Abstract
Despite of the increasing application of silica nanoparticles and identification of oral exposure as a major entry portal, we lack understanding of nanosilica effects in the gut. Thus, we investigated biointeractions of nanosilica with single intestinal cells. The invertebrate nematode Caenorhabditis elegans was chosen as model organism with a tractable intestine and realistic target organism of nanomaterials in the environment. We found that nanosilica impairs the intestinal uptake of oligopeptides. Downstream to absorption by the apical OPT-2/PEP-2 transporter dipeptides were trapped in aberrant vesicles that grow over time and reach diameters of ≥6 μm. The peptide vesicles do not correspond to known organelles such as gut granules and form independently of related gene products GLO-1 or GLO-3. Formation of aberrant peptide vesicles also occurred independently of insulin/IGF-I receptor (DAF-2) signaling and daf-2 loss of function mutants showed specific vesicle patterns including distinct localization along the apical membrane of single intestinal cells. As malnutrition of exposed C. elegans manifested in reduced growth and a petite phenotype similar to OPT-2/PEP-2 transporter deficient mutants, we conclude that nanosilica-induced peptide vesicles represent a new compartment of di- and tripeptide trapping which disrupts hydrolysis of nutrient peptides and metabolism.
Introduction
Their unique properties steadily augment the trade, use, and ultimate environmental deposition of nanomaterials. With a production volume of 541 318 tons/year in 2013 in Central Europe alone, nanosilica heads the list of manufactured nanoparticles (NPs, Wang and Nowack Citation2018). NP-flows are best described by probabilistic modeling that includes production, immediate, and in-stock use wear off from products to technical systems such as waste treatment plants or direct disposal to the environmental compartments air, soil, surface waters, and sediments (Oberdörster, Oberdörster, and Oberdörster Citation2005). The predicted median concentration of nanosilica in central European surface waters currently levels at 3.5 μg/L, whereas the median silica NP-concentrations of natural or urban soil are 160 μg/kg, sewage sludge-treated soil 390 and 490 μg/kg in landfill waste (Wang et al. Citation2016; Wang and Nowack Citation2018). Technically, soils and sediments are defined as environmental sinks; however, NPs are taken up by plants, including crops, and trophic transfer of NPs occurs along the aquatic and terrestrial food chains (Unrine et al. Citation2012; Skjolding, Winther-Nielsen, and Baun Citation2014; Gupta et al. Citation2016). Notably, specific formulations of agrochemicals, e.g. pesticides, include nanomaterials which are distributed into the environment by agricultural practice and hence enter the food chain (Pourzahedi, Vance, and Eckelman Citation2017). In addition to their environmental distribution, nanomaterials such as nanosilica and nano-TiO2 are used as food additives and therefore subject to oral uptake (Wang and Nowack Citation2018). The United States Food and Drug Administration (FDA) allows up to 2% per weight of silica additives to food, the European Union (EU) 1%, respectively (European Union Citation2011; US FDA Citation2015). It was shown that up to 80% of orally ingested food-grade silica is in the nano-sized range when reaching the intestinal lumen, e.g. the gut epithelium is most likely exposed to nanosilica (Peters et al. Citation2012).
Its dual role as a realistic target organism of nanomaterials in the environment and top animal model in the laboratory justifies the nematode roundworm Caenorhabditis elegans as a relevant study object to investigate biointeractions between NPs and the gut (von Mikecz Citation2018). The worms’ intestine represents a multifunctional organ involved in the absorption, metabolism, storage, and allocation of nutrients. Additionally, it provides for detoxification and innate immunity which is vital to the bacterivore (Pukkila-Worley and Ausubel Citation2012). The C. elegans intestine comprises 20 epithelial cells. Their apical domain faces the intestinal lumen and is characterized by microvilli that execute uptake of macromolecules from the environment. A number of genes integral to the worms’ microvilli were identified (McGhee Citation2007). Dynamin is highly expressed on the luminal surface of the intestine, consistent with the high rates of endocytosis necessary for the uptake of nutrients from the intestinal lumen (Labrousse, Shurland, and van der Bliek Citation1998). Likewise, the OPT-2/PEP-2 transporter is located in the apical cell membrane and serves as the major dipeptide transporter in C. elegans (Rubio-Aliaga and Daniel Citation2008). OPT-2/PEP-2 belongs to the proton-coupled transporter family (SLC15) of oligopeptide transporters that are integral membrane proteins and mediate absorption of dietary protein digestion products as well as peptide-like drugs from the intestinal lumen (Daniel Citation2004; Smith, Clémençon, and Hediger Citation2013; Newstead Citation2017). In contrast to the apical surface, the structure and expression of amino acid transporters in the basolateral domain of intestinal epithelial cells is less well characterized in C. elegans. The cytoplasm of intestinal cells contains vesicular organelles including lysosomes and numerous lysosome-related gut granules (GGs) that (i) are filled with autofluorescent material such as the kynurenine pathway product anthranilic acid, (ii) store zinc and (iii) express GLO-1, a homolog of mammalian small GTPase Rab38 (Roh et al. Citation2012; Coburn and Gems Citation2013). Gut granule loss mutants (glo-1,-3,-4) result in partial or complete loss of GGs and exhibit respective storage deficits (Rabbitts et al. Citation2008). Despite their prominent appearance in single intestinal cells, the function of GGs is still unsolved and concepts range from their role as storage sites of compounds that promote innate immunity to UV protection (Coburn and Gems Citation2013).
Co-feeding of C. elegans with bacteria and labeled nanomaterials showed early on that NPs traverse the pharynx and gut (Lim et al. Citation2006; Pluskota et al. Citation2009; Mohan et al. Citation2010). However, the interactions between NPs and nutrient transport in single intestinal cells and consequences for the worm’s metabolism remained unclear. Here, we show that silica NPs undergo intestinal uptake routes corresponding to the corona that is conditioned by the method of their synthesis. While all silica particles—BULK and nanosilica—translocate to the intestinal lumen, those NPs synthesized by Hartlen preparation are subjected to facilitated entry into intestinal epithelial cells and specifically concentrate in lysosome-like organelles, e.g. GGs. Intracellular localization of Hartlen-silica NPs in GGs is regulated by the peptide transporter OPT-2/PEP-2, because in mutants lacking the transporter, Hartlen-silica NPs remain in the gut lumen. Irrespective of their synthesis and uptake route, all silica nanomaterials significantly interfere with vesicular peptide trafficking and metabolism. Suggesting nanosilica-associated defects of peptide metabolism and consequent malnutrition, silica NP-treated worms show a petite phenotype similar to pep-2-deficient mutants. Dietary restriction by malnutrition due to silica NPs does not prolong C. elegans life span. In contrast, differently synthesized nanosilica promote a novel intestinal ballooning phenotype, impaired peptide trafficking, and protein homeostasis in young worms that normally does not occur until late midlife and comes along with a pleiotropic scenario of reduced health span.
Insulin/IGF-I receptor (DAF-2) signaling determines the longevity of adult worms as well as the occurrence of aging stigmata such as degeneration of protein homeostasis (Kenyon Citation2010; Walther et al. Citation2015). Daf-2 deletion mutants that disrupt insulin/IGF-I signaling in the nematode C. elegans are efficient in neutralizing age-related defects and thereby prolong life span (Kenyon Citation2010; Tank, Rodgers, and Kenyon Citation2011). Notably, in our study, primary level genetics indicate that disruption of the insulin/IGF-I receptor pathway is not sufficient to rescue the premature onset of intestinal ballooning or aberrant peptide trafficking induced by nanosilica.
Methods
Caenorhabditis elegans strains
All strains, except deletion mutant pep-2(lg601) that was kindly shared by Ralf Baumeister (Meissner et al. Citation2004), were purchased from Caenorhabditis Genetics Center (University of Minnesota, MN, US) and grown at 20 °C unless indicated otherwise. Worms were cultured on nematode growth medium (NGM) plates supplemented with yeast extract and fed with live Escherichia coli strain OP50 (Brenner Citation1974). C. elegans strains used included Bristol N2 (wild type), DR1572 daf-2(e1368) III, CB1370 daf-2(e1370) III, CF1038 daf-16(mu86) I, GH383 glo-3(zu446) X, GH403 glo-3(kx94) X (Rabbitts et al. Citation2008), JJ1271 glo-1(zu391) X (Hermann et al. Citation2005), LX975 vsls97 [tph-1p::DsRed2 + lin-15(+)] (Tanis et al. Citation2008) and BZ555 egls [dat-1p::GFP] (Nass et al. Citation2002). CB1370 daf-2(e1370) III were maintained at 15 °C on NGM plates.
Particles
Unlabeled Stoeber silica NPs (50 nm) and Stoeber BULK silica particles (500 nm) were purchased from Kisker (Steinfurt, Germany). Unlabeled 14 nm HTFH silica NPs and HTFH BULK silica particles (500–1000 nm) were from Sigma-Aldrich (Darmstadt, Germany). HTFH silica NPs of 12 and 20 nm were provided by Evonik Industries AG (Hanau, Germany). Rhodamine-labeled Hartlen silica NPs (25 and 80 nm) were kindly shared by Dr. Annette Kraegeloh (INM - Leibniz Institute for New Materials, Saarbrücken, Germany). COOH-polystyrene (PS) NPs (50 nm) and BULK PS particles (500 nm) were from Polysciences (Warrington, PA, US). Reference Ag NPs (15 nm, NM300K) were provided by the European Commission Joint Research Center (JRC, Ispra, Italy). Stock solutions of silica NPs and BULK silica particles were prepared by suspending 25 mg/mL powder in ddH2O. Table S1 lists the respective particle characterization. Particles were analyzed by transmission electron microscopy (TEM), dynamic light scattering (DLS), and laser Doppler velocimetry in ddH2O using the Zetasizer Nano-ZS (Malvern Instruments Ltd., Malvern, UK), and by live cell imaging (Hemmerich and von Mikecz Citation2013; Scharf, Piechulek, and von Mikecz Citation2013).
Table 1. Summary of particle effects on the morphology and function of intestinal epithelium in the nematode Caenorhabditis elegans.
Nanomaterial exposition in liquid media
Synchronized worms were exposed to nanomaterials in liquid medium (S-medium, pH 5.7, with 50 mg/mL carbenicillin and 0.1 mg/mL fungizone) on 96-well plates containing 12 mg/mL freshly prepared E. coli OP50 in a total volume of 150 μL per well (Petrascheck, Ye, and Buck Citation2007). Nematodes were seeded as L4 larvae and treated with 5-flouro-20-deoxyuridine (1.5 mM final concentration) to prevent self-fertilization. Nanomaterials were added on day 1 of adulthood at the indicated concentrations. All experiments were performed at 20 °C except for daf-2(e1370) that were kept at 15 °C.
Peptide trafficking
Nanomaterial-exposed animals or control worms (ddH2O) were washed with M9 buffer in 96-well plates and cultivated in M9 containing 1 mM ß-Ala-Lys-AMCA (Meissner et al. Citation2004). After 2–3 h, worms were washed at least four times with M9 and analyzed on 5% agar pads with 10 mM NaN3. Peptide distribution was observed by epifluorescence microscopy (see below) in single intestinal cells and is presented as pseudocolor intensity map using Metamorph 4.6 software (Molecular Devices, Sunnyvale, CA) and Photoshop (Redmond, WA). The formation of peptide vesicles was quantified in wild type animals (N2) by determining vesicle size, the number of vesicles per cell, and the number of affected worms. Vesicle formation was classified in apical or random distribution within single intestinal cells. Six to thirty-nine worms (n) were tested per condition per experiment. All experiments were performed triplicate or more.
Caenorhabditis elegans length
Worms were collected on day 4 of adulthood and cultivated with M9 supplemented with 10% NaN3 to stretch the worms. Length measurements were performed with a Nikon SMZ800 stereomicroscope (Nikon Instruments Europe BV, Amsterdam, Netherlands). Micrographs were taken with an epifluorescence microscope (Fluoview, IX70, Olympus, Tokyo, Japan) with a 10×/0.3 NA UPlanF objective. Twenty worms (n) were tested per condition per experiment. All experiments were performed three times.
Caenorhabditis elegans ballooning phenotype
Nanomaterial-exposed animals and control worms (ddH2O) were washed at least three times with M9 buffer in 96-well plates and observed on 5% agar pads with 10 mM NaN3. Worms showing a balloon-like structure of the gut were scored as intestinal ballooning. Per condition per experiment, 20–197 worms (n) were tested. All experiments were performed at least three times.
Microscopy
Living C. elegans were analyzed on 5% agarose pads containing 10 mM NaN3 at room temperature. Imaging was performed by epifluorescence microscopy (Fluoview, IX70, Olympus, Tokyo, Japan) with a 10er/0.3 NA and a 60×/1.4 NA Plan Apo objective. Rhodamine-labeled silica NPs and DsRed2 reporter worms were detected by 561 nm excitation/575–615 nm emission and GFP was detected by 488 nm excitation/510–550 nm emission. ß-Ala-Lys-AMCA staining was detected by 358 nm excitation/430 nm emission. Quantification of fluorescence intensity was performed with the Metamorph image analysis software package (Molecular Devices, Sunnyvale, CA).
Statistics
One-way ANOVA with Tukey's post-hoc test (Origin 8.5, Origin Lab Corporation, Northampton, MA) was used to determine statistical significance. p-values below 0.05 were considered as significant. Violin and Box-Plots were created with seaborn statistical data visualization 0.71 in Python 3 (https://pypi.org/project/seaborn/).
Results
Silica NPs induce alteration of intestinal morphology
To cover relevant nanomaterials for oral uptake, nanosilica was used to investigate interactions with the intestinal epithelium of adult C. elegans. The particles were generated by different methods including Hartlen preparation, the Stoeber process, and high-temperature flame hydrolysis (HTFH). Table S1 summarizes the respective particle characterization and properties including transmission electron micrographs (TEM) of nanosilica, and control NPs (polystyrene and silver; Table S1). While NPs possess diameters between 12 and 105 nm, larger BULK particles of 500 nm were also included to provide controls for size specificity. Irrespective of the mode of their synthesis, all characterized particles exhibited a negative surface net charge (zeta potential; Table S1).
Co-feeding of 1-day old hermaphrodite N2 worms with OP50 bacteria and particles showed that nanosilica specifically induce a morphological phenotype in the intestine that is characterized by shortening of the cell junction region between single epithelial cells and thus appears as ballooning (). All pristine silica NPs with a diameter of 25, 50, and 80 nm induced the intestinal ballooning phenotype within 24 h of exposure, whereas mock-treated worms (H2O) and worms exposed to larger BULK silica (500 nm) showed normal intestinal morphology (). Titration revealed that 50 nm silica NPs promote intestinal ballooning in the lowest observed effect level (LOAEL) concentrations of ≥160 μg/mL (Figure S1). Time kinetics indicated that the percentage of C. elegans that are affected by ballooning increases between 24 and 96 h of exposure with nanosilica to 50% (). The age-resolved analysis of untreated N2 adults showed that normally ballooning occurs in 4% of 7-day-old worms, 58% of 10-day-old worms, and 87% of 11-day-old worms, and thus represents an age-associated phenotype that is prematurely induced by the xenobiotic nanosilica (). Due to the involvement of aging, we next analyzed by first level genetics if daf-2 mutants that promote longevity and rescue certain age-related defects such as neurodegeneration (Kenyon Citation2010; Tank, Rodgers, and Kenyon Citation2011) could likewise rescue nanosilica-induced ballooning. However, different daf-2 mutants (daf-2(e1368); daf-2(e1370)), daf-16 mutants, and reporter worms for serotonergic (tph-1p::DsRed2) or dopaminergic (dat-1p::GFP) neurotransmission are equally vulnerable to intestinal ballooning by silica NPs as the wild type N2 (Figure S2).
Figure 1. Silica NPs induce untimely premature gut aging in young worms. (A) Schematic cartoon of the intestinal ballooning phenotype. Wild type (N2) worms were left untreated or fed with silica particles for 48 h. (B) Representative differential interference contrast (DIC) of a 2-day-old adult C. elegans that was mock-treated with H2O. (C–E) Representative micrographs of 2-day-old adult C. elegans that were fed with 0.2 mg/mL silica NPs. The worms show intestinal ballooning. (F) Representative micrograph of a 2-day-old adult C. elegans that was fed with 0.5 mg/mL BULK silica. Bar, 25 µm. (G) Representative micrograph of a 12-day-old adult C. elegans showing intestinal ballooning. Bar, 25 µm. (H) Quantification of the number of wild type (N2) worms showing intestinal ballooning (three independent experiments). Values represent means ± SD from at three independent experiments with n = 74–128 per experiment. (I–M) Violin plots for respective quantification of the number of worms showing ballooning. Significant differences (p < 0.01) after 48-h exposure for each group compared with H2O (I) and BULK silica (J). Values represent means ± SD from at least five independent experiments with n = 32–197 per condition per experiment.
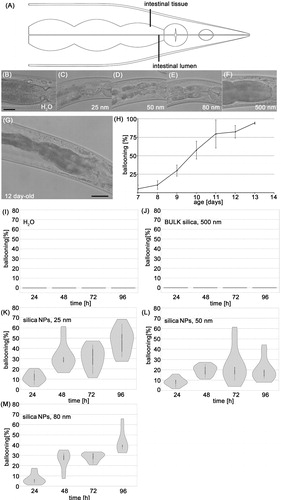
Figure 2. Uptake routes of silica particles. (A–C) Representative micrographs of 2-day-old adult C. elegans that were fed with 0.2 mg/mL rhodamine-labeled silica NPs for 48 h. Top row: epifluorescence (red) merged with differential interference contrast (DIC) microscopy; middle: epifluorescence was inverted to gray scale; bottom: epifluorescence was inverted to pseudocolor and intensity map. (A,C) Nanosilica synthesized by the Hartlen preparation. (B) Nanosilica synthesized by the Stoeber process. Bar, 15 µm. (D) Representative micrographs of 2-day-old adult C. elegans that were treated with 0.5 mg/mL rhodamine-labeled BULK silica particles for 48 h. Top: epifluorescence merged with DIC; middle: gray scale; bottom: pseudocolor and intensity map. (E) Top: schematic of the localization of Hartlen nanosilica (25 and 80 nm) in a single intestinal cell within gut granules (GGs, red); middle: blow up of a single (anteriormost) intestinal cell, merged epifluorescence (red) and DIC; bottom: schematic of an entire worm with colored gut (red) and inset that locates anteriormost intestinal cells. Bar 7.5 µm. (F–G) Representative micrographs of 2-day-old adult pep-2 mutants that were treated with 0.2 mg/mL rhodamine-labeled Hartlen silica NPs for 48 h. In OPT-2/PEP-2 deletion mutants Hartlen nanosilica locate in the gut lumen. Bar, 15 µm. GGs, gut granules; intensity, fluorescence intensity in arbitrary units; nm, nanometer; nucleus, cell nucleus; pep-2, OPT-2/PEP-2 deletion mutant. Note: Colour version of this figure is available online.
Taken together intestinal ballooning represents a novel aging phenotype that increases in aging adult hermaphrodites. Heterogeneous silica NPs promote intestinal ballooning untimely in young worms, as nanosilica synthesized by different methods induce the phenotype. Moreover, premature ballooning in young worms is nanosilica-specific, as no significant induction was observable after exposure with BULK silica, nanopolystyrene, BULK polystyrene, or nanosilver (Figure S3).
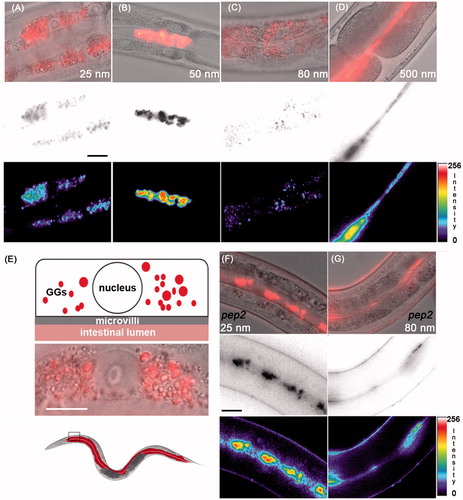
Figure 3. Nanosilica disrupts peptide trafficking in intestinal epithelial cells. Representative micrographs show the localization of the dipeptide reporter substrate ß-Ala-Lys-AMCA in intestinal cells of 4-day-old, adult C. elegans. Wild type worms (N2) were left untreated H2O (A, A′, A″) or exposed to silica NPs (50 nm; B, B′, B″) or BULK silica (500 nm; C, C′, C″) for 72 h and cultivated with the fluorescent dipeptide derivate ß-Ala-Lys-AMCA at 20 °C. ß-Ala-Lys-AMCA staining indicates peptide localization as epifluorescence micrographs (middle, blue) and as pseudocolor intensity map (bottom). Bar, 10 µm. (D, D′, D″) Daf-2 mutants (e1370) were fed with 50 nm silica NPs for 72 h and cultivated with ß-Ala-Lys-AMCA at 15 °C. Fluorescence staining (D′, blue) and pseudocolor intensity map (D″, red) shows formation of small vesicles along the apical domain. (E,F) Schematic visualization of ß-Ala-Lys-AMCA distribution after silica NPs exposition in single intestinal cells of wild type (N2) worms or (G) daf-2 mutants. (H) Quantification of the vesicle size in nanosilica-exposed N2 worms after 72 h. (I) Graph depicting the number of worms with 3 vesicles per cell (black) and number of vesicles per cell (blue) over time. Values represent means ± SD from three independent experiments with n = 16–80 animals per experiment. (J) For determination of the lowest observed effect level (LOAEL) of vesicle formation, adult C. elegans were mock-treated (H2O, white), exposed to increasing concentrations of silica NPs (gray) or to BULK silica (black). Values represent means ± SD from 3 to 5 independent experiments with n = 16–39 per condition per experiment. (K) Vesicle formation in 4-day-old adult wild type (N2) worms compared with age-matched daf-2 mutants or daf-16 mutants after silica exposition (200 µg/mL). Staining patterns of daf-2 mutants were categorized into the groups apical versus random localization. Values represent means ± SD from three independent experiments with n = 20–25 per condition per experiment. (L) Schematic of the daf-2 gene indicating the position and nature of different mutations. A mutation in the ligand-binding domain leads to a random localization of large nanosilica-induced vesicles in the cytoplasm. In contrast, the mutation in the tyrosine kinase domain of the daf-2 gene promotes formation of smaller vesicles in the apical domain (D′, D″, K). ß-Ala-Lys-AMCA, fluorescent di-peptide conjugate β-Ala-Lys-AMCA; *p < 0.05, **p < 0.01; h: hours; μg: microgram; mL: milliliter; NPs: nanoparticles. Note: Colour version of this figure is available online.
Intestinal uptake pathways of silica NPs
Next adult hermaphrodite C. elegans were fed with fluorescently labeled silica NPs and imaged to comparatively monitor intestinal uptake. Representative micrographs show that nanosilica uses uptake routes that correspond to the corona formed due to the mode of their synthesis (). Silica NPs produced by Hartlen preparation (25 and 80 nm) translocate to gut granules (GGs) and thus use a classical endocytic route to the lysosome-related organelles (). Surprisingly, the OPT-2/PEP-2 peptide transporter is decisively involved in the uptake of Hartlen nanosilica into intestinal epithelial cells, because epifluorescence imaging revealed that in pep-2 mutants lacking OPT-2/PEP-2, Hartlen synthesized nanosilica were retained in the intestinal lumen (). Pep-2(lg601) animals exposed to Hartlen silica NPs (25 or 80 nm) showed fluorescence throughout the gut lumen, indicative of a normal ingestion but lack of absorption by intestinal epithelial cells. Likewise, silica NPs synthesized by the Stoeber process (50 nm) and BULK silica particles predominately locate in the intestinal lumen and do not accumulate in GGs ().
Nanosilica interfere with vesicular peptide trafficking
The comparative analysis of nanomaterial effects on the intestine of adult hermaphrodite C. elegans showed that while different nanosilica use different uptake pathways, they all induce premature intestinal ballooning in young worms. Next, we asked whether the morphological changes indicate deficits of intestinal function and therefore analyzed peptide transport by OPT-2/PEP-2 that plays a role in the absorption of dietary protein digestion products as well as peptide-like drugs from the intestinal lumen (Daniel Citation2004; Nehrke Citation2003).
One-day-old C. elegans were fed with OP50 bacteria, unlabeled particles, and the fluorescent di-peptide conjugate β-Ala-Lys-AMCA that was previously shown to be a representative substrate of OPT2/PEP-2 (, blue; Figure S4; Groneberg et al. Citation2001). Representative micrographs of anteriormost intestinal cells show that in control worms (H2O) β-Ala-Lys-AMCA is taken up by OPT-2/PEP-2 and distributes diffusely in the cytoplasm (; Figure S4(D,E)). In nanosilica (50 nm)-treated worms, β-Ala-Lys-AMCA was likewise taken up by intestinal cells. However, we observed the formation of large vesicles that randomly distributed throughout the cytoplasm and suggest altered peptide trafficking (). Time kinetics indicate that the newly formed peptide vesicles grow over time. After 72 h, the size of the vesicles varies between 2 and 12 μm in diameter with a mean diameter of 6 μm (). Titration of LOAEL concentrations shows that effective nanosilica concentrations, as well as the time windows of peptide vesicle formation and ballooning, concur (). Consistent with the idea that altered peptide trafficking occurs specifically in response to nano-sized silica, BULK silica, nano polystyrene, BULK polystyrene, and nanosilver induced diffuse cytoplasmic staining of β-Ala-Lys-AMCA comparable to mock-treated control worms (BULK; and ). In contrast, the formation of peptide vesicles was induced by all different silica NPs tested, including food-grade nanosilica (Figure S5; ).
Figure 4. Nanosilica-induced peptide vesicles form independent of Glo-1 and Glo-3. (A) Representative micrographs show the uptake of rhodamine-labeled Hartlen silica NPs (25 nm, red, 200 µg/mL) into gut granules and the formation of ß-Ala-Lys-AMCA vesicles (blue) in wild type (N2) worms after 72 h. Bottom: The schematic visualizes different localization of silica NPs (red) and ß-Ala-Lys-AMCA vesicles (blue) in single intestinal cells. Bar, 13 µm. (B) Representative epifluorescence micrographs of ß-Ala-Lys-AMCA vesicles in silica NPs (50 nm) exposed wild type worms (N2), glo-1(zu391) mutants, glo-3(kx94) mutants and glo-3(zu446) mutants. Bar, 15 µm. (C) Respective quantification of worms with 2–3 peptide vesicles per cell (gray) or >2–3 peptide vesicles per cell (black) after silica exposition (200 µg/mL). Values represent means ± SD from at least four independent experiments with n = 6–28 per condition per experiment (one way ANOVA with Tukey’s post-hoc test). **p < 0.01; BULK, BULK silica particles; nm, nanometer; n.s., not significant. Note: Colour version of this figure is available online.
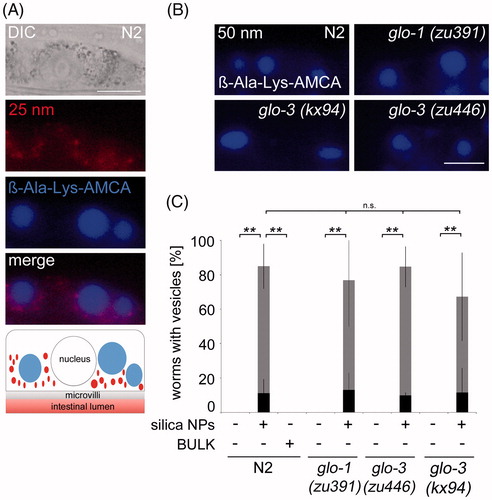
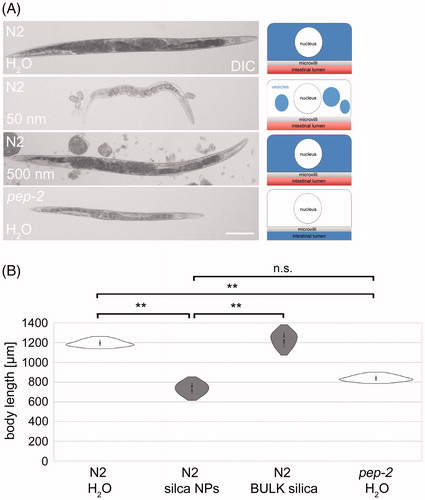
Figure 5. Nanosilica induces a ‘petite’ phenotype resembling OPT-2/PEP-2 deletion mutants. (A) Differential interference contrast (DIC) of stretched adult wild type (N2) worms that were left untreated H2O (mock control) or fed with silica NPs (200 µg/mL) or BULK silica (200 µg/mL) for 72 h at 20 °C. Bottom: pep-2(lg601) mutants were mock-treated with H2O for 72 h. Right column: schematic of respective ß-Ala-Lys-AMCA distribution within single intestinal cells. Bar, 150 µm. (B) Respective quantification of the body length. Data from three independent experiments with n = 20 per condition per experiment. **p < 0.01; n.s.: not significant; NPs: nanoparticles.
DAF-2 represents the exclusive C. elegans insulin/IGF-1 receptor, significantly prolongs life span and protects against a variety of cellular stressors (Kenyon Citation2010; Tank, Rodgers, and Kenyon Citation2011). Thus, we investigated whether reduced insulin/IGF-1 signaling suppresses the formation of peptide vesicles and monitored β-Ala-Lys-AMCA in daf-2 mutants exposed to nanosilica. Mutant worms fed with OP50 bacteria, β-Ala-Lys-AMCA and nanosilica showed peptide vesicles suggesting altered peptide trafficking (). Notably, the cytoplasmic localization of the nanosilica-induced peptide vesicles differed between different daf-2 mutants. While daf-2(e1368) with a mutation in the ligand-binding domain displayed peptide vesicles randomly distributed throughout the cytoplasm, daf-2(e1370) that has a mutated tyrosine kinase domain specifically showed smaller peptide vesicles located in-line along the apical domain of intestinal cells (; Figure S6). The cause for the diverse peptide vesicle patterns in relation to different daf-2 mutations is unknown, but are likely related to interactions between mutation of the tyrosine domain, e.g. respective signaling pathways and apical-specific trafficking defects.
Nanosilica-induced peptide vesicles form independent of GLO-1 and GLO-3
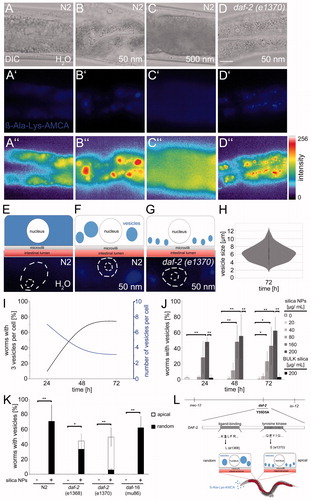
To learn more about the identity of the nanosilica-induced peptide vesicles, their formation was investigated in relation to gut granules. Wild type worms (N2) were co-fed with OP50 bacteria, rhodamine-labeled Hartlen silica NPs (25 nm) and β-Ala-Lys-AMCA. Representative fluorescence imaging of a single intestinal epithelial cell shows punctate staining of numerous foci throughout the cytoplasm excluding the nucleus (, red) and a few large round vesicles (, blue). Focal and vesicle staining does not overlap suggesting that peptide vesicles (blue) and gut granules filled with Hartlen silica NPs (red) represent separate entities. Moreover, the merged micrograph shows gut granules preferentially locating in the surroundings of the large vesicles which indicate that the peptide vesicles physically displace other cytoplasmic organelles (, merge and bottom).
We next analyzed silica NP-induced peptide vesicle formation in gut granule loss mutants. Intestinal cells of adult N2 animals contain hundreds of GGs, whereas glo-1 and glo-3 mutants contain none to <10 gut granules per cell (Hermann et al. Citation2005). Glo-1(zu391), glo-3(kx94) and glo-3(zu446) mutants were co-fed with OP50 bacteria, unlabeled nanosilica (50 nm) and β-Ala-Lys-AMCA. Fluorescence imaging shows that the numbers and shape of newly formed peptide vesicles are similar in wild type (N2) worms compared with glo-1 and glo-3 mutants (; Figure S7). The results suggest that induction, distribution and growth of peptide vesicles occurs independent of gut granule formation, e.g. GLO-1, the homolog of mammalian small GTPase Rab38, and the GG membrane protein GLO-3. Thus, peptide vesicles visualized by β-Ala-Lys-AMCA represent separate storage sites in intestinal epithelial cells that may be specialized and store dietary protein digestion products.
Peptide vesicles correlate with a petite phenotype
To characterize the nutritional consequences of peptide vesicle formation the body size of nanosilica-treated worms was compared to untreated controls (N2, H2O), BULK silica-treated worms (N2, 500 nm) and pep-2 deletion mutants lacking the OPT-2/PEP-2 peptide transporter (). Untreated and BULK silica-treated wild type C. elegans grow normally with a mean body length of 1194 μm and 1233 μm, respectively (). In contrast, nanosilica exposed N2 worms showed a small phenotype with a mean body length of 736 μm that we term ‘petite.’ This evidently equals the mean body length of pep-2 mutants (838 μm) that have a loss of transporter function and are unable to absorb di- or tri-peptides into intestinal cells (Daniel Citation2004). Next, we measured the body length in adult N2 worms exposed to different varieties of silica nanomaterials. Silica NPs reduced C. elegans size irrespectively of how they were synthesized, as a significant reduction of body length was observed after 72 h of treatment with 12 nm HTFH-, 25 nm Hartlen- and 50 nm Stoeber-nanosilica (Figure S8). Screening of different silica nanomaterials revealed that reduction of body size concurs with the formation of aberrant peptide vesicles. We conclude that while apparently the peptide transport via OPT-2/PEP-2 still works in nanosilica-treated worms, as the reporter β-Ala-Lys-AMCA is absorbed by intestinal cells, the newly formed vesicles apparently trap nutrient peptides and segregate them from further hydrolysis. In turn, di- and tri-peptides are not available for amino acid metabolism (Smith, Clémençon, and Hediger Citation2013). Thus, the outcome of disturbed peptide homeostasis is the same in pep-2 and nanosilica-treated N2 worms although having different cellular origins, namely, the lack of apical membrane transport (pep-2) or aberrant segregation of peptides in vesicles. Both scenarios result in malabsorption of di- and tri-peptides and abated allocation of nutrients which contributes to the petite phenotype.
Discussion
Intestinal ballooning
The fate of nanomaterials in the environment as eventual components of the food chain and their application as food additives prompted us to investigate NP biointeractions with intestinal function in the nematode C. elegans. Age-resolved observation of adult hermaphrodite worms showed that nanosilica induce the premature occurrence of a morphological phenotype which is characterized by shortening of cell junction regions between single intestinal cells and thus appears as ballooning. Intestinal ballooning normally affects 10- to 12-day-old wild type (N2) C. elegans which defines middle-aged worms as vulnerable concerning an intestinal phenotype that has not been described before (McGee et al. Citation2011). Nanosilica shifts this vulnerability to young adult, e.g. 2-day-old worms, and ballooning represents yet another age-related C. elegans phenotype with an early onset due to exposure with nanomaterials (Scharf, Piechulek, and von Mikecz Citation2013; Jung et al. Citation2015; Scharf, Gührs, and von Mikecz Citation2016; Piechulek and von Mikecz Citation2018). Notably, silica NP-induced intestinal ballooning occurs independent of the insulin/IGF-1 receptor DAF-2 pathway that otherwise results in longevity and rescues certain age-associated phenotypes (Lee and Ashrafi Citation2008; Tank, Rodgers, and Kenyon Citation2011; Chen et al. Citation2015; Figure S2). Ballooning is promoted by a broad spectrum of differently synthesized nanosilica, whereas BULK silica particles, nanosilver, nanopolystyrene, and BULK polystyrene (microplastic) are ineffective. This excludes general particle effects on the apical region of intestinal epithelium or an exclusive contribution of surface charge (see Table S1, zeta potential) and is consistent with the concept that nanomaterials are more biologically active than their BULK counterparts (Oberdörster, Oberdörster, and Oberdörster Citation2005; Oberdörster Citation2010).
Particle localization
Here, we used silica NPs that were generated by different synthesis methods, namely the Stoeber process, high-temperature flame hydrolysis (HTFH) and Hartlen preparation (Stoeber, Fink, and Bohn Citation1968; Hartlen, Athanasopoulos, and Kitaev Citation2008). Generally, different preparation methods of nanomaterials result in respective post-production particle coronas that influence their uptake and localization in living organisms and single cells (Charan et al. Citation2011; Fadeel et al. Citation2015). While fluorochrome-labeled Stoeber and HTFH silica NPs predominately located in the gut lumen, Hartlen nanosilica specifically used the endocytic route from the apical cell membrane to gut granules (this study). Pep-2 mutants showed that respective localization requires the OPT2/PEP-2 peptide transporter; however, interactions between OPT2/PEP-2 and Hartlen nanosilica remain unclear. The transporter belongs to the NTR1/PTR family, also known as POT family, of transporters that have a cone-shaped cavity with approximate dimensions of 11 Å × 11 Å × 21 Å (Newstead Citation2017) and thus are too small for the transport of monodisperse nanomaterials with diameters of 25–80 nm, e.g. the Hartlen silica NPs identified in GGs. The finding that all tested nanosilica-induced intestinal ballooning irrespective of their uptake route and cellular localization rather suggests specific interactions of silica nanomaterials with microvilli, e.g. the apical region of intestinal cells. With advancing imaging and proteomics techniques, future research should include the isolation and quantitative mass spectrometry of single intestinal epithelial cells from untreated and nanosilica-treated C. elegans to compare the respective proteomes (McGhee Citation2007; Hosp and Mann Citation2017).
Nonetheless, we previously showed that Stoeber silica NPs (50 nm) penetrate vulval epithelial cells and concentrate in the cell nucleus, e.g. nucleoli (Scharf, Piechulek, and von Mikecz Citation2013). Intracellular localization of NPs according to their particle corona was also observed by nanotomography and nanoscopic X-ray fluorescence imaging of cobalt NPs in single intestinal cells. Notably, two-dimensional X-ray mapping with Zn revealed that Co NPs did not concentrate in GGs (Cagno et al. Citation2017) confirming the specificity of the endocytic route taken by the Hartlen synthesized silica NPs in this study.
A practical outcome from our studies is the application of Hartlen silica NPs as selective markers of GGs which may aid investigation of GG-function, intestinal membrane trafficking, and absorption of nutrients. A similar endocytic route was identified previously by Nile red-coated silver NPs that likewise labeled GGs (Charan et al. Citation2011); however, Hartlen silica NPs bear the advantage of coupling dyes for any desired imaging application.
Intestinal function
We show here that intestinal nanosilica effects target OPT-2/PEP-2-dependent absorption of di- and tri-peptides. Generally, OPT-2/PEP-2 mediates absorption of dietary protein digestion products as well as peptide-like drugs from the intestinal lumen (Daniel Citation2004; Grant and Caplan Citation2008). Accordingly, the reporter substrate β-Ala-Lys-AMCA is absorbed by intestinal epithelial cells and distributes diffusely throughout the cytoplasm. The diffuse localization facilitates subsequent cytoplasmic hydrolysis to amino acids, basolateral efflux and further nutrient allocation (Silk, Grimble, and Rees Citation1985; Broer Citation2013). In contrast, nanosilica induces the aberrant formation of β-Ala-Lys-AMCA peptide vesicles that grow over time and show unique distribution in daf-2 mutants (Figure S6). Mutation of the tyrosine kinase domain (daf-2(e1370)) leads to vesicles accumulating along the apical membrane of intestinal epithelial cells which indicates an apical-specific trafficking defect. Formation of GGs does not play a critical role, since segregation of di- and tri-peptides into aberrant vesicles occurs independently of GLO-1 and GLO-3 (). Up to date trafficking defects have been described for the basolateral domain and RME-1 (Grant et al. Citation2001; Grant and Caplan Citation2008). Notably, rme-1 mutants develop a vacuolated intestine starting in L4 developmental stage and the size of the vacuoles increases in an age-associated manner similar to nanosilica-induced β-Ala-Lys-AMCA vesicles.
From our results, it seems unlikely that nanosilica-induced peptide vesicles mainly function as transport vesicles. In wild type (N2) worms they gradually grow over time to spherical structures of considerable size with average diameters of 6 μm (72 h; ). A more fitting idea is that β-Ala-Lys-AMCA vesicles represent aberrant vacuoles/vesicles that store and trap di- and tri-peptides immediately after transport from the intestinal lumen via OPT-2/PEP-2. Due to a lack of turnover, e.g. hydrolysis, the peptides accumulate and accordingly the vesicles grow. In turn, nutrient allocation is disturbed and the peptides are not available for supply of amino acids or peptide hormones and respective signaling. Generally, nutrition stress by amino acid deprivation leads to starvation-induced inactivation of the target of rapamycin (TOR) kinase and reduced transcription of ribosomal RNA (rRNA), decreased ribosome subunit production and reduction of translation (Long et al. Citation2002; Sengupta, Peterson, and Sabatini Citation2010; Spriggs, Bushell, and Willis Citation2010). In addition to TOR, the general amino acid control pathway (GAAC) is activated by amino acid starvation. Here, the cascade of accumulation of uncharged tRNA, activation of GCN2, and inactivation of translation initiation factor eIF2 by phosphorylation reduces global protein synthesis and reprograms translation (Zaborske et al. Citation2009; Spriggs, Bushell, and Willis Citation2010). Accordingly, polysome profiling in yeast showed changes in the translational state of >600 mRNAs during the global response to amino acid starvation and significant downregulation of transcripts belonging to the gene ontology (GO) group of ribosome biogenesis and translation (Smirnova et al. Citation2005).
On the level of protein homeostasis nanosilica induce global amyloid protein aggregation that involves segregation of components of the ubiquitin-proteasome system, protein folding, the translational machinery, ribosomal RNA processing, and metabolic processes into an insoluble aggregome (Scharf, Piechulek, and von Mikecz Citation2013; Scharf, Gührs, and von Mikecz Citation2016). The GO groups of rRNA processing, translation, and protein folding altogether constitute 55% of the nanosilica-induced aggregome (Scharf, Gührs, and von Mikecz Citation2016). Segregation of proteins involved in translation within the insoluble fraction likely amplifies translational repression by nutritional stress.
Taken together nanosilica exposed adult C. elegans face a scenario of perturbed peptide metabolism and protein homeostasis. The metabolic defects generate petite C. elegans that show an early onset of age-associated stigmata such as intestinal ballooning, aberrant peptide vesicle formation in intestinal cells, and widespread amyloid protein aggregation (this study, Scharf, Piechulek, and von Mikecz Citation2013; Walther et al. Citation2015; Scharf, Gührs, and von Mikecz Citation2016). Nanosilica-induced accelerated aging additionally manifests in premature neurodegeneration, impaired serotonergic neurosignaling and related reproductive or locomotion defects that all fit into a concept of pleiotropic phenotypes indicating reduced health span (Pluskota et al. Citation2009; Hartl Citation2016; Scharf, Gührs, and von Mikecz Citation2016). Consistent with this idea Jung et al. introduced the term population fitness. Using high-throughput analysis they showed that a panel of NPs, including nanosilica, did not significantly reduce life span when used in sublethal concentrations, but instead reduced the fitness of middle-aged worms concerning behavioral phenotypes such as locomotion (Jung et al. Citation2015). The concept emerges that chronic exposure to environmentally realistic concentrations of nanomaterials does not affect longevity, but rather accelerates age-related impairments and thereby decreases the health span and reproductive fitness of the nematode C. elegans (von Mikecz Citation2018). While there are significant differences concerning the organization of the gut between nematodes and men, the basic architecture of intestinal epithelium is remarkably similar (Clark and Walker Citation2018; McGhee Citation2007). Notably, recent work identifies reduced protein translation as a pathomechanism in the progeric Cockayne syndrome (Alupei et al. Citation2018). Thus, the identification of nanosilica-induced impairment of peptide homeostasis and metabolism in the roundworm C. elegans may fuel respective nanotoxicological investigation across species.
Consistent with this idea, we note that our panel of tested nanosilica included a food-grade nanomaterial that is synthesized by high-temperature flame hydrolysis (HTFH; 12 nm) and applied as an additive to powders such as table salt, tomato powder, and the galenic formulation of drugs. There remains a gap of knowledge if other silica nanomaterials that are currently applied in food likewise affect intestinal morphology and interfere with peptide transport. We also do not know if respective LOAEL concentrations manifest in the human intestine after consumption of silica NPs as a food additive. With this in mind, our results provide for an incentive to further investigate the bioavailability and intestinal effects of all food-grade nanosilica that are currently applied in consumer products to support safe nanomaterials.
Supplemental Material
Download MS Word (5.7 MB)Acknowledgments
The authors thank the members of NanoMILE for provision of reference particles and for critical discussions. They thank Dr Annette Kraegeloh (Leibniz Institute of new Materials, Saarbrücken, Germany) for sharing Hartlen synthesized nanosilica within the Leibniz Nanosafety Research Network, the Caenorhabditis Genetics Center (CGC, University of Minnesota, Minneapolis, MN, US) for providing C. elegans strains, Ralf Baumeister (University of Freiburg, Germany) for sharing the pep-2 deletion mutants, Katrin Buder and Peter Hemmerich (FLI-Leibniz Institute for Age Research, Jena) for TEM analyses, and Klaus Meerholz and Tanja Tegeder (University of Cologne, Germany) for enabling dynamic light scattering characterization.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alupei, M. C., P. Maity, P. R. Esser, I. Krikki, F. Tuorto, R. Parlato, M. Penzo, A. Schelling, V. Laugel, L. Montanaro, et al. 2018. “Loss of Proteostasis is a Pathomechanism in Cockayne Syndrome.” Cell Reports 23(6): 1612–1619. doi:10.1016/j.celrep.2018.04.041.
- Brenner, S. 1974. “The Genetics of Caenorhabditis elegans.” Genetics 77(1): 71–94.
- Broer, S. 2013. “Diseases Associated with General Amino Acid Transporters of the Solute Carrier 6 Family (SLC6).” Current Molecular Pharmacology 6(2): 74–87.
- Cagno, S., D. A. Brede, G. Nuyts, F. Vanmeert, A. Pacureanu, R. Tucoulou, P. Cloetens, G. Falkenberg, K. Janssens, B. Salbu, et al. 2017. “Combined Computed Nanotomography and Nanoscopic X-Ray Fluorescence Imaging of Cobalt Nanoparticles in Caenorhabditis elegans.” Analytical Chemistry 89(21): 11435–11442. doi:10.1021/acs.analchem.7b02554.
- Charan, S., F. C. Chien, N. Singh, C. W. Kuo, and P. Chen. 2011. “Development of Lipid Targeting Raman Probes for in Vivo Imaging of Caenorhabditis elegans.” Chemistry - A European Journal 17(18): 5165–5170. doi:10.1002/chem.201002896.
- Chen, P., M. R. DeWitt, J. Bornhorst, F. A. Soares, S. Mukhopadhyay, A. B. Bowman, and M. Aschner. 2015. “Age- and Manganese-Dependent Modulation of Dopaminergic Phenotypes in a C. elegans DJ-1 Genetic Model of Parkinson's Disease.” Metallomics 7(2): 289–298. doi:10.1039/C4MT00292J.
- Clark, R. I., and D. W. Walker. 2018. “Role of Gut Microbiota in Aging-Related Health Decline: Insights from Invertebrate Models.” Cellular and Molecular Life Sciences 75(1): 93–101. doi:10.1007/s00018-017-2671-1.
- Coburn, C., and D. Gems. 2013. “The Mysterious Case of the C. elegans Gut Granule: Death Fluorescence, Anthranilic Acid and the Kynurenine Pathway.” Frontiers in Genetics 4: 151. doi:10.3389/fgene.2013.00151.
- Daniel, H. 2004. “Molecular and Integrative Physiology of Intestinal Peptide Transport.” Annual Review of Physiology 66(1): 361–384. doi:10.1146/annurev.physiol.66.032102.144149.
- European Union. 2011. “Commission Regulation (EU) No 1129/2011 of 11 November 2011 Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives.” Official Journal of the European Union 295: 1–177.
- Fadeel, B., A. Fornara, M. S. Toprak, and K. Bhattacharya. 2015. “Keeping It Real: The Importance of Material Characterization in Nanotoxicology.” Biochemical and Biophysical Research Communications 468(3): 498–503. doi:10.1016/j.bbrc.2015.06.178.
- Grant, B. D., and S. Caplan. 2008. “Mechanisms of EHD/RME-1 Protein Function in Endocytic Transport.” Traffic 9(12): 2043–2052. doi:10.1111/j.1600-0854.2008.00834.x.
- Grant, B., Y. Zhang, M. C. Paupard, S. X. Lin, D. H. Hall, and D. Hirsh. 2001. “Evidence That RME-1, a Conserved C. elegans EH-Domain Protein, Functions in Endocytic Recycling.” Nature Cell Biology 3(6): 573–579. doi:10.1038/35078549.
- Groneberg, D. A., F. Döring, P. R. Eynott, A. Fischer, and H. Daniel. 2001. “Intestinal Peptide Transport: Ex Vivo Uptake Studies and Localization of Peptide Carrier PEPT1.” American Journal of Physiology Gastrointestinal and Liver Physiology 281(3): G697–704. doi:10.1152/ajpgi.2001.281.3.G697.
- Gupta, G. S., A. Kumar, R. Shanker, and A. Dhawan. 2016. “Assessment of Agglomeration, co-Sedimentation and Trophic Transfer of Titanium Dioxide Nanoparticles in a Laboratory-Scale Predator-Prey Model System.” Scientific Reports 6(1): 31422. doi:10.1038/srep31422.
- Hartl, F. U. 2016. “Cellular Homeostasis and Aging.” Annual Review of Biochemistry 85: 1–4. doi:10.1146/annurev-biochem-011116-110806.
- Hartlen, K. D., A. P. Athanasopoulos, and V. Kitaev. 2008. “Facile Preparation of Highly Monodisperse Small Silica Spheres (15 to >200 nm) Suitable for Colloidal Templating and Formation of Ordered Arrays.” Langmuir 24(5): 1714–1720. doi:10.1021/la7025285.
- Hemmerich, P. H., and A. H. von Mikecz. 2013. “Defining the Subcellular Interface of Nanoparticles by Live-Cell Imaging.” PLoS One 8(4): e62018. doi:10.1371/journal.pone.0062018.
- Hermann, G. J., L. K. Schroeder, C. A. Hieb, A. M. Kershner, B. M. Rabbitts, P. Fonarev, B. D. Grant, and J. R. Priess. 2005. “Genetic Analysis of Lysosomal Trafficking in Caenorhabditis elegans.” Molecular Biology of the Cell 16(7): 3273–3288. doi:10.1091/mbc.e05-01-0060.
- Hosp, F., and M. Mann. 2017. “A Primer on Concepts and Applications of Proteomics in Neuroscience.” Neuron 96(3): 558–571. doi:10.1016/j.neuron.2017.09.025.
- Jung, S. K., X. Qu, B. Aleman-Meza, T. Wang, C. Riepe, Z. Liu, Q. Li, and W. Zhong. 2015. “Multi-Endpoint, High-Throughput Study of Nanomaterial Toxicity in Caenorhabditis elegans.” Environmental Science & Technology 49(4): 2477–2485. doi:10.1021/es5056462.
- Kenyon, C. J. 2010. “The Genetics of Ageing.” Nature 464(7288): 504–512. doi:10.1038/nature08980.
- Labrousse, A. M., D. L. Shurland, and A. M. van der Bliek. 1998. “Contribution of the GTPase Domain to the Subcellular Localization of Dynamin in the Nematode Caenorhabditis elegans.” Molecular Biology of the Cell 9(11): 3227–3239. doi:10.1091/mbc.9.11.3227.
- Lee, B. H., and K. Ashrafi. 2008. “A TRPV Channel Modulates C. elegans Neurosecretion, Larval Starvation Survival, and Adult Lifespan.” PLoS Genetics 4(10): e1000213. doi:10.1371/journal.pgen.1000213.
- Lim, S. F., R. Riehn, W. S. Ryu, N. Khanarian, C. K. Tung, D. Tank, and R. H. Austin. 2006. “In Vivo and Scanning Electron Microscopy Imaging of up-Converting Nanophosphors in Caenorhabditis elegans.” Nano Letters 6(2): 169–174. doi:10.1021/nl0519175.
- Long, X., C. Spycher, Z. S. Han, A. M. Rose, F. Müller, and J. Avruch. 2002. “TOR Deficiency in C. elegans Causes Developmental Arrest and Intestinal Atrophy by Inhibition of mRNA Translation.” Current Biology 12(17): 1448–1461. doi:10.1016/S0960-9822(02)01091-6.
- McGee, M. D., D. Weber, N. Day, C. Vitelli, D. Crippen, L. A. Herndon, D. H. Hall, and S. Melov. 2011. “Loss of Intestinal Nuclei and Intestinal Integrity in Aging C. elegans.” Aging Cell 10(4): 699–710. doi:10.1111/j.1474-9726.2011.00713.x.
- McGhee, J. D. 2007. “The C. elegans intestine.” WormBook: The Online Review of C. elegans Biology, Mar 27. http://www.wormbook.org/chapters/www_intestine/intestine.html.
- Meissner, B., M. Boll, H. Daniel, and R. Baumeister. 2004. “Deletion of the Intestinal Peptide Transporter Affects Insulin and TOR Signaling in Caenorhabditis elegans.” Journal of Biological Chemistry 279(35): 36739–36745. doi:10.1074/jbc.M403415200.
- Mohan, N., C. S. Chen, H. H. Hsieh, Y. C. Wu, and H. C. Chang. 2010. “In Vivo Imaging and Toxicity Assessments of Fluorescent Nanodiamonds in Caenorhabditis elegans.” Nano Letters 10(9): 3692–3699. doi:10.1021/nl1021909.
- Nass, R., D. H. Hall, D. M. Miller, and R. D. Blakely. 2002. “Neurotoxin-Induced Degeneration of Dopamine Neurons in Caenorhabditis elegans.” Proceedings of the National Academy of Sciences of the United States of America 99(5): 3264–3269. doi:10.1073/pnas.042497999.
- Nehrke, K. 2003. “A Reduction in Intestinal Cell pHi Due to Loss of the Caenorhabditis elegans Na+/H + Exchanger NHX-2 Increases Life Span.” The Journal of Biological Chemistry 278(45): 44657–44666. doi:10.1074/jbc.M307351200.
- Newstead, S. 2017. “Recent Advances in Understanding Proton Coupled Peptide Transport via the POT Family.” Current Opinion in Structural Biology 45: 17–24. doi:10.1016/j.sbi.2016.10.018.
- Oberdörster, G. 2010. “Safety Assessment for Nanotechnology and Nanomedicine: Concepts of Nanotoxicology.” Journal of Internal Medicine 267(1): 89–105.
- Oberdörster, G., E. Oberdörster, and J. Oberdörster. 2005. “Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles.” Environmental Health Perspectives 113(7): 823–839. doi:10.1289/ehp.7339.
- Peters, R., E. Kramer, A. G. Oomen, Z. E. Rivera, G. Oegema, P. C. Tromp, R. Fokkink, A. Rietveld, H. J. Marvin, S. Weigel, et al. 2012. “Presence of Nano-Sized Silica during in Vitro Digestion of Foods Containing Silica as a Food Additive.” ACS Nano 6(3): 2441–2451. doi:10.1021/nn204728k.
- Petrascheck, M., X. Ye, and L. B. Buck. 2007. “An Antidepressant That Extends Lifespan in Adult Caenorhabditis elegans.” Nature 450(7169): 553–556. doi:10.1038/nature05991.
- Piechulek, A., and A. von Mikecz. 2018. “Life Span-Resolved Nanotoxicology Enables Identification of Age-Associated Neuromuscular Vulnerabilities in the Nematode Caenorhabditis elegans.” Environmental Pollution 233: 1095–1103. doi:10.1016/j.envpol.2017.10.012.
- Pluskota, A., E. Horzowski, O. Bossinger, and A. von Mikecz. 2009. “In Caenorhabditis elegans Nanoparticle-Bio-Interactions Become Transparent: Silica-Nanoparticles Induce Reproductive Senescence.” PLoS One 4(8): e6622. doi:10.1371/journal.pone.0006622.
- Pourzahedi, L., M. Vance, and M. J. Eckelman. 2017. “Life Cycle Assessment and Release Studies for 15 Nanosilver-Enabled Consumer Products: Investigating Hotspots and Patterns of Contribution.” Environmental Science & Technology 51(12): 7148–7158. doi:10.1021/acs.est.6b05923.
- Pukkila-Worley, R., and F. M. Ausubel. 2012. “Immune Defense Mechanisms in the Caenorhabditis elegans Intestinal Epithelium.” Current Opinion in Immunology 24(1): 3–9. doi:10.1016/j.coi.2011.10.004.
- Rabbitts, B. M., M. K. Ciotti, N. E. Miller, M. Kramer, A. L. Lawrenson, S. Levitte, S. Kremer, E. Kwan, A. M. Weis, and G. J. Hermann. 2008. “Glo-3, a Novel Caenorhabditis elegans Gene, Is Required for Lysosome-Related Organelle Biogenesis.” Genetics 180(2): 857–871. doi:10.1534/genetics.108.093534.
- Roh, H. C., S. Collier, J. Guthrie, J. D. Robertson, and K. Kornfeld. 2012. “Lysosome-Related Organelles in Intestinal Cells are a Zinc Storage Site in C. elegans.” Cell Metabolism 15(1): 88–99. doi:10.1016/j.cmet.2011.12.003.
- Rubio-Aliaga, I., and H. Daniel. 2008. “Peptide Transporters and Their Roles in Physiological Processes and Drug Disposition.” Xenobiotica 38(7–8): 1022–1042. doi:10.1080/00498250701875254.
- Scharf, A., K. H. Gührs, and A. von Mikecz. 2016. “Anti-Amyloid Compounds Protect from Silica Nanoparticle-Induced Neurotoxicity in the Nematode C. elegans.” Nanotoxicology 10(4): 426–435. doi:10.3109/17435390.2015.1073399.
- Scharf, A., A. Piechulek, and A. von Mikecz. 2013. “Effect of Nanoparticles on the Biochemical and Behavioral Aging Phenotype of the Nematode Caenorhabditis elegans.” ACS Nano 7(12): 10695–10703. doi:10.1021/nn403443r.
- Sengupta, S., T. R. Peterson, and D. M. Sabatini. 2010. “Regulation of the mTOR Complex 1 Pathway by Nutrients, Growth Factors, and Stress.” Molecular Cell 40(2): 310–322. doi:10.1016/j.molcel.2010.09.026.
- Silk, D. B., G. K. Grimble, and R. G. Rees. 1985. “Protein Digestion and Amino Acid and Peptide Absorption.” Proceedings of the Nutrition Society 44(1): 63–72. doi:10.1079/PNS19850011.
- Skjolding, L. M., M. Winther-Nielsen, and A. Baun. 2014. “Trophic Transfer of Differently Functionalized Zinc Oxide Nanoparticles from Crustaceans (Daphnia magna) to Zebrafish (Danio rerio).” Aquatic Toxicology 157: 101–108. doi:10.1016/j.aquatox.2014.10.005.
- Smirnova, J. B., J. N. Selley, F. Sanchez-Cabo, K. Carroll, A. A. Eddy, J. E. McCarthy, S. J. Hubbard, G. D. Pavitt, C. M. Grant, and M. P. Ashe. 2005. “Global Gene Expression Profiling Reveals Widespread yet Distinctive Translational Responses to Different Eukaryotic Translation Initiation Factor 2B-Targeting Stress Pathways.” Molecular and Cellular Biology 25(21): 9340–9349. doi:10.1128/MCB.25.21.9340-9349.2005.
- Smith, D. E., B. Clémençon, and M. A. Hediger. 2013. “Proton-Coupled Oligopeptide Transporter Family SLC15: Physiological, Pharmacological and Pathological Implications.” Molecular Aspects of Medicine 34(2–3): 323–336. doi:10.1016/j.mam.2012.11.003.
- Spriggs, K. A., M. Bushell, and A. E. Willis. 2010. “Translational Regulation of Gene Expression during Conditions of Cell Stress.” Molecular Cell 40(2): 228–237. doi:10.1016/j.molcel.2010.09.028.
- Stoeber, W., A. Fink, and E. Bohn. 1968. “Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range.” Journal of Colloid and Interface Science 26: 62–69. doi:10.1016/0021-9797(68)90272-5.
- Tanis, J. E., J. J. Moresco, R. A. Lindquist, and M. R. Koelle. 2008. “Regulation of Serotonin Biosynthesis by the G Proteins Galphao and Galphaq Controls Serotonin Signaling in Caenorhabditis elegans.” Genetics 178(1): 157–169. doi:10.1534/genetics.107.079780.
- Tank, E. M., K. E. Rodgers, and C. Kenyon. 2011. “Spontaneous Age-Related Neurite Branching in Caenorhabditis elegans.” Journal of Neuroscience 31(25): 9279–9288. doi:10.1523/JNEUROSCI.6606-10.2011.
- Unrine, J. M., W. A. Shoults-Wilson, O. Zhurbich, P. M. Bertsch, and O. V. Tsyusko. 2012. “Trophic Transfer of Au Nanoparticles from Soil along a Simulated Terrestrial Food Chain.” Environmental Science & Technology 46(17): 9753–9760. doi:10.1021/es3025325.
- US FDA 2015. “Code of Federal Regulations Title 21, 21CFR172.480. 2015.” United States Food and Drug Administration. Accessed 25 Apr 2016. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=172.480.
- von Mikecz, A. 2018. “Lifetime Eco-Nanotoxicology in an Adult Organism: Where and When Is the Invertebrate C. elegans Vulnerable?” Environmental Science: Nano 5(3): 616–622. doi:10.1039/C7EN01061C.
- Walther, D. M., P. Kasturi, M. Zheng, S. Pinkert, G. Vecchi, P. Ciryam, R. I. Morimoto, C. M. Dobson, M. Vendruscolo, M. Mann, et al. 2015. “Widespread Proteome Remodeling and Aggregation in Aging C. elegans.” Cell 161(4): 919–932. doi:10.1016/j.cell.2015.03.032.
- Wang, Y., A. Kalinina, T. Sun, and B. Nowack. 2016. “Probabilistic Modeling of the Flows and Environmental Risks of Nano-Silica.” Science of the Total Environment 545–546: 67–76. doi:10.1016/j.scitotenv.2015.12.100.
- Wang, Y., and B. Nowack. 2018. “Dynamic Probabilistic Material Flow Analysis of nano-SiO2, Nano Iron Oxides, nano-CeO2, nano-Al2O3, and Quantum Dots in Seven European Regions.” Environmental Pollution 235: 589–601. doi:10.1016/j.envpol.2018.01.004.
- Zaborske, J. M., J. Narasimhan, L. Jiang, S. A. Wek, K. A. Dittmar, F. Freimoser, T. Pan, and R. C. Wek. 2009. “Genome-Wide Analysis of tRNA Charging and Activation of the eIF2 Kinase Gcn2p.” The Journal of Biological Chemistry 284(37): 25254–25267. doi:10.1074/jbc.M109.000877.
