ABSTRACT
Phosphate-based glasses (PBG) have low melting temperatures and can be obtained by melt-quenching and sol-gel methods. The most significant characteristic of PBG is their ability to dissolve completely in aqueous solution within different timeframes. This solubility can reduce the need for revision surgeries and makes PBG well-suited for soft tissue regeneration. Phosphate glass fibres (PGF) due to their geometry and volume–surface area ratio are the subject of a growing number of studies on resorbable composites. Medical applications include bone fixation devices, nerve tissue scaffolds and wound healing. PBG can be doped with various ions to enhance their biological, chemical and structural properties allowing the preparation of fibres with designed properties and with the ability to release biologically active ions upon degradation. The aim of this review is to look in detail at the influence of different dopants on PGF behaviour, both at a structural and biological level.
Phosphate-based glasses
Phosphate-based glasses (PBG) may be divided into three groups according to their P2O5 content. Ultraphosphate glasses (2.5 ≤ [O]/[P] < 3) contain more than 50 mol-% of P2O5, while metaphosphate glasses ([O]/[P] = 3) contain 50 mol-% of P2O5 in the glass. The structure of these two types of glasses is built upon chains and rings however chains represent more of a polymeric linear structure than a 3-D network. Polyphosphate glasses with [O]/[P] > 3 have less than 50 mol-% of P2O5 and the glass network preferentially forms chains and rings terminated by Q1 diamers [Citation1,Citation2]. Invert glasses are a class of phosphate glasses called ‘pyrophosphate’ with less than 33.3 mol-% of P2O5 formed by orthophosphate and pyrophosphate groups. Invert phosphate glasses are more resistant to hydrolysis but difficult to manufacture due to a narrow processing window [Citation3] (define as the onset of crystallisation temperature decrease by glass transition temperature [Citation4]).
In glass technology, oxides can be divided into three groups: (i) network forming oxides (e.g. SiO2, P2O5, B2O3), (ii) network modifying oxides (e.g. CaO, MgO, SrO, BaO, PbO, ZnO, Na2O, Li2O, K2O) and (iii) intermediate oxides (e.g. oxides of: Ga, Ti, C, V, Bi, Mo, W, S, Se, Te) [Citation5] which can act as both network formers and network modifiers. In addition, many other elements can be used as dopants to improve the properties of PBG. Crystallisation tendency, mechanical properties and stability against hydrolytic attack are all features which are dependent upon the charge-to-size ratio of the network modifier ion. This ratio determines the strength of the ionic cross-links between two non-bridging oxygens. A large charge-to-size ratio results in creating bonds more resistant to hydrolysis and lowers the crystallisation tendency. When the phosphate structure is more disrupted, and the phosphate structural domains are smaller, the tendency for the glass to crystallise is higher. Moreover, a decrease into the cross-linking of PBG results in a lowering of the glass transition temperature.
The ability to control properties is a great challenge when designing materials for medical applications as they need to have very specific chemical composition and physical and biological properties. PBG with the appropriate composition can be highly soluble so that when they are implanted into the body they will dissolve completely after a certain period of time. Indeed, phosphate glasses can be considered as a reservoir of biologically active ions, which can be released in the body upon dissolution of the glass.
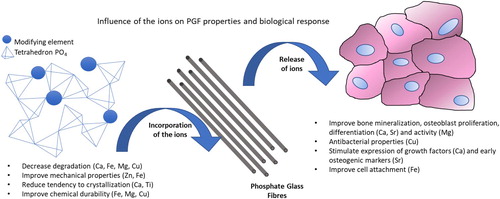
The inclusion of trace elements into PBG not only improves their properties but can also have beneficial effects from a biological perspective (). Trace element biology relates to a number of various chemical elements that occur naturally in very small amounts in organisms and are essential for many physiological and biochemical processes [Citation6]. In addition to the active research in the areas of pharmaceutics and medical devices, there are examples of the application of phosphate glasses in the agriculture and veterinary fields [Citation7]. The trace elements such as copper, cobalt or selenium have been added to PBG to obtain a soluble glass bolus for the veterinary application. Molybdenum deficiency in plants has been fought through controlled release of ions from PBG [Citation8].
Phosphate glass fibres
Phosphate glass fibres (PGF) have an interesting morphology which gives them an advantage over traditional bulk glasses. This morphology means they exhibit great potential as materials for regeneration of tissue with a medium to high anisotropy like muscle or ligament [Citation5].
In the work of Massera et al. [Citation9] the authors focused on the differences between glass fibres and bulk glasses in the composition 50P2O5–40CaO–10Na2O (mol-%). The fibres were obtained via the melt–drawn spinning process. Both bulk glass samples and fibres were incubated in simulated body fluid and Tris(hydroxymethyl)aminomethane (TRIS) solution. The following observations were made based on DTA thermograms and Raman analysis: (i) fibre drawing leads to preferential orientation of P–O–P bond (most likely along the axis) and (ii) tendency to crystallisation is lower for fibres than for bulk materials [Citation9].
No other significant differences were observed proving that fibres have the same structural properties as bulk glasses. However, the main difference between them is the thermal history considering that melt-spun fibres undergo fast cooling (which helps avoid crystallisation) but bulk glasses are annealed to control their cooling rate [Citation10]. Those results have been confirmed by Munoz et al. [Citation11] who showed no significant structural differences between bulk glasses and glass fibres.
Solubility of PBG and PGF
PBG and PGF can resorb completely in aqueous solution [Citation12]. However, for practical reasons glasses with very limited durability are not always desired. For example, PBG with very fast dissolution rates has been shown to decrease proliferation [Citation13] and disturb the expression of the bone-associated proteins [Citation13]. The high solubility of PBG is caused by the presence of Q3 tetrahedra characteristic of pure vitreous P2O5. Introducing R2O oxides results in depolymerisation of the Q3 units and the formation of new Q2 units, however the incorporation of Na2O does not reduce the solubility of binary PBG. As an alternative, the RO/P2O5 system has been developed and investigated by Toyoda et al. [Citation14] who showed that the viscosity of glass decreases with the ionic radius of the glass modifier. Despite improving the durability, CaO–P2O5 glasses are difficult to process due to their high thermal expansion coefficients. To overcome the described difficulties associated with binary systems, the P2O5–CaO–Na2O ternary composition has been extensively investigated showing both promising biological properties [Citation15–18] and reasonable degradation rates [Citation19]. Several studies have been carried out on this material proving its potential for use in medical applications [Citation13].
Biological response to PBG and PGF
The influence on soft and hard tissue of PBG has been comprehensively investigated using glasses in the system: 50P2O5–(48–30) CaO–(20–2) Na2O (mol-%) [Citation20]. Four different human cell lines were used: MG63 cells (human osteosarcoma delivery cell line) as representative of osteoblastic behaviour, human oral osteoblast (HOB), human oral fibroblast (HOF) and human tendon fibroblast (HTF). Tests showed that glasses with less than 40% CaO do not support MG63 attachment while the best cell attachment was observed for glasses with 48% of CaO. Despite the fact that round HOB cells were observed on all glasses, only fibres with composition 50P2O5–48CaO–2Na2O (mol-%) led to the best response of bone sialoprotein and osteonectin – the proteins responsible for bone mineralisation. Similar results were reported for HOF and primary cells, where adhesion was not observed for all glasses apart for those with the highest amount of calcium oxide. Spread morphology and cytoskeleton of HOF cells were also observed to be similar to those of the positive control. It has been proven that ternary PBG with a high level of CaO (45 mol-%) and 50% of P2O5 are suitable for both hard and soft tissue regeneration by supporting cell attachment, proliferation and further survival of the cell [Citation20]. The studies have been confirmed by Salih et al. [Citation13,Citation21] who investigated ternary phosphate glass compositions on two human osteoblast cell lines, MG63 and HOS (TE85). The results showed that a higher amount of CaO (40 mol-%) in glasses with fixed 45 mol-% of P2O5 is beneficial for adhesion and cell proliferation. Skelton et al. [Citation15] performed studies on PBG with 50 mol-% of P2O5 investigating their role in osteoblast and osteoblast-like proliferation, differentiation and death using human bone marrow stromal cells (hBMSCs) and human foetal osteoblast 1.19 (HFOB). Again, a strong correlation between dissolution rate and cell response was reported. Degradation and processes related to it like ion release, hydration and hydrolysis have a great impact on cells. Moreover, the loss of surface integrity associated with glass dissolution can disturb adhesion of cells leading to poor proliferation or differentiation. Nevertheless, glasses with 48 mol-% CaO have been proven to support osteogenic proliferation and early differentiation [Citation15] and other studies presented in this review suggest promising biological properties of PGF of different elemental compositions.
Manufacturing and modifications methods
There are several fibre manufacturing methods however not all phosphate glass compositions allow successful preparation of continuous fibres [Citation22]. For example, glasses with higher P2O5 content (for example 50, 55 mol-%) have a higher amount of infinitely long Q2 chains which make them easier to be pulled as opposed to glasses with many short Q1 units [Citation22].
In general, PGF are obtained by three methods: (a) melt-drawing spinning process, (b) preform technique and (c) pulling from melting solution (). Only by using drums with controlled rotation speeds can the fibres be produced with consistent, controllable diameters, with faster rotation delivering smaller diameters: 40 µm (300 rev min−1), 40–23 µm (400 rev min−1), 25–20 µm (880 rev min−1), 20–15 µm (1200 rev min−1), 15–8 µm (1600 rev min−1) and ∼10 µm (2000 rev min−1) [Citation17,Citation19,Citation23–31].
Figure 2. Techniques for PGF manufacturing: (a) melt-drawing spinning process, (b) preform technique, (c) pulling from melting solution, adapted from Colquhoun and Tanner [Citation32]. CopyRights CC BY 3.0. DOI:10.1088/1748-6041/11/1/014105.
![Figure 2. Techniques for PGF manufacturing: (a) melt-drawing spinning process, (b) preform technique, (c) pulling from melting solution, adapted from Colquhoun and Tanner [Citation32]. CopyRights CC BY 3.0. DOI:10.1088/1748-6041/11/1/014105.](/cms/asset/7a907d66-468f-4edb-8546-dc32a5067543/yaac_a_1564413_f0002_oc.jpg)
Delivery of therapeutic ions – component ions
Most PGF developed for medical application are based on ternary phosphate glasses in the system: Na2O–CaO–P2O5. This base composition is interesting because it contains only elements present in the human body (P, Ca, Na 1, 1.5 and 0.2 wt-% respectively) with important biological roles. Moreover, it is chemically similar to the inorganic phase of human bone [Citation33].
Phosphorus
Orthophosphate PO43− is the most abundant form of phosphorus in the body and it is mostly present in bone where it forms hydroxyapatite. Moreover, PO43− plays an important role in the cell signalling system, regulation of protein synthesis, skeleton development and bone integrity [Citation34]. Phosphorus is also present in adenosine triphosphate (ATP) and Guanosine-5′-triphosphate (GTP) – the biomolecules responsible for energy transport. In addition, phosphorus is a component of the phospholipids which are important in the development of the cell membrane and stimulate the expression of matrix Gla protein (MGP), a key regulator in bone formation [Citation35].
Other important properties and roles of phosphorus include linkage units of the sugar esters of DNA and RNA; coenzymes such as FMN, NADH, NADPH; signalling molecules such as CAMP [Citation36].
As a network forming oxide P2O5 plays a crucial role in the glass manufacturing process. A glass network is made by linkages between the phosphate tetrahedra with the addition of metal oxides causing depolymerisation of the phosphorus-oxygen bond in P–O–P [Citation37]. Pure P2O5 glass is rarely used because of its high solubility since P–O–P bonds hydrolyse easily. However, the degradation products are non-toxic and well known to the body due to their natural presence [Citation18]. The addition of Ca and Na results in cleavage of P–O–P linkages due to the creation of non-bonding oxygens (NBO). Even when calcium ions are in contact with two NBO, a link with stronger effects on the network connectivity is created [Citation19].
Calcium
Calcium (Ca) takes part in many biochemical reactions and in every metabolic pathway there is a calcium-dependent step. It controls the mechanical stability of cell walls and membranes and the filament tension in many cells [Citation36]. Intercellular Ca2+ level controls processes like muscle contraction, transport processes, cell division and growth, enzyme activation and metabolic processes. Ca is intimately associated with foetal development and hormonal activities [Citation38]. Calcium is an important component of mineralised biological tissues such as shells, teeth and bone (the largest reservoir of calcium in the body) and is typically expressed as calcium salts, carbonates and phosphate-based biominerals. Also, important organelles like mitochondria and chloroplasts depend on a highly controlled calcium level. Calcium controls neuromuscular excitability, proper heart beating, blood clotting, and the degree of hydration of the cytoplasm. Addition of calcium into PBG results in favoured osteoblast proliferation and differentiation, ECM mineralisation and the activation of Ca sensing receptors in osteoblast cells to increase the expression of growth factors such as IGF-I or IGF-II [Citation33].
In phosphate glass networks, calcium has two structural functions, it can both modify the primary glass structure and provide inter-chain cross-linking. In PGF specifically calcium ions provide the cross-link between non-bonding oxygens of the different chains making the fibres more durable, given that Ca decreases degradation and improves the mechanical properties of the glass. Increasing calcium oxide (CaO) can reduce the tendency of crystallisation [Citation2], however only to a certain level. Glasses with 35–40 mol-% of CaO and low sodium level (<15 mol-%) are prone to crystalise [Citation27]. CaO appears in almost all known phosphate glasses for biomedical applications as one of the major components and the Ca amount differs according to the application. High levels of CaO result in Ca/P ratios that are similar to that of the mineral phase of bone, Ca/P ratio is equal to 1.67. Binary calcium phosphate fibres are a popular group of materials which support bone ingrowth [Citation39].
Sodium
Sodium (Na) is an important element in the human body. Na plays many roles: it controls osmotic pressure in conjunction with K+ and Cl−, controls the electrolytic balance, membrane potential, and condensation of polyelectrolytes. Na along with K takes part in passive diffusion, active transport and ion exchange across membranes. Sodium is also an important component of gastric fluid [Citation36]. Na2O is important for glass manufacturing because it is an effective flux and reduces the viscosity of the glass melt. However, Na2O reduces the resistance to chemical attack and weakens the mechanical properties of glasses [Citation11]. According to Van Wazer’s reorganisation theory monovalent oxide modifiers like Na2O disturb the structure of P2O5 by breaking bridging oxygen bonds and forming non-bridging oxygens [Citation40]. On the other hand, Na2O increases the thermal expansion of glass [Citation41], while also increasing solubility and the tendency to crystallise [Citation2].
Delivery of therapeutic ions – dopants
The ternary system ‘P2O5–CaO–Na2O’ has been widely investigated to determine the correlation between composition and cellular activity. However, to achieve better flexibility or durability, incorporation of a quaternary component is usually considered. Indeed some interesting PGF contain five or more different oxides to not only improve biocompatibility or mechanical properties but also to increase the processing windows [Citation2].
Some of the well-known dopants are able to change the biological features of phosphate glasses improving: cell attachment and proliferation and in the downstream steps: tissue regeneration, surface properties of the material, degradation rate and dissolution rate.
The high dissolution rate of PBG and ion release can cause potential risk of allergic reaction or toxicity of dopants. Still little is known about the function of some elements and especially about their safe dose limits; the same element may cause a positive effect in small amounts and become dangerous at higher concentrations [Citation42]. In recent years numerous research activities have taken place to find possible new applications for PGF doped with different oxides, especially as scaffolds for tissue regeneration or as reinforcement of bone fixation devices [Citation43,Citation44].
summarises the different elements assessed in the baseline ‘P2O5–CaO–Na2O’ compositions. A commentary on each element follows in which their role in the human body and their impact on phosphate glass fibre properties are discussed.
Table 1. Summary of studies on phosphate glass fibres with different compositions intended for biomedical applications.
Iron in PGF
Iron (Fe) is often used as a dopant in PGF. Iron plays an essential role in the human body by being responsible for a number of biological processes: Fe is a component of haemoglobin, takes part in cell division and respiratory processes [Citation67]. Iron oxide (Fe2O3) has a large impact on the properties of phosphate glasses such as solubility or degradation, however in industry Fe2O3 has been used mostly as a colourant [Citation68].
Fe increases Tg (glass transition) and Ts (temperature of softening), chemical durability, glass density and decreases solubility or ion release [Citation56,Citation69]. Total surface energy also decreased with increasing Fe2O3 content. At low temperatures, iron increases the viscosity of the glass melt especially at higher phosphate levels, however, it is still the amount of phosphate which retains the greatest influence on glass viscosity [Citation70]. Biological properties of Fe2O3 have shown that PGF with 4 or 5 mol-% of Fe2O3 in a glass network provide better cell attachment as well as proliferation and differentiation compared to undoped fibres. However, the improved biocompatibility of Fe-PGF is attributed to their enhanced durability. Fe replaces P in P–O–P network and creates strong cross-linking P–O–Fe units which also reduce the tendency to crystallise [Citation69]. It is not clear if Fe(II) or Fe(III) is present in the network, nevertheless it has been proven that Fe(III) is more likely to behave as a network former than as a network modifier [Citation27].
The medical applications are bone fixation devices, dental application and nerve tissue regeneration. Fe-doped PGF were used with coupling agents to improve bone-ligament integration [Citation30] and to treat traumas covering not only bone damage but also the surrounding soft tissue. Moreover, Fe-PGF has been used to investigate the role of annealing in slowing down the degradation of PGF by 50% [Citation63].
As a scaffold, Fe-doped PGF were used for muscle delivery system to treat Duchenne Muscular Dystrophy [Citation27]. To date the only effective treatment is muscle transplantation which delivers the normal gene into a diseased area, however the described procedure has two main disadvantages: risk of rejection and low survival rate of implanted cells. Furthermore, the mobility of injected myoblasts is limited [Citation27]. Application of Fe-doped PGF is beneficial considering the predictable dissolution time and relative high solubility. Additionally, implantation of long fibres allows better cell attachment. Glasses with 50P2O5–(30, 35, 40)CaO–(20–x)Na2O–xFe2O3 (x = 1–5) (mol-%) composition have been proven to have good cell attachment and enhance proliferation and differentiation of muscle precursor cells [Citation27].
Magnesium in PGF
Next to Fe2O3, magnesium oxide (MgO) is most often used as a dopant in PGF, and similar to iron, magnesium (Mg) is a trace element in the human body. In contrast to Fe2O3, the main role of MgO is to improve the biological properties of PBG. In glass technology magnesium oxide is mostly used as a colourant (giving grey colour), moreover MgO, like CaO, can lower the annealing point of doped glasses [Citation68].
Seventy-five per cent of magnesium in the human body resides in bone but it is also present in skeletal muscle and soft tissues. Indeed, most Mg-dependent functions are related with bone tissues: stimulating new bone formation and increasing bone cell adhesion and stability [Citation33,Citation56]. Mg increases alkaline phosphatase (ALP) activity and short-term oral magnesium supplementation can minimise bone turnover markers in young adult males and post-menopausal osteoporotic women [Citation42]. Moreover, Mg plays numerous important roles in cell functions: stabilisation of lipid membranes, nucleic acids and ribosomes [Citation71] chelation of anions to prevent them taking part in hydrolysis or assisting chemical reactions; structure stabilisation and maintaining the electrical potential of the cell membranes of neuromuscular cells. Magnesium, together with calcium, is present in membranes where it provides a cross-linking activity for carboxylated and phosphorylated anionic polymers. Finally, Mg takes part in transport processes and ion pumping processes in cells.
The structural and biological properties of MgO-doped PBG were exhaustively described by Ahmed et al. [Citation72]. Glasses were doped with 10, 20 and 30 mol-% MgO; increasing MgO content results in linear increases in Tg, cross-link density and the chemical durability. Degradation of these glasses decreases with increasing MgO content as monovalent Na+ is substituted by divalent cation Mg2+ in the glass network. Glasses with 30% MgO tended to crystallise on the surface upon cooling to room temperature. However, rapid changes in coordination number of Mg2+ ions have an anomalous effect on the physical properties of phosphate glasses [Citation69]. Biological tests have shown higher osteoblast activity for glasses with 20–30 mol-% MgO, however no apparent relationship between glass composition and cell attachment has been observed in correlation to MgO content. PGF with MgO have been used for reinforcement of bone fixation devices, in nerve tissue regeneration and as drug delivery systems [Citation29].
Copper in PGF
Copper oxide (CuO) has been used in glass technology for many years. The antibacterial properties of copper have also been well exploited since ancient times as evidenced by its use to sterilise water [Citation73]. Of more current relevance, CuO influences the properties of PBG, in particular, the degradation rate or ion release.
Cu is a biologically important element. Significant amounts of cellular copper are found in human endothelial cells when undergoing angiogenesis, promoting synergic stimulation effects on angiogenesis when associated with angiogenic growth factor FGF-2. Cu stimulates proliferation of human endothelial cells and induces higher differentiation of mesenchymal stem cells towards the osteogenic lineage [Citation33]. The important oxidase enzyme ceruloplasmin, present in plasma and used to transport proteins, requires Cu where it helps to metabolise iron. Deficiency of copper results in iron overload in the tissues of the brain and liver, however high doses of copper may result in its accumulation in the liver [Citation73]. Cu is a cofactor for superoxide dismutase (enzyme catalyses O2- into O2 or H2O2) and lysyl oxidase (critical role in biogenesis of connective tissue). The GHK-Cu complex increases messenger RNA production of various extracellular matrices such as collagen, elastin, proteoglycan or glycosaminoglycans in fibroblasts. Inorganic copper was used in implants and research proved that 56 ng of copper sulphate per implant achieves a positive effect on blood vessel ingrowth [Citation42,Citation73]. Copper is essential for cross-linking of collagen and elastin which is important to form a strong, flexible connective tissue in skin and bone and also plays an important role in bone resorption. It has been proven that osteoclasts produce Cu/Zn superoxide dismutase (SOD), a decrease of Cu/Zn SOD activity due to inadequate copper levels leads to increasing bone resorption [Citation73].
Cu-PGF were used to obtain materials for wound healing application. PGF with 10 mol-% of CuO have shown antibacterial properties by killing the opportunistic pathogen Staphylococcus epidermidis [Citation54]. Many PBG with antibacterial dopants like Ag, Zn, Cu, Ga, etc. [Citation74–76] have been reported however only one example of incorporation of copper into PGF is available [Citation54].
Research by Knowles et al. has proven that addition of CuO increases the Tg of the glass, as well as Ts, hardness and the chemical durability. In the glass, instead of P–O–Na bonds, P–O–Cu bonds were formed due to the higher electronegativity of copper ions vs. sodium ions. Substitution of Cu results in an increase in cross-link density and a decrease in degradation rate [Citation54].
Boron in PGF
Boron oxide (B2O3) is one of the network-former oxides, however, it is also often used as a flux. Addition of boron (B) improves both chemical resistance and mechanical properties of glasses. Glasses containing B2O3 are characterised by low thermal expansion and resistance of thermal shock [Citation41]. It has been shown that crystallisation temperature increases with increasing B2O3 content [Citation73,Citation77].
In organisms, B stimulates RNA synthesis in fibroblast cells, stimulates bone formation, and is responsible for expression of RUNX2, a growth factor for osteoblastic differentiation and bone formation [Citation78]. Boron works together with calcium and is present in the bone and neural systems. One of boron’s main roles is to metabolise the major minerals: calcium, magnesium and phosphates. Deficiency causes a reduction in the urinary excretion of Ca, Mg and P, elevates the serum concentration of estradiol-17β and testosterone and can even cause calcium loss / bone demineralisation in postmenstrual women. Boric acid reduces the time required in intensive wound care by 66% due to an ability to activate the key enzymes involved in fibroblast formation which improves extracellular-matrix turnover [Citation78]. B significantly improves wound healing, an anti-inflammatory effect from boron was observed as a result of inhibition of the oxidative burst by leukocytes and excessive activity by neutrophils [Citation78]. Addition of borate improves the bioactivity of PBG by promoting hydroxyl group formation at the surface of glasses and some borate glasses have been proven to bond to bone at levels comparable to Bioglass 45S5 [Citation77].
Addition of 5 mol-% B2O3 into PGF with composition: 45P2O5–16CaO–24MgO–5Na2O (mol-%) significantly increases tensile strength (50%) and modulus (11%). However, similar increases were reported from a synergistic effect of adding 5 mol-% of B2O3 and 3 mol-% of Fe2O3. A 22% decrease in the dissolution rate was observed by adding either 5 mol-% of B2O3 or 3 mol-% Fe2O3 while in glasses doped with both B2O3 and Fe2O3 there was only an 11% decrease in dissolution rate. Both B and Fe-doped PGF show similar degradation rate of 20 µm diameter fibre samples. Despite this B2O3 significantly improves the mechanical properties of the fibres, whereas the glasses with iron have shown better biological properties on human osteosarcoma [Citation64]. Lower biocompatibility of high-boron phosphate glasses is associated with extensive release of B3+ ions [Citation69].
Strontium in PGF
Strontium (Sr) is an alkaline earth metal which has found application in osteoporosis treatment [Citation79]. Strontium reduces bone resorption, stimulates bone formation and increases replication of preosteoblastic cells [Citation79,Citation80]. Moreover, a correlation between an increase in the amount of Sr in bone and compressive strength has been found [Citation81]. There are several similarities between calcium and strontium: they have similar metabolic pathways by gastrointestinal tract (absorption) and the urinary systems (extraction) and both are concentrated in the bone [Citation80]. However, high similarities may lead to complication due to replacement of calcium with strontium in bone [Citation79]. Nevertheless, many soluble phosphate glasses have been proposed as a system of controlled strontium release [Citation79,Citation80,Citation82,Citation83].
Several studies have shown the influence of strontium on PBG. The substitution of calcium with strontium results in increased glass density and molar volume due to the larger ionic radius of Sr2+ compared to Ca2+. Weaker Sr–O bonds lead to a slight decrease in Tg while Tm was significantly lower [Citation79]. High amounts of Sr decrease the processing window and can cause problems with fibre drawing [Citation79] however according to Massera et al., glasses doped with 20 mol-% of Sr were characterised with good stability and possibility to draw fibres [Citation84].
Studies on 40P2O5–25CaO–5Na2O–(30-x)MgO–xSrO [mol-%] phosphate glasses have shown an improved cell proliferation (1% of Sr), hMSC osteogenic differentiation (5%) and an increase in early osteogenic markers such as COL1 and RUNX and later markers ALP and OC [Citation83]. Based on this evaluation, the optimal Sr concentration in PBG has been set as 5 mol-% [Citation83].
PGF doped with strontium were used to produce porous scaffolds for bone tissue regeneration as proposed by Zheng et al. [Citation85,Citation86].
Potassium in PGF
Sodium and potassium oxides (Na2O, K2O) impart similar properties to glassy materials being powerful fluxing (melting) alkaline materials. However, replacing with sodium and potassium results in melts of higher viscosity and lower thermal expansion [Citation68].
Addition of mixed alkali oxides to glasses results in higher durability and electrical resistance [Citation41]. Increasing the amount of alkali ions in PBG is known to decrease connectivity of the glass structure by creating non-bridging oxygens, hence increasing the thermal expansion coefficient and solubility [Citation56].
Potassium is an essential element for human health. The main roles for K are regulation of acid–base balance, energy metabolism, controlling blood pressure, membrane transport, distribution of fluid within the body, transmission of nerve impulses and maintaining cardiac function [Citation87]. K controls the electric potential of cell membranes as one of the outer membrane ions. It also takes part in regulating osmotic balance.
K2O has been used to produce PGF for nerve tissue regeneration. PGF labelled as TiPS 2.5 contained 50P2O5–30CaO–12Na2O–3SiO2–2.5K2O–2.5TiO2 (mol-%). PGF were suitable for glial cells and axonal growth. Neonatal olfactory bulb ensheathing cells (NOBEC) were well spread on the fibre surface and active proliferation was also observed. Some of the cells presented cytoplasmic processes extending along the fibres. TiPS 2.5 were also used to test Dorsal Root Ganglia neurons which showed a pseudo-unipolar morphology [Citation26]. Moreover, K2O was used to obtain hollow glass fibres for a controlled release system [Citation29].
Silica in PGF
Silicon oxide (SiO2) is mainly used as a network-former. Silica-based bioactive glasses are being extensively studied for their potential to bond to hard and soft [Citation88] tissue and their ability to release biologically active ions [Citation33]. A particular silica-based glass called Bioglass 45S5 has great biological potential [Citation89]. Silica-based glasses produced by both melt-quench and sol-gel are less soluble than the PBG described in this review.
Silicon (Si) is also an important element in human health, it is essential for metabolic processes and formation and calcification of bone tissue [Citation33]. Dietary intake of Si increases bone mineral density, while Si–O in an aqueous environment induces HA precipitation and Si(OH)4 stimulates collagen I formation and osteoblastic differentiation [Citation33]. Si along with Ca plays an important role in the early stages of calcification [Citation42]. Silica can be used as a dopant to modify the properties of PBG.
Glasses with composition 50P2O5–40CaO–5SiO2–5Fe2O3 (mol-%) degrade faster than those without silica. Si–O–P bonds are more sensitive to hydrolytic activity because inclusion of Si acts to disconnect the network of phosphate glasses. It also results in faster ion release in water in line with the dissolution rate [Citation42,Citation90].
PGF containing Si have been used as materials for bone fixation devices, in soft tissue regeneration, in muscle delivery systems and scaffolds for nerve tissue regeneration [Citation90].
Titanium in PGF
Titanium (Ti) is a popular and widely investigated element in biomaterials [Citation91]. Titanium alloys are the most widely used metals in orthopaedic implants. Titanium oxide (TiO2) has been incorporated into different glasses, both silica and phosphate types, due to the expected improvement of the biological response. However, TiO2 also has other effects on glass properties, Ti: improves the resistance to acid attack, inhibits crystallisation, decreases solubility and increases the processing window [Citation2]. Owing to a high charge-to-size ratio of Ti4+, titanium has strong ionic strength. Phosphate glasses with a lower content of P2O5 and shorter chains were shown to crystallise more easily, while an addition of Ti4+ with strong ionic cross-linking prevents it [Citation2].
The viscosity of glass melts increases with TiO2 content [Citation26] due to the formation of covalent Ti–O–P bonds or ionic cross-links between phosphate chains. Ti stabilises glass networks, increases glass characteristic temperatures, increases density and prevents lowering of pH during dissolution [Citation26].
The lower solubility of Ti-doped PBG improves cell adhesion which results in superior proliferation assays with murine pre-osteoblast cells [Citation44]. Titanium has been used in PGF (TiPS) for nerve tissue regeneration and muscle delivery systems. Additionally, Ti is used in invert PGF with composition 35P2O5–27.5CaO–22.5Na2O–9.5MgO–5.5TiO2 (mol-%) as a bone regeneration material [Citation44].
Aluminium in PGF
In spite of the fact that aluminium (Al) cannot form a glass alone, Al takes part in the glass network as an intermediate oxide [Citation68]. Aluminium increases chemical durability has no effect on thermal expansion coefficient and induces a lower dissolution rate [Citation28,Citation56]. Aluminium can work as a network former by creating AlO4 units which can bond to P=O bonds, causing double bond removal (reduction) with the ‘O’ changing to a bridging oxygen. Addition of Al2O3 into a glass network and substituting sodium with alumina can increase the strength of PBG [Citation56]. Aluminium is essential for plant development, e.g. club moss, hydrangea and tea plant but there is no definite general biological role in humans or higher animals [Citation92].
Al-doped PGF with composition 62.9P2O5–21.9Al2O3–15.2ZnO (mol-%) were used as a muscle delivery system [Citation46]. Moreover, different fibres containing Al2O3 have been considered as reinforcement for medical fixation devices [Citation56].
Zinc in PGF
In traditional glass manufacturing, zinc (Zn) has been used as a flux [Citation68]. Recent research has proven that zinc is beneficial for osteoblast attachment but only up to certain levels after which it becomes toxic for cells [Citation93]. Wang et al. [Citation94] proved that zinc concentration within range from 0 to 0.59 ± 0.03 mg−1 was confirmed to promote bone growth in vivo.
Zinc has many advantages: it shows an anti-inflammatory effect and stimulates bone formation in vitro by activation of protein synthesis in osteoblasts and increased ATPase activity. It regulates transcription of osteoblast differentiation genes, collagen I, ALP, osteopontin and osteocalcin [Citation33]. Zn plays a role in bone healing and the absence of it may cause blindness. Zinc is the most common trace element metal ion present in the cytoplasm of advanced aerobic cells. The structural role of zinc is to stabilise filamentary, keratin-like structures for the organisation of chromosomes [Citation36].
Mechanical tests on PGF have shown doubling of the tensile strength values (from range between 2.8–4.2 GPa and 4.2–7.2 GPa) when substituting Na with Zn [Citation56]. Also, a significant decrease in the degradation rate has been observed. Zn-doped PGF were used in regeneration of craniofacial muscle tissue [Citation46] and reinforcing materials for bone fixation devices [Citation27].
Applications of phosphate glass fibres
PGF are used both for hard and soft tissue regeneration, as a scaffold, wound dressing or reinforcement for bone fixation devices. The application is strongly correlated with the glass composition. summarises the use of PGF for medical applications.
Table 2. Summary of PGF according to their medical applications. PGF have been used both for hard and soft tissue regeneration depending on their composition.
PGF for hard tissue regeneration
In bone tissue regeneration applications, bioactive glasses and ceramic materials are frequently studied because of their interesting ability to bond to bone – their bioactivity [Citation97]. PBG are not an exception, however PGF, because of their geometrical characteristics have been mainly studied for bone fixation devices. Numerous articles describe the advantages of using PGF in combination with resorbable polymers such as PLA, PCL or PLGA [Citation17,Citation43,Citation44,Citation50,Citation51,Citation55,Citation58,Citation59,Citation62,Citation90,Citation98–101] The aim is to obtain bone fixation devices with a modulus similar to that of natural bone, which also disappears completely after the healing process is completed. This disappearance will necessarily be associated with a decrease in mechanical properties that will be offset by the presence of newly formed bone. PLA, PCL and PLGA have much lower mechanical properties than cortical or cancellous bone so reinforcing PGF are used to improve the mechanical performance of such polymers. The measured values for polymers are as follows: PLA tensile modulus 4.4 GPa; PCL tensile strength 190 MPa; PGA tensile strength 57 MPa, tensile modulus 6.5 GPa [Citation102], while mechanical properties of cortical bone reaches values of 50–150 MPa and 7–30 GPa, for flexure strength and elastic modulus, respectively [Citation32]. Composites obtained from resorbable polymers and PGF allow the creation of materials more suitable for bone application [Citation103]. Bioresorbable screws, rods or nails are examples of PGF-based composites for bone regeneration [Citation27,Citation57,Citation90]. Such components are also a convenient alternative for metallic implants which commonly have elastic moduli which are too high. Mismatch in elastic modulus may cause stress shielding in the surrounding bone and absence of mechanical stimulation [Citation32], which results in a weakening of the bone [Citation104]. The most common fibres used in these applications are Fe-doped PGF and B-doped PGF. Those dopants have been shown to improve mechanical properties and to decrease solubility of PGF. The most suitable duration for composites for bone application before complete dissolution should be around 6–8 weeks [Citation32].
Novajra et al. [Citation21] and Zheng et al. [Citation86] suggested using PGF-based 3D porous scaffolds for bone regeneration. Both systems are promising materials for bone regeneration due to their suitable mechanical properties (yield stress 0.38 and modulus 2.84 MPa) similar to cancellous and trabecular bone (compressive strengths of 0.15 MPa and 2–3.5 MPa respectively), good cell response and stimulation of hydroxyapatite formation. Chen et al. proposed a modification of stainless steel bone implants by embedding a short PGF on their surface using electrophoretic deposition (EPD). Addition of fibres is expected to improve bone-to-implant bonding as it enhances proliferation and migration of MC3T3-E1 cells [Citation61].
PGF for soft tissue regeneration
PGF are frequently studied for use in soft tissue regeneration, especially in nerve tissue regeneration, wound healing and muscle delivery systems. However, compared to their application as hard tissue regeneration materials, their use in soft tissue healing is not as widespread. Nonetheless, the unique properties of PGF have been appreciated and are slowly receiving recognition also in soft tissue regeneration.
An especially interesting and promising application of PGF is in nerve regeneration [Citation23,Citation25,Citation52]. Reported by Joo et al. for the first time, treatment with PGF resulted in full regeneration after spinal cord injury in a rat model. 18 µm fibres with composition 50P2O5–40CaO–5Na2O–5Fe2O3 (mol-%) were used to produce 3D collagen scaffolds which were implanted into the animal transected spinal cords. High levels of Ca and Fe in the glasses enabled the preparation of less soluble fibres which better supported neuronal growth. After 12 weeks, the locomotion ability was improved and the result for PGF-collagen scaffolds was better than for PGF-free ones. The positive impact of PGF on neuronal growth was confirmed due to the capacity of aligned PGF to provide directional guidance for outgrowing axons [Citation52].
50P2O5–30CaO–9Na2O–3SiO2–3MgO–(5–x)K2O–xTiO2 (mol-%) fibres were used for nerve regeneration and it was proven that neurites outgrew along the direction of the fibre axis. The fibre surfaces provided support for cell attachment and proliferation due to higher stabilisation (lower solubility) and stiffness. Additionally, the tendency for growth along the fibre axis increased with decreasing fibre diameter [Citation26]. Similar studies were performed on a peripheral nerve injury which can heal unaided but only in a very limited way [Citation23]. The fibre/collagen scaffolds were manufactured using 50P2O5–40CaO–5Na2O–5Fe2O3 (mol-%) glass fibres. To investigate the influence of the fibres on nerve regeneration an animal model (12-week adult rats) was used. After 12 weeks, the results for collagen (Coll) and PGF/Coll were similar however the first sign of regeneration appeared 2 weeks earlier for scaffolds with fibres (6 instead of 8 weeks) [Citation23].
PGF for both hard and soft tissue regeneration
Properties to support both soft and hard tissue regeneration are especially important in applications where both bone and ligament regeneration must be supported in the tissue interface regeneration filed. Many traumas cover not only bone damage but also the surrounding soft tissue [Citation30]. For example, three-dimensional scaffold using PGF was made to produce soft-hard tissue integration at the bone-ligament interface. 50P2O5–46CaO–(4–1)Na2O–(1–3)Fe2O3 (mol-%) fibres were produced and tested with HOB and HOF cells. During these studies, it was proven that 3% of Fe2O3 supports cell attachment and proliferation. Cells of both types were flat and showed spread morphology which suggested very good attachment and integration with the material’s surface [Citation30].
Other biomedical applications of PGF
Hollow 50P2O5–30CaO–9Na2O–3SiO2–3MgO–2.5K2O–2.5TiO2 (mol-%) PGF were used as a controlled release system that offered an alternative for oral drug administration to avoid liver damage caused by drugs absorbed through the gastrointestinal tract. Such fibres act as capillaries and are able to transport solutions and release them from extremities (70% after 5 h and 100% after 24 h). Such a system may be especially interesting to transport biologically active molecules [Citation29].
A similar system has been investigated using 50P2O5–30CaO–(17–15)Na2O–(3–5)Fe2O3 (mol-%) PGF with diameter 32 ± 7 µm. PGF have been fabricated which degrade upon soaking to spontaneously form capillary channels. The process seems to start after 1 week when cracks appeared and after 3 months fibres were replaced by hollow tubes that formed over the following 6 to 18 months. Incubation in deionised water caused depolymerisation of the glass network due to a combination of surface hydration and internal hydrolysis reactions. Another explanation of the observed behaviour is the difference in chain strength between the outer and the core part of the fibres. The rapid cooling during manufacturing of fibres tends to create stronger bonds on the outer layer. Such fibres have potential application as controlled drug delivery systems. Moreover, PGF showed congruent degradation with zero order release which makes them suitable materials for antibacterial applications in their own right, as potential sources of antibacterial elements such as Ag or Cu [Citation24].
A unique application of soluble 50P2O5–30CaO–20Na2O (mol-%) PGF has been reported by Alshomer et al. [Citation105] who used them as a sacrificial material for scaffolds for tendon regeneration. To produce a patterned surface, PGF have been put on the interface of liquid polymer Polyhedral Oligomeric Silsesquioxane Poly (Carbonate-urea) Urethane (POSS-PCU). The scaffold was placed in deionised water until complete dissolution of the PGF and demonstrated good cell response [Citation105].
Summary
PGF are interesting materials with a wide range of medical applications which can be used in contact with both hard and soft tissues. PGF offer useful variation in shape (high aspect ratio) which hold great promise in medical applications. Modification of fibre composition can be employed to enhance the mechanical properties of PGF, used for example in bone fixation devices, or increase their solubility for application as drug delivery systems. Moreover, addition of certain ions can improve the biological response and expand the possible applications: nerve tissue regeneration, wound healing, muscle cell delivery system, bone regeneration and bone-ligament regeneration are all examples which are highlighted and discussed. Owing to their potential high solubility, PGF can be considered as a source of therapeutic ions which can be beneficial for cells, but in higher doses may bring about undesirable effects. Appropriate design of the chemical and physical properties of PGF requires an informed awareness of the possible impacts upon a patient’s body. This information is critically important to mitigate the hazardous effects of certain metallic ions in order to broaden the medical applications of PGF.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Agata Lapa http://orcid.org/0000-0002-2130-9643
Mark Cresswell http://orcid.org/0000-0002-5636-3797
Aldo R. Boccaccini http://orcid.org/0000-0002-7377-2955
Additional information
Funding
References
- Brow RK. Review: the structure of simple phosphate glasses. J Non-Cryst Solids. 2000;263–264:1–28.
- Brauer DS. Phosphate glasses. In: JR Jones, A Clare, editors. Bio-glasses: an introduction. John Wiley & Sons Ltd; 2012. p. 45–64.
- Walter G, Vogel J, Hoppe U, et al. The structure of CaO-Na2O-MgO-P2O5 invert glass. J Non Cryst Solids. 2001;296(3):212–223.
- Zhu C, Ahmed I, Parsons A, et al. Structural, thermal, in vitro degradation and cytocompatibility properties of P2O5-B2O3-CaO-MgO-Na2O-Fe2O3 glasses. J Non-Cryst Solids. 2017;457:77–85.
- Knowles JC. Phosphate based glasses for biomedical applications. J Mater Chem. 2003;13(10):2395–2401. doi:10.1039/B307119G
- Scheiber I, Dringen R, Mercer JFB. Copper: effects of deficiency and overload. In: A Sigel, H Sigel, RKO Sigel, editors. Interrelations between essential metal ions and human diseases. Dordrecht: Springer Netherlands; 2013. p. 359–387.
- Kendall NR, Mackenzie AM, Telfer SB. Effect of a copper, cobalt and selenium soluble glass bolus given to grazing sheep. Livest Prod Sci. 2001;68(1):31–39.
- Pyare R, Lai LJ, Joshi VC, et al. Leachability of Molybdenum from ternary phosphate glasses. J Am Ceram Soc. 1996;79(5):1329–1334.
- Massera J, Ahmed I, Petit L, et al. Phosphate-based glass fiber vs. bulk glass: change in fiber optical response to probe in vitro glass reactivity. Mater Sci Eng C. 2014;37(1):251–257.
- Rinehart JD, Taylor TD, Tian Y, et al. Real-time dissolution measurement of sized and unsized calcium phosphate glass fibers. J Biomed Mater Res. 1999;48(6):833–840.
- Muñoz-Senovilla L, Muñoz F, Tricot G, et al. Structure–properties relationships in fibre drawing of bioactive phosphate glasses. J Mater Sci. 2017;52(15):9166–9178.
- Abou Neel EA, Chrzanowski W, Pickup DM, et al. Structure and properties of strontium-doped phosphate-based glasses. J R Soc Interface. 2009;6(34):435–446.
- Salih V, Franks K, James M, et al. Development of soluble glasses for biomedical use part II: the biological response of human osteoblast cell lines to phosphate-based soluble glasses. J Mater Sci Mater Med. 2000;11(10):615–620.
- Toyoda S, Fujino S, Morinaga K. Density, viscosity and surface tension of 50RO-50P2O5 (R: Mg, Ca, Sr, Ba, and Zn) glass melts. J Non-Cryst Solids. 2003;321(3):169–174.
- Skelton KL, Glenn JV, Clarke SA, et al. Effect of ternary phosphate-based glass compositions on osteoblast and osteoblast-like proliferation, differentiation and death in vitro. Acta Biomater. 2007;3(4):563–572.
- Bitar M, Salih V, Knowles JC, et al. Iron-phosphate glass fiber scaffolds for the hard-soft interface regeneration: the effect of fiber diameter and flow culture condition on cell survival and differentiation. J Biomed Mater Res Part A. 2008;87(4):1017–1026.
- Ahmed I, Cronin PS, Neel EA, et al. Retention of mechanical properties and cytocompatibility of a phosphate-based glass fiber/polylactic acid composite. J Biomed Mater Res Part B Appl Biomater. 2009;89(1):18–27.
- Abou Neel EA, Ahmed I, Blaker JJ, et al. Effect of iron on the surface, degradation and ion release properties of phosphate-based glass fibres. Acta Biomater. 2005;1(5):553–563.
- Ahmed I, Lewis M, Olsen I, et al. Phosphate glasses for tissue engineering: part 1. Processing and characterisation of a ternary-based P2O5-CaO-Na2O glass system. Biomaterials. 2004;25(3):491–499.
- Bitar M, Salih V, Mudera V, et al. Soluble phosphate glasses: In vitro studies using human cells of hard and soft tissue origin. Biomaterials. 2004;25(12):2283–2292.
- Novajra G, Boetti NG, Lousteau J, et al. Phosphate glass fibre scaffolds: Tailoring of the properties and enhancement of the bioactivity through mesoporous glass particles. Mater Sci Eng C. 2016;67:570–580.
- Sharmin N, Parsons AJ, Rudd CD, et al. Effect of boron oxide addition on fibre drawing, mechanical properties and dissolution behaviour of phosphate-based glass fibres with fixed 40, 45 and 50 mol% P2O5. J Biomater Appl. 2014;29(5):639–653.
- Young-Phil Kim JKH, Lee G-S, Kim J-W, et al. Phosphate glass fibres promote neurite outgrowth and early regeneration in a peripheral nerve injury model. J Tissue Eng Regen Med. 2015;9:236–246.
- Abou Neel EA, Young AM, Nazhat SN, et al. A facile synthesis route to prepare microtubes from phosphate glass fibres. Adv Mater. 2007;19(19):2856–2862.
- Parsons AJ, Ahmed I, Haque P, et al. Phosphate glass fibre composites for bone Repair. J Bionic Eng. 2009;6(4):318–323.
- Vitale-Brovarone C, Novajra G, Lousteau J, et al. Phosphate glass fibres and their role in neuronal polarization and axonal growth direction. Acta Biomater. 2012;8(3):1125–1136.
- Ahmed I, Collins CA, Lewis MP, et al. Processing, characterisation and biocompatibility of iron-phosphate glass fibres for tissue engineering. Biomaterials. 2004;25(16):3223–3232.
- Cozien-Cazuc S, Parsons AJ, Walker GS, et al. Real-time dissolution of P40Na20Ca16Mg24 phosphate glass fibers. J Non-Cryst Solids. 2009;355(50–51):2514–2521.
- Novajra G, Lousteau J, Milanese D, et al. Resorbable hollow phosphate glass fibres as controlled release systems for biomedical applications. Mater Lett. 2013;99:125–127.
- Bitar M, Knowles JC, Lewis MP, et al. Soluble phosphate glass fibres for repair of bone-ligament interface. J Mater Sci Mater Med. 2005;16(12):1131–1136.
- Hasan MS, Ahmed I, Parsons AJ, et al. The influence of coupling agents on mechanical property retention and long-term cytocompatibility of phosphate glass fibre reinforced PLA composites. J Mech Behav Biomed Mater. 2013;28:1–14.
- Colquhoun R, Tanner KE. Mechanical behaviour of degradable phosphate glass fibres and composites – a review. Biomed Mater. 2015;11(1):014105.
- Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774.
- Penido MG, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27(11):2039–2048.
- Julien M, Khoshniat S, Lacreusette A, et al. Phosphate-dependent regulation of MGP in osteoblasts: role of ERK1/2 and Fra-1. J Bone Miner Res. 2009;24(11):1856–1868.
- Frausto da Silva JJR, Williams RJP. The principles of the uptake and chemical speciation of the elements in biology. In: The biological chemistry of the elements: the inorganic chemistry of life. 2nd ed. New York: Oxford Univiersity Press; 2001. p. 29–82.
- Hoppe U. A structural model for phosphate glasses. J Non-Cryst Solids. 1996;195(1–2):138–147.
- Forsen S, Kordel J. Calcium in biological systems. In: Bertini I, Gray HB, Lippard SJ, editors. Bioinorganic chemistry. 1st ed. Mill Valley (CA): University Science Book; 2007. p. 121.
- Mouthuy PA, Crossley A, Ye H. Fabrication of calcium phosphate fibres through electrospinning and sintering of hydroxyapatite nanoparticles. Mater Lett. 2013;106:145–150.
- Brow RK, Kirkpatrick RJ, Turner GL. The short range structure of sodium phosphate glasses I. MAS NMR studies. J Non-Cryst Solids. 1990;116(1):39–45.
- Consolidated B. Glasses Borax Consolidated Ltd. 2nd ed., p. 8–22; 1965.
- Habibovic P, Barralet JE. Bioinorganics and biomaterials: bone repair. Acta Biomater. 2011;7(8):3013–3026.
- Felfel RM, Ahmed I, Parsons AJ, et al. Cytocompatibility, degradation, mechanical property retention and ion release profiles for phosphate glass fibre reinforced composite rods. Mater Sci Eng C. 2013;33(4):1914–1924.
- Brauer DS, Rüssel C, Vogt S, et al. Degradable phosphate glass fiber reinforced polymer matrices: mechanical properties and cell response. J Mater Sci Mater Med. 2008;19(1):121–127.
- Lin ST, Krebs SL, Kadiyala S, et al. Development of bioabsorbable glass fibres. Biomaterials. 1994;15(13):1057–1061.
- Shah R, Sinanan ACM, Knowles JC, et al. Craniofacial muscle engineering using a 3-dimensional phosphate glass fibre construct. Biomaterials. 2005;26(13):1497–1505.
- Ahmed I, Lewis M, Olsen I, et al. Phosphate glasses for tissue engineering: part 2. Processing and characterisation of a ternary-based P2O5–CaO–Na2O glass fibre system. Biomaterials. 2004;25(3):501–507.
- Ruhul CDR, Khan A, Parsons AJ, et al. Surface treatment of phosphate glass fibers using 2-hydroxyethyl methacrylate: fabrication of poly(caprolactone)-based composites. J Appl Polym Sci. 2013;21(7):449–456.
- Cozien-Cazuc S, Parsons AJ, Walker GS, et al. Effects of aqueous aging on the mechanical properties of P40Na20Ca16Mg24 phosphate glass fibres. J Mater Sci. 2008;43(14):4834–4839.
- Ahmed I, Jones IA, Parsons AJ, et al. Composites for bone repair: phosphate glass fibre reinforced PLA with varying fibre architecture. J Mater Sci Mater Med. 2011;22(8):1825–1834.
- Han N, Ahmed I, Parsons AJ, et al. Influence of screw holes and gamma sterilization on properties of phosphate glass fiber-reinforced composite bone plates. J Biomater Appl. 2013;27(8):990–1002.
- Joo NY, Knowles JC, Lee GS, et al. Effects of phosphate glass fiber-collagen scaffolds on functional recovery of completely transected rat spinal cords. Acta Biomater. 2012;8(5):1802–1812.
- Alani A, Knowles JC, Chrzanowski W, et al. Ion release characteristics, precipitate formation and sealing ability of a phosphate glass-polycaprolactone-based composite for use as a root canal obturation material. Dent Mater. 2009;25(3):400–410.
- Abou Neel EA, Ahmed I, Pratten J, et al. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials. 2005;26(15):2247–2254.
- Kobayashi HYLS, Brauer DS, Rüssel C. Mechanical properties of a degradable phosphate glass fibre reinforced polymer composite for internal fracture fixation. Mater Sci Eng C. 2010;30(7):1003–1007.
- Karabulut M, Melnik E, Stefan R, et al. Mechanical and structural properties of phosphate glasses. J Non-Cryst Solids. 2001;288(1–3):8–17.
- Felfel RM, Ahmed I, Parsons AJ, et al. Bioresorbable screws reinforced with phosphate glass fibre: manufacturing and mechanical property characterisation. J Mech Behav Biomed Mater. 2013;17:76–88.
- Haque P, Barker IA, Parsons A, et al. Influence of compatibilizing agent molecular structure on the mechanical properties of phosphate glass fiber-reinforced PLA composites. J Polym Sci Part A Polym Chem. 2010;48(14):3082–3094.
- Haque P, Parsons AJ, Barker IA, et al. Interfacial properties of phosphate glass fibres/PLA composites: effect of the end functionalities of oligomeric PLA coupling agents. Compos Sci Technol. 2010;70(13):1854–1860.
- Haque P, Ahmed I, Parsons A, et al. Degradation properties and microstructural analysis of 40P2O5–24MgO–16CaO–16Na2O–4Fe2O3 phosphate glass fibres. J Non-Cryst Solids. 2013;375:99–109.
- Chen Q, Jing J, Qi H, et al. Electric field-assisted orientation of short phosphate glass fibers on stainless steel for biomedical applications. ACS Appl Mater Interfaces. 2018;10(14):11529–11538.
- Chen M, Parsons AJ, Felfel RM, et al. In-situ polymerisation of fully bioresorbable polycaprolactone/phosphate glass fibre composites: In vitro degradation and mechanical properties. J Mech Behav Biomed Mater. 2016;59:78–89.
- Choueka J, Charvet JL, Alexander H, et al. Effect of annealing temperature on the degradation of reinforcing fibers for absorbable implants. J Biomed Mater Res. 1995;29(11):1309–1315.
- Sharmin N, Hasan MS, Parsons AJ, et al. Cytocompatibility, mechanical and dissolution properties of high strength boron and iron oxide phosphate glass fibre reinforced bioresorbable composites. J Mech Behav Biomed Mater. 2016;59:41–56.
- Zhu C, Liu J, Huang S, et al. The structure, degradation and fibre drawing properties of phosphate based glasses fibre: the effects of Fe₂O₃ and B₂O₃ addition. Ceram Silikaty. 2018;62(2):1–10.
- Tan C, Ahmed I, Parsons AJ, et al. Effects of Fe2O3 addition and annealing on the mechanical and dissolution properties of MgO-and CaO-containing phosphate glass fibres for bio-applications. Biomed Glas. 2018;4(1):57–71.
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164–174.
- Taylor JR, Bull AC, and Institute of Ceramics. Available raw materialsCeramics glaze technology. Stoke on trent: Cookson Ceramics & Anatomy Ltd; 1986. p. 15–49.
- Tan C, Ahmed I, Parsons AJ, et al. Structural, thermal and dissolution properties of MgO- and CaO-containing borophosphate glasses: effect of Fe2O3 addition. J Mater Sci. 2017;52(12):7489–7502.
- Parsons AJ, Sharmin N, Shaharuddin SIS, et al. Viscosity profiles of phosphate glasses through combined quasi-static and bob-in-cup methods. J Non-Cryst Solids. 2015;408(May 2016):76–86.
- Chellan P, Sadler PJ. The elements of life and medicines. Philos Trans R Soc A Math Phys Eng Sci. 2015;373(2037):20140182.
- Ahmed I, Parsons A, Jones A, et al. Cytocompatibility and effect of increasing MgO content in a range of quaternary invert phosphate-based glasses. J Biomater Appl. 2010;24(6):555–575.
- Goudouri OM, Kontonasaki E, Lohbauer U, et al. Antibacterial properties of metal and metalloid ions in chronic periodontitis and peri-implantitis therapy. Acta Biomater. 2014;10(8):3795–3810.
- Valappil SP, Knowles JC, Wilson M. Effect of silver-doped phosphate-based glasses on bacterial biofilm growth. Appl Environ Microbiol. 2008;74(16):5228–5230.
- Valappil SP, Ready D, Abou Neel EA, et al. Antimicrobial gallium-doped phosphate-based glasses. Adv Funct Mater. 2008;18(5):732–741.
- Valappil SP, Ready D, Abou Neel EA, et al. Controlled delivery of antimicrobial gallium ions from phosphate-based glasses. Acta Biomater. 2009;5(4):1198–1210.
- Sharmin N, Hasan MS, Parsons AJ, et al. Effect of boron addition on the thermal, degradation, and cytocompatibility properties of phosphate-based glasses. Biomed Res Int. 2013;2013:1–12.
- Pizzorno L. Nothing boring about boron. Integr Med (Encinitas). 2015;14(4):35–48.
- Patel U, Moss RM, Hossain KMZ, et al. Structural and physico-chemical analysis of calcium/strontium substituted, near-invert phosphate based glasses for biomedical applications. Acta Biomater. 2017;60:109–127.
- Lakhkar N, Abou Neel EA, Salih V, et al. Titanium and strontium-doped phosphate glasses as vehicles for strontium ion delivery to cells. J Biomater Appl. 2011;25(8):877–893.
- Massera J, Petit L, Cardinal T, et al. Thermal properties and surface reactivity in simulated body fluid of new strontium ion-containing phosphate glasses. J Mater Sci Mater Med. 2013;24(6):1407–1416.
- Qiu K, Zhao XJ, Wan CX, et al. Effect of strontium ions on the growth of ROS17/2.8 cells on porous calcium polyphosphate scaffolds. Biomaterials. 2006;27(8):1277–1286.
- Stefanic M, Peroglio M, Stanciuc A-M, et al. The influence of strontium release rate from bioactive phosphate glasses on osteogenic differentiation of human mesenchymal stem cells. J Eur Ceram Soc. 2018;38(3):887–897.
- Massera J, Bourhis K, Petit L, et al. Effect of the glass composition on the chemical durability of zinc-phosphate-based glasses in aqueous solutions. J Phys Chem Solids. 2013;74(1):121–127.
- Zheng K, Yang S, Wang J, et al. Characteristics and biocompatibility of Na2O–K2O–CaO–MgO–SrO–B2O3–P2O5 borophosphate glass fibers. J Non-Cryst Solids. 2012;358(2):387–391.
- Zheng K, Wu Z, Wei J, et al. Preparation and characterization of fibrous chitosan-glued phosphate glass fiber scaffolds for bone regeneration. J Mater Sci Mater Med. 2015;26(8):224.
- Smith SM, Zwart SR, Heer M. Human adaptation to space flight: the role of nutrition. Huston (Texas): National Aeronautics and Space Administration; 2009. p. 29–44.
- Baino F, Novajra G, Miguez-Pacheco V, et al. Bioactive glasses: special applications outside the skeletal system. J Non-Cryst Solids. 2016;432:15–30.
- Hench LL, Splinter RJ, Allen WC, et al. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;5(6):117–141.
- Mohammadi MS, Ahmed I, Muja N, et al. Effect of Si and Fe doping on calcium phosphate glass fibre reinforced polycaprolactone bone analogous composites. Acta Biomater. 2012;8(4):1616–1626.
- Lakhkar NJ, Park JH, Mordan NJ, et al. Titanium phosphate glass microspheres for bone tissue engineering. Acta Biomater. 2012;8(11):4181–4190.
- World Health Organization. AluminiumTrace elements in human nutrition and health. Geneva: World Health Organization; 1996. p. 221–223.
- Balasubramanian P, Strobel LA, Kneser U, et al. Zinc-containing bioactive glasses for bone regeneration, dental and orthopedic applications. Biomed Glas. 2015;1(1):51–69.
- Wang X, Li X, Ito A, et al. Synthesis and characterization of hierarchically macroporous and mesoporous CaO-MO-SiO2-P2O5(M = Mg, Zn, Sr) bioactive glass scaffolds. Acta Biomater. 2011;7(10):3638–3644.
- Barrera Betanzos F, Gimeno-Fabra M, Segal J, et al. Cyclic pressure on compression-moulded bioresorbable phosphate glass fibre reinforced composites. Mater Des. 2016;100:141–150.
- Mazali IO, Barbosa LC, Alves OL. Preparation and characterization of new niobophosphate glasses in the Li2O-Nb2O5-CaO-P2O5 system. J Mater Sci. 2004;39(6):1987–1995.
- Bohner M, Lemaitre J. Can bioactivity be tested in vitro with SBF solution? Biomaterials. 2009;30(12):2175–2179.
- Prabhakar RL, Brocchini S, Knowles JC. Effect of glass composition on the degradation properties and ion release characteristics of phosphate glass - Polycaprolactone composites. Biomaterials. 2005;26(15):2209–2218.
- Ahmed I, Parsons AJ, Palmer G, et al. Weight loss, ion release and initial mechanical properties of a binary calcium phosphate glass fibre/PCL composite. Acta Biomater. 2008;4(5):1307–1314.
- Lassila LVJ, Nohrström T, Vallittu PK. The influence of short-term water storage on the flexural properties of unidirectional glass fiber-reinforced composites. Biomaterials. 2002;23(10):2221–2229.
- Felfel RM, Ahmed I, Parsons AJ, et al. Initial mechanical properties of phosphate-glass fibre-reinforced rods for use as resorbable intramedullary nails. J Mater Sci. 2012;47(12):4884–4894.
- Daniels AU, Chang MK, Andriano KP. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J Appl Biomater. 1990;1(1):57–78.
- Rezwan K, Chen QZ, Blaker JJ, et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–3431.
- Frost HM. Wolff’s law and bone’s structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994;64(3):175–188.
- Alshomer F, Chaves C, Serra T, et al. Micropatterning of nanocomposite polymer scaffolds using sacrificial phosphate glass fibers for tendon tissue engineering applications. Nanomed Nanotechnol Biol Med. 2017;13(3):1267–1277.