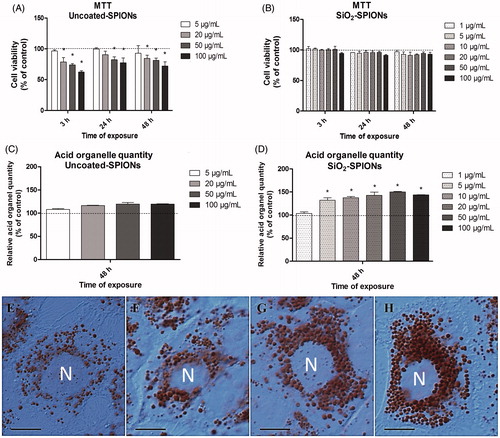
The MTT assay showed a significant reduction of cell viability after exposure to uncoated-SPIONs at exposure concentrations above 20 μg/mL, already after 3 h of treatment (). Similar results were obtained also for a 24 and 48 h treatment (). In contrast to the results of uncoated-SPIONs, even the highest exposure concentration of SiO2-SPIONs (100 μg/mL) did not significantly affect cell viability, as measured by the MTT assay, regardless of the duration of exposure ().
Figure 2. Cytotoxicity results and acid organelle quantity in A549 cells exposed to SPIONs. The MTT assay revealed a significant decrease in cell viability in A549 cells exposed to uncoated-SPIONs (A), but not to SiO2-SPIONs (B). Uncoated-SPIONs did not significantly increase the quantity of acid organelles in A549 cells (C). In contrast, SiO2-SPIONs significantly increased the cellular quantity of acid organelles (D). Data for graphs A–D is presented as the mean (+SEM) percentage of untreated control (dashed line) in a representative experiment performed twice in five replica wells. Asterisk presents a significant difference with respect to the negative control cells (* equals p < 0.05; Student’s t-test). (E–H) Figures of differential interference contrast (DIC) microscopy confirmed that SiO2-SPIONs after a 48 h treatment significantly increased the cellular quantity of acid organelles in terms of increasing their number and size. (E) Untreated control cell, (F) 5 μg/mL SiO2-SPION treated cell, (G) 20 μg/mL SiO2-SPION treated cell and (H) 50 μg/mL SiO2-SPION treated cell. Unstained nuclei (N) are surrounded by acid organelles stained red. Scale bar = 10 μm.
Cellular quantity of acid organelles and phospholipid rich organelles
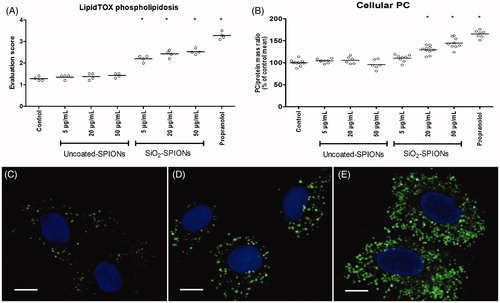
When A549 cells were exposed to uncoated-SPIONs for 48 h, only a slight insignificant increase in Neutral red fluorescence per cell protein content was observed, which indicates that the quantity of acid organelles was unchanged (). In contrast, results from A549 cells exposed to SiO2-SPIONs for 48 h indicate that the quantity of acid organelles was significantly higher than that in control cells (). Data obtained by spectrofluorometric measurements () were confirmed by microscopic observation (). The increased cellular quantity of acid organelles is a characteristic of lysosomotropism, which is a known phenomenon leading to phospholipidosis (Bauch et al., Citation2015). In order to examine the potential of SPIONs to induce phospholipidosis, we used the LipidTOX phospholipidosis reagent. The treatment of A549 cells with uncoated-SPIONs for 48 h did not show any differences in LipidTOX staining, while SiO2-SPIONs significantly increased LipidTOX fluorescence ().
Figure 3. Effects of SPIONs on LipidTOX staining and on cellular amount of phosphatidylcholine (PC). (A) Microscopic evaluation of LipidTOX stained A549 cells after a 48 h exposure to uncoated-SPIONs and SiO2-SPIONs. Every circle (o) in graph A represents the average evaluation score of one independent sample, where at least 100 cells were evaluated. For each treatment condition, 4 independent repeats have been performed. (B) Relative amount of PC in A549 cells after a 48 h exposure to uncoated-SPIONs and SiO2-SPIONs. Every circle (o) in graph B represents one independent measurement. For each treatment conditions, at least six independent repeats were performed. Horizontal lines in graphs (A) and (B) represent the average value for each treatment condition. Data are presented as percentages of untreated controls. Asterisk presents a significant difference with respect to the untreated control cells (*equals p < 0.05; one-way ANOVA with Bonferroni’s post test). (C–E) Epifuolorescence micrographs of LipidTOX stained A549 cells after a 48 h exposure to SiO2-SPIONs. (C) Untreated control cells, (D) cells treated with 20 μg/mL SiO2-SPIONs, (E) cells treated with 50 μg/mL SiO2-SPION. Fluorescence represents phospholipid rich organelles and cell nuclei. Scale bar = 10 μm.
Amount of phosphatidylcholine (PC) and lipid droplets in A549 cells
In A549 cells treated with uncoated-SPIONs, the amount of PC did not differ from the untreated control cells, while the treatment with SiO2-SPIONs (above 20 μg/mL) significantly increased the cellular amount of PC (). In contrast to the increased amount of PC in A549 cells treated with SiO2-SPIONs, we did not observe any changes in the amount of lipid droplets in these cells (Figure S1).
Ultrastructure of SiO2-SPION-exposed A549 cells
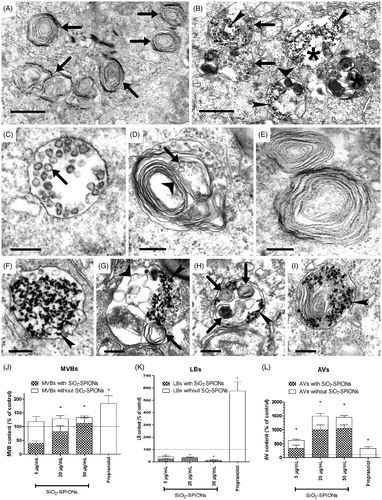
Despite the observed increase in acid organelle quantity, LipidTOX fluorescence, and cellular PC levels in SiO2-SPION exposed cells, the observation with TEM () did not confirm increased cytosolic accumulation of LBs, which is the main characteristic of phospholpidosis (Anderson & Borlak, Citation2006). The quantity of LBs in cells exposed to SiO2-SPIONs was actually significantly reduced and the majority of LBs were filled with SiO2-SPIONs (). In contrast, the cellular quantity of MVBs, organelles involved in LB biogenesis, was elevated (). We also observed a significantly increased number of autophagic vacuoles (AVs) (), and majority of them contained LBs (). In SiO2-SPION exposed cells, we observed an extensive Golgi apparatus and frequent contacts between the endoplasmic reticulum and the mitochondria.
Figure 4. TEM micrographs and a semi-quantitative analysis of organelles in A549 cells exposed to SiO2-SPIONs for 48 h. Representative TEM images of (A) untreated control A549 cells with lamellar bodies (LBs; arrows) and (B) SiO2-SPION-treated cells with multivesicular bodies (MVBs; arrows), endosomes (asterisk), and the autophagic vacuole (AVs; wide arrowheads). The majority of vesicles are filled with electron-dense SiO2-SPIONs (narrow arrowheads). In untreated cells (C–E), structures associated with LB biogenesis were observed: (C) MVB with small intraluminal vesicles (ILVs; arrow); (D) immature LB with ILVs (arrow) and membrane whorls (wide arrowhead); (E) LBs with regularly, tightly and concentrically packed whorled membranes. In SiO2-SPION exposed cells (F–I), structures associated with LB biogenesis were frequently filled with SiO2-SPIONs (narrow arrowheads). (F) MVB with SiO2-SPIONs; (G) vesicle with a small membrane whorl (arrow), disorganized membranes (wide arrowhead) and SiO2-SPIONs; (H) double-membrane-surrounded AV with membrane whorls at different stages of degradation (arrows) and SiO2-SPIONs (narrow arrowheads); (I) LB filled with SiO2-SPIONs (narrow arrowheads). Scale bar = 2 μm for A, B and 0.5 μm for C–I. A semi-quantitative analysis with TEM revealed that SiO2-SPION-treated cells have a significantly increased cellular quantity of MVBs (J), a significantly decreased cellular quantity of LBs (K), and a significantly increased cellular quantity of AVs (L). The majority of organelles contained SiO2-SPIONs. A semi-quantitative analysis has been performed for at least 30 cells per each treatment. Values on the graphs are means ± SEM. Data is presented as the mean (+SEM) percentage of untreated controls (dashed line). The asterisk represents a significant difference with respect to the negative control cells (*equals p < 0.05; Student’s t-test).
Gene expression related to lipid surfactant metabolism in A549 cells exposed to SiO2-SPIONs
The exposure of A549 cells to SiO2-SPIONs led to significant time- and dose-dependent changes in the expression of genes involved in lipid metabolism (CHKA, PCYT1A, PCYT1B, LPCAT1, ABCA3, PLTP and the transcription factor PPARG). Interestingly, the expression of genes for de novo PC synthesis and remodeling was elevated already after 6 h of treatment, the most significant being the ∼3-fold increase in CHKA transcript levels. However, after a prolonged exposure (up to 48 h), the expression of most genes involved in de novo PC synthesis gradually decreased to control levels, or even lower ( and Table S2). In fact, 48 h of exposure of A549 cells to 50 μg/mL of SiO2-SPIONs led to a down-regulation of most of the analyzed genes, suggesting a strong suppression of surfactant lipid metabolism. Interestingly, the SiO2-SPION treatment did not alter the expression of genes encoding for the lipogenic transcription factor SREBP-1c (SREBF1) and for fatty acid synthase (FAS; a product of the FASN gene), the key enzyme responsible for fatty acid synthesis. Prior to its final decline, we observed a transient increase (at 24 h of treatment) in the expression of the SFTPC gene, coding for the surfactant protein SP-C. In line with our data showing no alterations in lipid droplet formation in A549 cells exposed to SiO2-SPIONs (Figure S1), there were no significant changes in the expression of the PLIN2 gene, encoding the lipid droplet-coating protein perilipin 2.
Figure 5. Dose- and time-dependent gene expression changes in A549 cells exposed to SiO2-SPIONs. The color green indicates suppressed mRNA transcript levels and the color red elevated mRNA levels relative to untreated control cells. Relative expression values were calculated using the results of at least two independent experiments performed in duplicate. Data for the heatmap can be found in Table S2. Abbreviations: CHKA, CHKB: choline kinase ɑ and β; PCYT1A, PCYT1B: CTP: phosphocholine cytidylyltransferase ɑ and β; LPCAT1, LPCAT2: lysophosphatidylcholine acyltransferase 1 and 2; ABCA3: ATP-binding cassette transporter 3; PLTP: phospholipid transfer protein; PPARA, PPARG: peroxisome proliferator-activated receptor ɑ and γ; SREBF1: sterol regulatory element binding transcription factor 1; FASN: fatty acid synthase; PLIN2: perilipin2; SFTPC: surfactant protein C

Surface tension of the hypophase
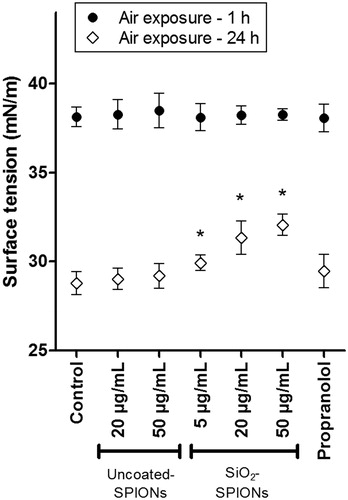
The 48 h treatment of A549 cells to uncoated-SPIONs, SiO2-SPIONs and 30 μM propranolol did not influence the surface tension of the liquid lining layer, when measured after 1 h air-exposure (when surfactant film is not yet formed). In comparison to the surface tension after 1 h air-exposure, we observed that the surface tension of the hypophase after 24 h air-exposed cells decreased in all cell samples, but nevertheless, treatment of A549 cells with SiO2-SPIONs, led to a significantly higher surface tension of the hypophase ().
Figure 6. A549 hypophase surface tension. A549 cells were treated with uncoated-SPIONs, SiO2-SPIONs and 30 μM propranolol for 48 h and the surface tension of the liquid lining layer was measured after the cells were exposed to air for either 1 h (before the formation of the surfactant film) or for 24 h (when surfactant film in untreated control cells reduces surface tension). In contrast to results after 1 h air-exposure, where surface tension of hypophase was unaffected by different cell treatments, results after 24 h air-exposure are showing that treatment of A549 cells with SiO2-SPIONs cause higher surface tension of the hypophase. Values present means (+SD) of at least four independent repetitions. Asterisk presents a significant difference with respect to the negative control cells (*equals p < 0.05; one-way ANOVA with Bonferroni’s post test).
Discussion
In accordance with data reported in the literature (Baber et al., Citation2011), we confirmed that silica coating prevents the cytotoxicity of SPIONs as measured by the MTT assay. In our study, exposure to uncoated-SPIONs did not increase the quantity of acid organelles, while their cytotoxic action was detected at concentrations above 20 μg/mL. In contrast, exposure to SiO2-SPIONs already at concentration 5 μg/mL significantly increased the quantity of acid organelles, while no cytotoxicity was detected up to 100 μg/mL. It is possible that an equivalent particle concentration in cell medium results in different cell doses (Hinderliter et al., Citation2010). In our study we observed that the sedimentation of uncoated-SPIONs was more intense (visual observation), resulting in a higher particle amount reaching the cell surface within the experimental time frame. The cytotoxicity of SPIONs can be explained by the dissolution of ions from endocytosed NPs in low pH intracellular compartments (Wahajuddin, Citation2012). High intracellular iron ion concentrations potentially lead to oxidative stress generated cytotoxicity (Sarkar et al., Citation2014). It is suggested that silica coating serves as a protective barrier that prevents iron ion dissolution from the SPIONs and thus lowers the cytotoxicity of SiO2-SPIONs (Baber et al., Citation2011). One possible explanation for different cellular effects of uncoated- and SiO2-SPIONs could be the fact that uncoated-SPIONs are internalized and degraded in lysosomes, which releases the toxic amount of iron ions, while the internalization of persistent SiO2-SPIONs did not lead to the release of a toxic amount of ions, but instead resulted in the intracellular accumulation of SiO2-SPIONs in acid organelles, which is a known outcome when cells endocytose hardly degradable material (Pentchev et al., Citation1980). Moret et al. (Citation2015) suggested that the most relevant factor determining the cytotoxicity of NPs in A549 cells is their localization in the LBs. However, in our study and in the study of Esquivel-Gaon et al. (Citation2015), the presence of NPs in LBs was detected even in the absence of cytotoxicity. This suggests that the accumulation of NPs in LBs per se is not responsible for cytotoxicity.
In SiO2-SPION-treated cells we observed a significant increase in LipidTOX fluorescence and the cellular amount of PC. The elevated amount of intracellular phospholipids has previously been interpreted as an adaptive response to prevent the toxicity of foreign matter by sequestering them away from other cellular sites (Shayman & Abe, Citation2013). However, in our study, we observed ultrastructural and metabolic changes indicating significant cellular alteration that can be deleterious even at non-cytotoxic concentrations and can affect the main function of ATII cells, which is the production of lung surfactant.
Several previous studies reported on an altered cellular quantity of LBs in A549 cells due to NP exposure. Davoren et al. (Citation2007) reported that the exposure of A549 to carbon nanotubes (CNT) resulted in an increased number of LBs, an increased amount of lipid droplets and a reduction in microvilli. Simon-Deckers et al. (Citation2008) showed the internalization of NPs (TiO2, Al2O3 and CNT), but they report an increased cellular quantity of LBs only for CNT. Wang & Petersen (Citation2013) showed that lipid-coated Au NPs promoted LB formation. An increase of LBs was also reported by Esposito et al. (Citation2012); however, for sub-micron particles (PM1.0). Similar to the observations made by Wang & Petersen (Citation2013), who found Au NPs in MVBs and LBs, our TEM examination revealed that A549 cells internalize SiO2-SPIONs predominantly inside MVBs and LBs. However, we observed that although SiO2-SPIONs caused an increase in the quantity of MVBs, they did not elevate the LB content. In fact, the quantity of LBs in SiO2-SPION exposed cells was even decreased when compared to untreated cells. These results are consistent with the observations of Moret et al. (Citation2015) and Stearns et al. (Citation2001), who reported a reduced quantity of LBs after treating A549 cells with PEG-ORMOSIL NPs and cytochalasin D (inhibitor of actin polymerization), respectively. All these studies confirm that the endo-lysosomal compartments are the most common intracellular sites of NP sequestration (Stern et al., Citation2012). In ATII cells, these compartments are involved in surfactant synthesis, recycling, and storage, which make NP interference with surfactant turnover unavoidable. The contradictory literature data about LB quantity in A549 cells exposed to NPs can be explained by the fact that NP cellular interaction is controlled by the adsorbed protein and lipid corona, which depends on NP characteristics and the composition of cell culture media (Lesniak et al., Citation2013). The disturbance of LB biogenesis is thus NP specific.
A549 cells exposed to SiO2-SPIONs showed typical ultrastructural characteristics, similar to the control A549 cells, which are: a well-developed endomembrane system, an abundant network of intermediate filaments (tonofilaments), normal mitochondria and microvilli on the apical surface. However, A549 cells exposed to SiO2-SPIONs showed a markedly enlarged Golgi apparatus and an increased quantity of AVs. It seems that the internalization of SiO2-SPIONs is a signal for a rapid synthesis of lysosomal enzymes to degrade internalized material. In SiO2-SPION exposed cells, we frequently observed contacts between the endoplasmic reticulum and the mitochondria, which are known to play important roles in several biological processes, including intracellular trafficking, the regulation of lipid synthesis and autophagy (Marchi et al., Citation2014). The internalization of SiO2-SPIONs probably interferes with the normal turnover of surfactant components, leading to a defective formation of lamellae and LBs, and consequently into intense autophagy. Autophagy was previously proposed as an adaptive mechanism of A549 cells used to survive the stress caused by SiO2 NPs and to avoid apoptosis (Nowak et al., Citation2014) by clearing oxidatively damaged proteins and organelles, or sequestering and degrading foreign or aberrant cellular material (Stern et al., Citation2012).
We observed a significant but transient increase in gene expression for enzymes involved in both the de novo synthesis of PC (the Kennedy pathway) and the PC remodeling pathway (Land’s cycle). This is consistent with the measured increased cellular amount of PC. Furthermore, we have detected a changed expression of genes responsible for proteins involved in the cellular PC transport trafficking LBs (PLTP and ABCA3). However, we did not observe any significant changes in regulator of fatty acid synthesis (SREBF1), in fatty acid synthase (FASN) expression, and in the cellular lipid droplet amount, indicating that de novo fatty acid synthesis and triglyceride lipolysis are not crucial pathways for PC synthesis. It follows that A549 cells exposed to SiO2-SPIONs, in order to satisfy the increasing demand for fatty acids needed for PC synthesis, obtain fatty acids through alternative routes, such as membrane remodeling, fatty acid uptake, or autophagy. The initial rise in the expression of most genes involved in PC metabolism was followed by its decline, resulting in lower gene expression as in control cells. This may indicate a failure of the cells to complete formation of LBs, and accordingly no necessity for de novo PC synthesis. Similar to our results indicating that SiO2-SPIONs interfere with lipid metabolism without inducing cytotoxicity, Przybytkowski et al. (Citation2009) observed that NPs (uncoated- and ZnS-coated quantum dots) induce changes in lipid metabolism without compromising cellular.
In A549 cells exposed to SiO2-SPIONs, we also found an altered expression of the gene encoding the surfactant protein C (SP-C, a protein encoded by the SFTPC gene). SP-C is a hydrophobic protein, which is expressed exclusively by ATII cells, important for the formation and maintenance of the surfactant film in alveoli (Weaver et al., Citation2002). SP-C is not involved in the packaging of the surfactant into the whorled membranes of LBs, since SP-C-deficient mice have normal LBs (Glasser et al., Citation2001). However, SP-C deficiency is associated with the development of chronic lung disease in humans (Perez-Gil & Weaver, Citation2010). We observed a decreased SFTPC expression due to SiO2-SPION exposure after 6 and 48 h, and an increased expression after 24 h, both in a dose-dependent manner. Our results are consistent with the observations of Moret et al. (Citation2015), who also noticed that A549 cells had a lower amount of SP-C after treatment with PEG-ORMOSIL NPs, indicating reduced surfactant production.
Pulmonary surfactant has an important role in reducing surface tension in lungs and it is crucial for the maintenance of normal lung volumes during the respiratory cycle (Whitsett et al., Citation2010). The law of LaPlace (P = 2γ/r; P = pressure in the bubble; γ = surface tension; r = radius of the bubble) indicates that when the radius of the alveoli is decreased, the surface tension also needs to be decreased, in order to allow inflation of smaller alveoli and to prevent alveolar collapse (Haitsma, Citation2007). Lower surface tension in smaller (deflated) alveoli is achieved by the surfactant. Deflated alveoli have a smaller surface area and a higher surfactant concentration, resulting in a lower surface tension in comparison to the surface tension in larger (inflated) alveoli. The value of surface tension in alveoli increases up to 40 mN/m during inspiration and decreases to 15 mN/m during expiration (Doursout et al., Citation2016). In our study, the surface tension of the hypophase after 24 h air-exposed A549 cells was 28.8 ± 0.6 mN/m for the control samples and 32.1 ± 0.6 mN/m for the samples where cells were pretreated with 50 μg/mL SiO2-SPIONs. Although we did not study the dynamics of surface tension during stretching/shrinking of surfactant film, we observed that SiO2-SPION pretreated A549 cells have an impaired ability to reduce hypophase surface tension (), which in in vivo conditions can increase the risk of developing airway disorders. If surfactant homeostasis and surface tension lowering capacity are affected then this can lead to severe pulmonary diseases, which were, until recently, considered idiopathic (Whitsett et al., Citation2010).
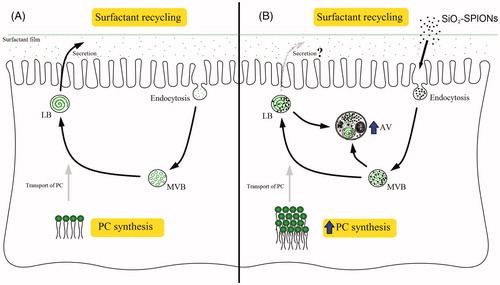
We explain that SiO2-SPIONs are endocytosed by A549 in the surfactant recycling process (). SiO2-SPIONs could not be internalized as bare particles, but with a corona composed of biological molecules probably containing components of surfactants. The endocytosed SiO2-SPIONs are placed into endocytic vesicles, which subsequently form MVBs. As MVBs deliver PC and other surfactants into the LBs, SiO2-SPIONs may enter the LBs and disturb the LB lamella formation. Defected LBs are recognized by the cellular self-degradative process (autophagy), which commences with the clearing of damaged LBs. It is our explanation that SiO2-SPIONs presumably affect the biogenesis of LBs by the adsorption of surfactant components outside the cell before they are internalized and recycled, by changing lipid metabolism, and probably also protein surfactant metabolism, and by the physical interference of SiO2-SPIONs in the process of phospholipid assembly into the whorled membranes of LBs. This interference affects the A549 cells ability to reduce hypophase surface tension (), which can lead to lung function impairment.
Figure 7. SiO2-SPION interference with lamellar body (LB) biogenesis in A549 cells. (A) In the control cells, the surfactant is recycled by endocytosis, where multivesicular bodies (MVBs) are formed. MVBs deliver the recycled phosphatidylcholine (PC) into the LBs. PC is also synthesized by the cell and transported into the LBs. (B) Cells exposed to SiO2-SPIONs have an increased quantity of MVBs and an elevated PC synthesis, which was previously suggested to be a mechanism reducing particle toxicity. However, SiO2-SPIONs interfered with the normal packaging of the surfactant into LBs, which resulted in their sequestration in autophagic vacuoles (AVs).
In our study we used A549 cells as an in vitro model of ATII cells to study SiO2-SPION impact on lipid surfactant metabolism and LB biogenesis. ATII cells are especially susceptible for NP interactions because they continuously use endocytic pathways to recycle a considerable proportion of lung surfactant (Andreeva et al., Citation2007). SiO2-SPIONs in our study had a corona presumably composed of excreted surfactant and other molecules from the cell medium, which may differ under in vivo conditions. The need to investigate the influence of surfactant coating on NPs was pointed out and discussed by Schleh et al. (Citation2013). These authors suggest the use of air–liquid interface models with a surfactant film to study interactions of NPs with alveolar cellular systems. Although we showed that SiO2-SPIONs influence surfactant homeostasis in A549 cells, there are still open questions regarding the influence of surfactant corona and how these NPs affect ATII cells in cell co-culture and in in vivo conditions, where alveolar macrophages and ATI cells are also present.
Conclusions
We showed that the exposure of A549 cells to SiO2-SPIONs induces changes in the surfactant metabolism, LB biogenesis and disturbs the cells ability to reduce hypophase surface tension without compromising cellular viability. In addition, SiO2-SPIONs induce autophagy in a dose-dependent manner. ATII cells are susceptible to NP exposure due to their high endocytic activity during the process of surfactant recycling. This fact could be used beneficially in medical imaging and therapy, but has to be taken into account in safety assessment. In order to ensure safe use of NPs for intrapulmonary delivery, we suggest that potential NP interference with lung surfactant metabolism is evaluated. To what extent is the surfactant metabolism disturbance cell, NP and NP-corona specific is a matter of further in vitro and in vivo research.
Kononenko-et-al-Supplementary-material.docx
Download MS Word (374.2 KB)Acknowledgments
We are grateful to Prof. Dr. Kenneth A. Dawson for providing us with A549 cells. We acknowledge Prof. Dr. Kristina Sepčić for the help with the PC measurement. We thank Neža Repar and Doc. Dr. Anita Jemec for helpful comments on the manuscript.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Agassandian M, Mallampalli RK. 2013. Surfactant phospholipid metabolism. Biochim Biophys Acta 1831:612–25.
- Anderson N, Borlak J. 2006. Drug-induced phospholipidosis. FEBS Lett 580:5533–40.
- Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. 2007. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 293:L259–71.
- Arick DQ, Choi YH, Kim HC, Won YY. 2015. Effects of nanoparticles on the mechanical functioning of the lung. Adv Colloid Interface Sci 225:218–28.
- Baber O, Jang M, Barber D, Powers K. 2011. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro BEAS-2B cells. Inhal Toxicol 23:532–43.
- Bauch C, Bevan S, Woodhouse H, Dilworth C, Walker P. 2015. Predicting in vivo phospholipidosis-inducing potential of drugs by a combined high content screening and in silico modelling approach. Toxicol In Vitro 29:621–30.
- Blank F, Rothen-Rutishauser BM, Schurch S, Gehr P. 2006. An optimized in vitro model of the respiratory tract wall to study particle cell interactions. J Aerosol Med 19:392–405.
- Currie E, Schulze A, Zechner R, Walther TC, Farese RV. 2013. Cellular fatty acid metabolism and cancer. Cell Metab 18:153–61.
- Davoren M, Herzog E, Casey A, Cottineau B, Chambers G, Byrne HJ, Lyng FM. 2007. In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol In Vitro 21:438–48.
- Doursout MF, Deshpande S, Williams GW. 2016. Respiratory physiology. Basic anesthesiology examination review. Oxford: Oxford University Press, 271.
- Dwivedi M, Sachan AK, Galla HJ. 2014. Interaction of nanoparticles with lipid monolayers and lung surfactant films. Measuring biological impacts of nanomaterials. Basel: Springer International Publishing, 109–33.
- Escamilla-Rivera V, Uribe-Ramirez M, Gonzalez-Pozos S, Velumani S, Arreola-Mendoza L, De Vizcaya-Ruiz A. 2016. Cytotoxicity of semiconductor nanoparticles in A549 cells is attributable to their intrinsic oxidant activity. J Nanopart Res 18:85.
- Esposito V, Lucariello A, Savarese L, Cinelli M, Ferraraccio F, Bianco A, et al. 2012. Morphology changes in human lung epithelial cells after exposure to diesel exhaust micron sub particles (PM1.0) and pollen allergens. Environ Pollut 171:162–7.
- Esquivel-Gaon M, Anguissola S, Garry D, Gallegos-Melgar AD, Saldana JM, Dawson KA, et al. 2015. Bismuth-based nanoparticles as the environmentally friendly replacement for lead-based piezoelectrics. Rsc Adv 5:27295–304.
- Glasser SW, Burhans MS, Korfhagen TR, Na CL, Sly PD, Ross GF, et al. 2001. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc Natl Acad Sci USA 98:6366–71.
- Griese M, Reinhardt D. 1998. Smaller sized particles are preferentially taken up by alveolar type II pneumocytes. J Drug Target 5:471–9.
- Haitsma JJ. 2007. Physiology of mechanical ventilation. Crit Care Clin 23:117–34.
- Hinderliter PM, Minard KR, Orr G, Chrisler WB, Thrall BD, Pounds JG, Teeguarden JG. 2010. ISDD: a computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part Fibre Toxicol 7:36.
- Kralj S, Rojnik M, Romih R, Jagodic M, Kos J, Makovec D. 2012. Effect of surface charge on the cellular uptake of fluorescent magnetic nanoparticles. J Nanopart Res 14:1151.
- Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C. 2013. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc 135:1438–44.
- Ma JY, Zhao H, Mercer RR, Barger M, Rao M, Meighan T, et al. 2011. Cerium oxide nanoparticle-induced pulmonary inflammation and alveolar macrophage functional change in rats. Nanotoxicology 5:312–25.
- Mansour HM, Rhee YS, Wu X. 2009. Nanomedicine in pulmonary delivery. Int J Nanomedicine 4:299–319.
- Marchi S, Patergnani S, Pinton P. 2014. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta 1837:461–9.
- Moret F, Selvestrel F, Lubian E, Mognato M, Celotti L, Mancin F, Reddi E. 2015. PEGylation of ORMOSIL nanoparticles differently modulates the in vitro toxicity toward human lung cells. Arch Toxicol 89:607–20.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival – application to proliferation and cyto-toxicity assays. J Immunol Methods 65:55–63.
- Mukherjee SG, O'Claonadh N, Casey A, Chambers G. 2012. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol In Vitro 26:238–51.
- Nowak JS, Mehn D, Nativo P, Garcia CP, Gioria S, Ojea-Jimenez I, et al. 2014. Silica nanoparticle uptake induces survival mechanism in A549 cells by the activation of autophagy but not apoptosis. Toxicol Lett 224:84–92.
- Patel B, Gupta N, Ahsan F. 2015. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur J Pharm Biopharm 89:163–74.
- Penno A, Hackenbroich G, Thiele C. 2013. Phospholipids and lipid droplets. Biochim Biophys Acta 1831:589–94.
- Pentchev P, Gal A, Booth A, Omodeo-Sale F, Fours J, Neumeyer B, et al. 1980. A lysosomal storage disorder in mice characterized by a dual deficiency of sphingomyelinase and glucocerebrosidase. Biochim Biophys Acta 619:669–79.
- Perez-Gil J, Weaver TE. 2010. Pulmonary surfactant pathophysiology: current models and open questions. Physiology (Bethesda) 25:132–41.
- Prentki M, Madiraju SRM. 2008. Glycerolipid metabolism and signaling in health and disease. Endocr Rev 29:647–76.
- Przybytkowski E, Behrendt M, Dubois D, Maysinger D. 2009. Nanoparticles can induce changes in the intracellular metabolism of lipids without compromising cellular viability. FEBS J. 276:6204–17.
- Pucer A, Brglez V, Payre C, Pungercar J, Lambeau G, Petan T. 2013. Group X secreted phospholipase A(2) induces lipid droplet formation and prolongs breast cancer cell survival. Mol Cancer 12:111.
- Ratoi M, Hoet PHM, Crossley A, Dobson P. 2014. Impact of lung surfactant on wettability and cytotoxicity of nanoparticles. Rsc Adv 4:20573–81.
- Repetto G, del Peso A, Zurita JL. 2008. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3:1125–31.
- Sadhukha T, Wiedmann TS, Panyam J. 2013. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 34:5163–71.
- Sarkar A, Ghosh M, Sil PC. 2014. Nanotoxicity: oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J Nanosci Nanotechnol 14:730–43.
- Schleh C, Kreyling WG, Lehr CM. 2013. Pulmonary surfactant is indispensable in order to simulate the in vivo situation. Part Fibre Toxicol 10:6.
- Shayman JA, Abe A. 2013. Drug induced phospholipidosis: an acquired lysosomal storage disorder. Biochim Biophys Acta 1831:602–11.
- Simon-Deckers A, Gouget B, Mayne-L'Hermite M, Herlin-Boime N, Reynaud C, Carriere M. 2008. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 253:137–46.
- Singh S, Shi TM, Duffin R, Albrecht C, van Berlo D, Hoehr D, et al. 2007. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol Appl Pharm 222:141–51.
- Stearns RC, Paulauskis JD, Godleski JJ. 2001. Endocytosis of ultrafine particles by A549 cells. Am J Respir Cell Mol Biol 24:108–15.
- Stern ST, Adiseshaiah PP, Crist RM. 2012. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol 9:20.
- Wahajuddin AS. 2012. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine 7:3445–71.
- Wang MJ, Petersen NO. 2013. Lipid-coated gold nanoparticles promote lamellar body formation in A549 cells. Biochim Biophys Acta 1831:1089–97.
- Weaver TE, Na CL, Stahlman M. 2002. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol 13:263–70.
- Whitsett JA, Wert SE, Weaver TE. 2010. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med 61:105–19.
