Abstract
Many new interventions are being created to address health problems of the developing world. However, many developing countries have fragile health systems and find it difficult to accommodate change. Consequently, it is essential that new interventions are well aligned with health systems and their users. Establishing target product profiles (TPPs) is a critical, early step towards tailoring interventions to suit both of these constituencies. Specific analyses can help identify and establish relevant TPP criteria such as optimal formulation, presentation and packaging. Clinical trials for a new intervention should be designed to address both TPP-specific questions and anticipated use of the intervention in target countries. Examples are provided from research on malaria vaccines that are also applicable to other new public health interventions.
Introduction
Health systems in developing countries (DCs) are more fragile than those in the developed world. In the context of large and diverse disease burdens, major challenges include limited health infrastructure, inconsistent energy supply and highly constrained financing. Unless new interventions are specifically designed to facilitate integration into such systems, and be acceptable to their users, these challenges may be exacerbated.
There is a welcome commitment to creating new means of addressing public health problems in DCs. The G-Finder report, which tracks funding for interventions that target neglected diseases, lists 31 diseases and 134 product areas, including drugs, vaccines, microbicides, diagnostics and vector control (George Institute for International Health Citation2009). The specific contexts of DCs must be considered from the earliest stages of development in order to avoid implementation delays after regulatory approval.
Frost and Reich (Citation2008), and others (Mahoney et al. Citation2007, Obrist et al. Citation2007, Brooks et al. Citation2010), suggest that several issues affect access to health interventions after regulatory approval; particularly availability, affordability, adoption decisions and effective coordination across these issues. Availability relates to manufacture, storage and distribution. Affordability represents the cost to purchasers and end-users. Effective adoption requires an intervention that is widely acceptable, thus leading to a series of positive decisions made by governments, providers and individuals.
All three considerations – availability, affordability and adoption – are directly influenced by decisions taken and studies conducted by developers and collaborators well before regulatory approval. To achieve public health impact in the context of DC health systems, an available intervention must have characteristics that are acceptable to end-users and which facilitate implementation; for example, suitability for tropical climates and a low logistical burden. Affordability is not simply limited to initial purchase price; it also takes account of total delivery costs, including storage, transport, health worker time and the quantity of unused or wasted product. Adoption partly depends upon providing data that responds to questions specific to DC health systems and populations.
If these three key issues are not considered during development, additional studies and investment may be needed subsequent to regulatory approval; expending time and money that might have been used more efficiently during the development phase. The lost public health impact of delayed implementation – probably years – can be even more significant. The GAVI Alliance invested approximately US$100 million to address supply constraints and outstanding research questions in support of implementing Haemophilus influenzae type B (Hib), pneumococcal, and rotavirus vaccines (Milstien et al. Citation2007). The Hib vaccine prevents one of the most important causes of pneumonia and meningitis in infants. GAVI's expenditure began after Hib was offered free to many DCs in the year 2000. Countries were unfamiliar with the disease and lacked data on its burden, a situation that could have been foreseen and addressed while the vaccine was still in research and development (R&D). Consequently, few countries adopted the vaccine, and few children were protected. GAVI had to make additional investments to strengthen the evidence base to inform the use of Hib, and to prevent a similar situation with pneumococcal and rotavirus vaccines.
This article presents an applied research strategy to foresee challenges and align interventions with DC health systems from the early stages of development. It is targeted at product-development partnerships (PDPs), researchers, for-profit organisations and others developing interventions for use in DCs. Published literature is used to complement Frost's framework. Examples demonstrating the application of this approach are drawn from a number of public health interventions and the work of the PATH Malaria Vaccine Initiative (MVI), a PDP. The second section of this article proposes a target product profile (TPP) template that should be utilised for public health interventions intended for DCs.
Since the ideal target for every attribute in a TPP may not be readily apparent, the third section of this article provides an example of research efforts to determine the optimal formulation, presentation, storage and packaging of a malaria vaccine candidate. These attributes influence availability, affordability and decisions on adoption. A similar analysis was conducted during the establishment of the TPP for the US$1.5 billion advance market commitment for pneumococcal vaccines (PATH Citation2007).
The fourth section of this article considers research questions specific to the data requirements for adoption in DCs. Such requirements may not be explicitly stated in a TPP, but highlight the need for careful alignment of research studies with TPP targets and desired health impact. The fifth section of this article discusses the implications of the previous sections, prior to the conclusion.
Taken together, this article proposes a practical strategy to align new public health interventions with the contexts of DCs.
Target product profiles
Background
There is no universal understanding of a TPP and its use. At its most generic, a TPP can be a list of the attributes of an intervention. A TPP can be a formal document used by private sector or non-profit developers for discussions with regulators, helping summarise the anticipated label claims for a product (Food and Drug Administration 2007, Yu Citation2008, Raw et al. Citation2011). It can also be used by the private sector, to compare a product with that of a competitor and to set pricing strategies (Lee and McGlone Citation2010). Alternatively, a TPP can be used as a tool that transparently identifies the major characteristics of a public health intervention, in order to focus effort by all those working on the intervention so as to achieve the intended health impact (Tebbey and Rink Citation2009, Vaccine Presentation and Packaging Advisory Group 2009, Lee and Burke Citation2010, Alonso et al. Citation2011). Several documents provide guidance on potential structures or categories when developing TPPs; although, most relate to experience from the developed world and are tailored to for-profit companies focused on market share (Ellis Citation2001, Garg et al. Citation2003, Food and Drug Administration Citation2007, Yu Citation2008, Tebbey and Rink Citation2009, Vaccine Presentation and Packaging Advisory Group 2009, Lambert Citation2010, Lee and Burke Citation2010, Alonso et al. Citation2011).
Target product profiles are living documents; they evolve over time as research, analyses and consultations clarify the ideal targets, and as interventions move from the pre-clinical to the late development stage (Garg et al. Citation2003, Lambert Citation2010). Within an organisation or consortium there should be a formal mechanism for approving a TPP template, individual TPPs for specific classes of interventions, and a formal change control system for on-going revisions to the template and to individual TPPs. One way to manage product evolution is initially to identify an acceptable range for each characteristic, and subsequently to indicate where an individual intervention's characteristics fall within the chosen ranges as it enters late development. If its characteristics stray outside any of the acceptable ranges, an assessment must be made to determine whether continued development is justified.
Case study: TPP development
The PATH Malaria Vaccine Initiative began a process in 2009–2010 to formalise both the development of TPPs for the candidate malaria vaccines it was developing, and the role of TPPs in guiding the work of the organisation. A multi-disciplinary team was led by experts on policy and access, accountable for envisioning the eventual implementation process for malaria vaccines. The team included members with clinical, regulatory and commercial expertise. It adapted, with permission, a TPP format that was developed by the Bill and Melinda Gates Foundation (Gates Foundation). In parallel, the malaria eradication research agenda (malERA) initiative agreed upon a TPP template which was informed by MVI and the Gates Foundation templates (Alonso et al. Citation2011). Following iterative modifications to the Gates Foundation template, a final MVI TPP template was agreed upon by team members, and formally approved by MVI's Portfolio Management Committee (PMC), which oversees development of the organisation's vaccine candidates. MVI decided that the PMC would approve all TPPs and any significant changes, with review oversight provided by MVI's external advisory body. Approved TPPs were shared with collaborators and made publicly available.
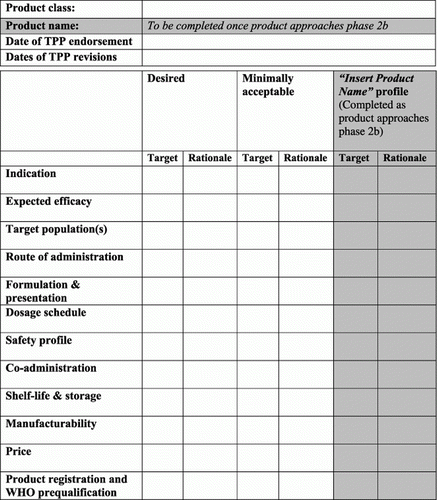
The PATH Malaria Vaccine Initiative's target product profile template was a table that identified a range of target characteristics, from desired to minimally acceptable, for each class of malaria vaccines, as well as the attributes of a specific product as it moved into late development (). Targets were intended to be concrete, evidence-based, and/or measurable. Definitions and examples are provided in and .
A distinguishing attribute of MVI's TPP was that each target was accompanied by a rationale. The rationale could be based upon published studies, on modelling or, at a minimum, logical justification of the chosen target. This rationale enabled others to understand the driving forces behind individual targets; this was particularly important because development activities can take years, teams often evolve, and it can be challenging to ensure that consistent assumptions are made about TPP targets.
Table 1. Target product profile (TPP) template definitions: structural elements.
The PATH Malaria Vaccine Initiative's target product profile explicitly included affordability in the form of purchase price, or relative cost-effectiveness. It also included the preferred route of administration, formulation and presentation, dosage schedule, co-administration, shelf-life and storage and ease of manufacture. These are among the characteristics that will eventually inform country adoption decisions (WHO Citation2005, PATH Malaria Vaccine Initiative Citation2011).
Table 2. Target product profile (TPP) template definitions: characteristic categories.
Formulation, presentation, shelf-life and storage
Background
Once an intervention becomes available, the extent to which its final characteristics comply with the TPP will directly impact affordability and adoption decisions.
Some public-private collaborations, intervention developers and advocates seek to align product characteristics with the contexts of DCs (Vaccine Presentation and Packaging Advisory Group Citation2009). For example, a public-private partnership recently formulated and packaged an appropriately flavoured and easy to swallow dispersible artemisinin-based combination therapy for the treatment of malaria in children (Abdulla and Sagara Citation2009). Similarly, vaccines have been formulated to combine multiple antigens into single injections (Andre Citation1999, Di Fabio and de Quadros Citation2001). The number of doses in a vaccine vial can be optimised for DC immunisation programmes (Drain et al. Citation2003). Novel pharmaceuticals can be evaluated under hot and humid storage conditions common in DCs (Bott and Oliveira Citation2007, Kerdpanich et al. Citation2011). Packaging can be minimised to reduce shipping and handling costs (Schreuder et al. Citation1997). Research has considered the perceptions of malaria and vaccines, and the acceptability of malaria vaccines, to users of health services (Ojakaa et al. Citation2011). Each of the aforementioned considerations relates to one or more aspects of a TPP that responds to the preferences of the public health community and of DCs.
Systematic alignment of interventions with the contexts of DCs builds upon an analytic foundation supported by clearly stated rationales and trade-off comparisons; particularly when there is collaboration with private sector partners. Most of the authors above do not report analysing and/or quantifying the trade-offs. Good alignment is more likely when preferred characteristics are integrated into the TPP and product-development plans early in the development process; this may be years before anticipated availability. If an intervention is modified in late development, or after licensure, this may cause delay and impose additional costs; for example, additional/extended clinical trials, modifications to manufacturing systems and requirements for health worker retraining.
One set of interrelated characteristics that lends itself to analysis are the optimal targets for formulation, presentation and shelf-life and storage of vaccines based on direct trade-offs between vaccine wastage and health system costs. For example, a multi-dose vial of vaccine formulated without a preservative cannot be reused from one immunisation session to the next if unused doses remain in a vial; this leads to more vaccine wastage than a multi-dose vial with a preservative (WHO Citation2000). Presentation of a vaccine in a multi-dose vial is more space-efficient for transport and storage; consequently when a single- or few-dose, preservative-free vaccine is adopted in order to reduce wastage, this will inevitably generate greater logistics costs. Similarly, a vaccine with a short shelf-life will often lead to increased wastage if health care workers have to destroy expired, unopened-vials. A mandatory requirement for refrigerated storage and transport also increases costs, particularly if the packaging is not optimised for DC distribution systems.
Case study: Public sector preferences for RTS,S/AS01(RTS,S) malaria vaccine formulation, presentation, shelf-life and storage
RTS,S, under development since the 1980s, has progressed over the last decade through a collaboration between GlaxoSmithKline (GSK) and MVI, a non-profit PDP. MVI, with grants from the Gates Foundation, entered into a public-private partnership with GSK in 2001 to co-finance the development of RTS,S for use in DCs, collaborate on many technical and management decisions, and provide training and support for infrastructure development at the participating clinical trial sites. The partnership is jointly committed to making RTS,S – if approved for use – available to infants and young children living in malaria-endemic regions in sub-Saharan Africa. Its phase III clinical trial began in 2009 (Vekemans et al. Citation2009, RTS,S Clinical Trials Partnership Citation2011). If all goes well in phase III testing, WHO has indicated that a policy recommendation for RTS,S is possible as early as 2015, paving the way for implementation in countries through the Expanded Program on Immunization (EPI). Defining the targets for a number of RTS,S characteristics evolved into a five-step process, from November 2006 to September 2007.
Step 1 – Structured discussions with a public–private sector working group
A working group from WHO, MVI and GSK was established in 2006, agreeing on a terms of reference to systematically analyse and/or quantify public sector preferences in order to:
| 1. | Align RTS,S presentation and packaging with WHO/UNICEF procurement specifications and previous experience with other childhood vaccines used in EPI to:
| ||||||||||||||||||||||||||||||||||||||||
| 2. | Determine the factors governing the choice of preferred vial size(s). | ||||||||||||||||||||||||||||||||||||||||
| 3. | Consider the use of preservatives in the vaccine. | ||||||||||||||||||||||||||||||||||||||||
| 4. | Consider thermostability issues. | ||||||||||||||||||||||||||||||||||||||||
| 5. | Consider implications of liquid versus lyophilised formulations. | ||||||||||||||||||||||||||||||||||||||||
| 6. | Consider health worker training and workload issues. | ||||||||||||||||||||||||||||||||||||||||
Step 2 – Quantitative analysis using the vaccine presentation assessment tool (VPAT)
An Excel-based VPAT was developed and refined over the course of the project to provide the working group with quantitative analyses of trade-offs among characteristics (Garnett Citation2007). It assessed the volumetric impact of alternative formulations and presentations of a vaccine, and associated commodities (syringes and safety boxes), with those for a typical immunisation schedule in sub-Saharan Africa. The tool used as its unit the Fully Immunised Target Group (FITG), which comprised a fully immunised child plus his/her mother's tetanus toxoid immunisation.
In addition to this volumetric analysis, the tool incorporated sufficient cost data to perform a ‘break-even’ analysis. This analysis indicated which presentations were likely to have implementation costs comparable to, or cheaper than, a baseline presentation of the same vaccine in a single-dose vial. The tool used a goal-seeking algorithm to calculate the wastage rates at which the total cost per administered dose (vaccine purchase + vaccine storage + vaccine distribution + consumables purchase + consumables distribution) for each presentation was equal to the baseline presentation. The calculated break-even wastage rates for each possible presentation and vaccine purchase price point were then compared with wastage rates for similar presentations achieved in the field. If the calculated break-even wastage rate was higher than that typically achieved in the field, the presentation/price combination was considered to be potentially viable.
Step 3 – Incorporating data from African experts and WHO normative materials
A questionnaire on product profile options for RTS,S was developed in consultation with the working group. This questionnaire was administered at two regional immunisation programme managers’ meetings held in Zimbabwe and Burkina Faso during March 2007 to determine the preferences of immunisation experts in Africa. At each session a presentation on malaria vaccine development was given before the questionnaire was administered. The 71 respondents included 35 country staff and 36 international agency staff from 31 malaria-endemic African nations. Responses were consolidated and analysed in Excel.
The working group reviewed WHO materials relevant to the study analyses in parallel with the survey-related activities. Applicable recommendations and norms were synthesised according to the study's topics and carefully referenced.
Step 4 – Discussions by public sector experts and endorsement by WHO staff
A complete report was drafted synthesising the findings from the aforementioned steps and giving conclusions on formulation, presentation, shelf-life, storage and packaging. A public sector expert group drawn from PATH and WHO discussed it. The report was updated in response to the comments received and to take into account improvements in the break-even modelling arising from a parallel GAVI Alliance-commissioned PATH study on pneumococcal conjugate vaccines (PATH Citation2007). The updated report was then reviewed and conclusions formally endorsed by senior immunisation staff in WHO at a meeting in September 2007.
Step 5 – Sharing public sector recommendations with the manufacturer
The final project report set out the agreed upon public sector priorities for the presentation, shelf-life and storage of RTS,S (PATH Citation2008). This report was shared with GSK representatives in September 2007. The manufacturer considered its findings, along with other constraints, such as production challenges and process validation, in the determination of the final product profile for RTS,S.
A complete list of conclusions endorsed at a meeting with WHO representatives is available in the final report, including the detailed methodologies associated with each step summarised earlier. The conclusions related to vial size are presented in to illustrate the complexity of aligning an intervention with the needs of DCs. Factors contributing to the optimal vial size for RTS,S included price per dose, anticipated usage, vial dimensions and estimates of likely wastage. The optimal vial size for RTS,S was found to be a two-dose vial if the price was above US$2.50 per dose or a three-dose vial if the price was lower than US$2.50.
Table 3. Public sector preferences for RTS,S vial size.
Anticipating supplemental research questions for the developing world when designing clinical trials
Background
The targets in a TPP help to define the research questions that need to be addressed in trials and studies in order to be confident that an intervention meets expectations. The targets also assist in identifying data that may be sought by policy-makers to inform adoption decisions. Clinical trials are generally designed to address indication, efficacy and safety, although these may not be straightforward to define or evaluate in the DC context. Less apparent research questions may arise from characteristics in the TPP, which also require explicit consideration in trial designs.
There are many examples in the literature that highlight the important challenge of foreseeing research questions specific to DCs; these may not be as relevant in developed countries, and might, therefore, be overlooked (Deen and Clemens Citation2006, Milstien et al. Citation2010). A partially efficacious, preventive intervention may not be of interest to developed countries, but may be used in DCs where treatment is less accessible and the disease burden is higher (Moorthy et al. Citation2007). Given the diverse disease load carried by many individuals in DCs, interventions may have indirect effects on unrelated pathogens (Shann et al. Citation2010). The safety and efficacy of interventions in HIV-positive individuals needs to be evaluated given its prevalence in Africa and the challenge of screening people (Sartori Citation2004, Steele et al. Citation2009, Mangtani et al. Citation2010). Studies assessed whether the birth dose of Bacillus Calmette-Guerin vaccination should be delayed in low birth weight newborns (Roth et al. Citation2004). Studies also evaluated the impact of breastfeeding on the efficacy of rotavirus vaccine (Moon et al. Citation2010).
There is no formal requirement for international and national policy-making bodies, particularly WHO, to inform or approve clinical development plans. However, it seems prudent to design pivotal studies that anticipate and address as many policy-related questions as possible. Although it is desirable to be comprehensive during the clinical development programme, it may not be practical to address all research questions arising from a TPP; developers may decide that some questions are best answered in studies which take place after regulatory approval.
Case study: TPPs and clinical trial design for malaria vaccines
The PATH Malaria Vaccine Initiative, GSK and partners developed iterative plans for the phase III trial of RTS,S from 2005 through 2008. At the beginning of the process, there was no clear agreement on the trial endpoints or the best way to measure them. WHO, with support from MVI, organised an international consultation in 2006, culminating in a consensus position that a primary study endpoint for licensure of uncomplicated, clinical malaria was appropriate for submission to regulators and policy-makers, and that additional data on efficacy against severe malaria might be useful (Moorthy et al. Citation2007). Once the primary endpoint was defined, WHO convened a consultation to establish standards for measuring efficacy of a malaria vaccine against uncomplicated, clinical malaria (Moorthy et al. Citation2009). The measurement is less straightforward for malaria than for other diseases because individuals can have multiple episodes while developing natural immunity over time.
The World Health Organization's efforts were complemented by MVI. MVI reviewed past policy recommendations for vaccines and malaria interventions to anticipate the policy process and to consider what data might be needed for a policy recommendation (Milstien et al. Citation2010); findings were reported to WHO's Malaria Vaccine Advisory Committee (MALVAC). MVI, WHO and others also worked with African countries to identify what data were needed for policy decisions (PATH Malaria Vaccine Initiative Citation2011).
Planning for policy processes, with WHO's leadership, was critical to ensuring that targeted characteristics were aligned with what could be measured and be requested by regulators and policy-makers. Among the study questions were efficacy in settings with different malaria epidemiology; efficacy against severe, hospitalised malaria and all-cause mortality; duration of protection to 30 months and beyond; co-administration with current and anticipated vaccines and need for a booster dose.
The target population and safety requirements identified in the TPP also led to specific research questions. RTS,S is intended for infants in Africa. It was determined that the phase III study should be undertaken in as representative a population as ethically feasible. Therefore, the study design included subjects that would often be removed from typical trials. For example, only subjects that were acutely malnourished or had late-stage AIDS were excluded, while infants and children with more mild forms or low birth weight were included. An additional safety study was planned in HIV-positive infants and children in parallel with the phase III efficacy study, rather than after regulatory approval. Including these subjects and collecting robust data during phase III studies will enable regulatory agencies to advise on its use in these higher-risk groups in parallel with use in healthy infants. Without such data, universal immunisation in countries could be overshadowed by the unknown risk to these vulnerable segments of the population.
Discussion
The approaches described herein reflect lessons, which, if implemented, will help ensure that new interventions incorporate characteristics suited to the contexts of DCs. The approaches also reflect the links between intervention characteristics, affordability and decisions on adoption in DCs.
These lessons led to the following recommendations for PDPs, researchers, for-profit organisations and others developing interventions for DCs:
| 1. | Target product profiles should state a clear rationale for each of the desired characteristics (e.g., efficacy, safety, formulation) to ensure continuity throughout the intervention development period, and to increase the likelihood of achieving optimum public health impact. | ||||
| 2. | When feasible, rationales should be the outcomes of research activities that quantify trade-offs related to characteristics; particularly when collaborating with the private sector. | ||||
| 3. | Trials need to be designed to be consistent with the TPP, and the unique policy questions and challenges of DCs. | ||||
While the TPP template may vary according to the type of intervention and between organisations (e.g., for those developing diagnostic tests), the critical importance of including components reflecting the unique contexts of DCs seems universally applicable. The inclusion of explicit, evidence-based rationales should also be applicable across organisations.
There are many challenges in developing TPPs. They can be complex documents with many different targets, making it difficult to ensure that the targets are mutually consistent. For example, if one target is to be used in a large proportion of a population, such as part of a disease eradication initiative, the intervention would likely need to be inexpensive, easily stored and transported at ambient temperatures, require one or few-doses, and be produced by a manufacturing process that is scalable. It follows that targets in a TPP interact with and influence each other, thus an interdisciplinary team is optimal to develop TPPs, perhaps led by someone familiar with the implementation of interventions. Determining who represents the DC constituency and how to obtain their input into a TPP may also be challenging. This article proposes a systematic approach, which, at minimum, seeks input from programme managers and technical partners like WHO.
Questions may arise as to who bears which costs for achieving TPP targets and whether these targets can be addressed after regulatory approval; this forces developers to define their responsibilities. For example, developers could spend additional resources and time striving to improve product thermostability because this would save money for countries and donors later. Alternatively, they may decide to seek regulatory approval as quickly as possible; leaving the costs associated with cold chain expansion and associated implementation to others. If developers assume that their TPP role ends at regulatory approval, this may lead to inconsistently defined targets and lack of accountability because important outstanding questions have to be addressed by others in later studies. For characteristics that can be foreseen, the public sector must make its preferences and requirements clear, perhaps five years, or more, ahead of regulatory approval, and it cannot postpone alignment of interventions with DC contexts until after regulatory approval.
The World Health Organization can play an important role in standard-setting during the R&D process. Regulatory agencies, international financing organisations and donors and DC governments look to WHO for guidance. It takes years to put such standards into place. Partners involved in the development of interventions can support WHO in its role, without influencing its neutrality. WHO should clarify what type of data it will need to set policies, prior to finalising the design of late phase clinical trials and years before regulatory approval of interventions, as was largely done for malaria vaccine candidates.
Conclusion
Misalignment of novel interventions with DC contexts can delay access to essential innovations, adding years to their implementation. The literature suggests interventions need to be available, affordable and adopted in order to realise the promised health impact in DCs. TPPs ensure that interventions are aligned with the contexts of DCs for each of these considerations. PDPs, researchers and other product developers should establish well-structured and carefully reasoned TPPs. The rationale behind a TPP is as, or more, important than the TPP target itself. PDPs and others, particularly those working with private sector partners, should agree on TPPs that are aligned with DC contexts years before regulatory approval to increase the likelihood of success. This can be accomplished by supporting public sector requirements and preferences with solid data. Developers should work with WHO to identify the research questions needed to address TPP characteristics, which helps developers efficiently schedule their studies to tackle those questions. This approach will help minimise the length of time between the development and successful implementation of new interventions.
Acknowledgements
The authors thank WHO and GSK for their collaboration on many activities in this document. The authors thank MVI's Leander Lauffer for his contributions to TPPs, and David Poland and Carla Botting for their input into the manuscript. This work was supported by a grant from the Bill and Melinda Gates Foundation. The donor had no role in publication decisions.
References
- Abdulla , S. and Sagara , I. , 2009 . Dispersible formulation of artemether/lumefantrine: specifically developed for infants and young children . Malaria Journal , 8 Suppl. 1 , S7 .
- Alonso , P.L. , Brown , G. , Arevalo-Herrera , M. , Binka , F. , Chitnis , C. , Collins , F. , Doumbo , O.K. , Greenwood , B. , Hall , B.F. , Levine , M.M. , Mendis , K. , Newman , R.D. , Plowe , C.V. , Rodríguez , M.H. , Sinden , R. , Slutsker , L. and Tanner , M. 2011 . A research agenda for malaria eradication: vaccines . Public Library of Science Medicine , 8 ( 1 ) : e1000398
- Andre , F.E. 1999 . Development and clinical application of new polyvalent combined paediatric vaccines . Vaccine , 17 ( 13–14 ) : 1620 – 1627 .
- Bott , R.F. and Oliveira , W.P. 2007 . Storage conditions for stability testing of pharmaceuticals in hot and humid regions . Drug Development and Industrial Pharmacy , 33 ( 4 ) : 393 – 401 .
- Brooks , A.D. , Wells , W.A. , McLean , T.D. , Khanna , R. , Coghlan , R. , Mertenskoetter , T. , Privor-Dumm , L.A. , Krattiger , A. , Mahoney , R.T. , and Innovation Strategy Today , 2010 . Ensuring that developing countries have access to new healthcare products: the role of product development partnerships . Innovation Strategy Today , 3 , 1 – 5 .
- Deen , J.L. and Clemens , J.D. 2006 . Issues in the design and implementation of vaccine trials in less developed countries . Nature Reviews Drug Discovery , 5 ( 11 ) : 932 – 940 .
- Di Fabio , J.L. and de Quadros , C. 2001 . Considerations for combination vaccine development and use in the developing world . Clinical Infectious Diseases , 33 ( Suppl. 4 ) : S340 – S345 .
- Drain , P.K. , Nelson , C.M. and Lloyd , J.S. 2003 . Single-dose versus multi-dose vaccine vials for immunization programmes in developing countries . Bulletin of the World Health Organisation , 81 ( 10 ) : 726 – 731 .
- Ellis , R.W. 2001 . Product development plan for new vaccine technologies . Vaccine , 19 ( 13–14 ) : 1559 – 1566 .
- Food and Drug Administration , 2007 . DRAFT guidance for industry and review staff. Target product profile – a strategic development process tool . Washington , DC : US Department of Health and Human Services .
- Frost , L.J. and Reich , M.R. 2008 . Access: how do good health technologies get to the poor people in poor countries? , Cambridge , MA : Harvard Center for Population and Development Studies .
- Garg , S. , Tambwekar , K.R. , Vermani , K. , Kandarapu , R. , Garg , A. , Waller , D.P. and Zaneveld , L.J. 2003 . Development pharmaceutics of microbicide formulations. Part II: formulation, evaluation, and challenges . AIDS Patient Care STDS , 17 ( 8 ) : 377 – 399 .
- Garnett , A. , 2007 . Vaccine Presentation Assessment Tool [online]. Available from: http://www.technet21.org/index.php/tools/view-document-details/1086-vaccine-presentation-assessment-tool-vpat-program-files.html [Accessed 26 May 2011] .
- George Institute for International Health , 2009 . The G-FINDER report: neglected disease research and development: new times, new trends [online]. Available from: https://studies.thegeorgeinstitute.org/g-finder/index.jsp [Accessed 26 May 2011] .
- Kerdpanich , A. , Chokephaibulkit , K. , Watanaveeradej , V. , Vanprapar , N. , Simasathien , S. , Phavichitr , N. , Bock , H.L. , Damaso , S. , Hutagalung , Y. and Han , H.H. 2011 . Immunogenicity of a human rotavirus vaccine (RIX4414) after storage at 37 degrees C for seven days . Human Vaccines , 7 ( 1 ) : 74 – 80 .
- Lambert , W.J. 2010 . Considerations in developing a target product profile for parenteral pharmaceutical products . American Association of Pharmaceutical Scientists PharmSciTech , 11 ( 3 ) : 1476 – 1481 .
- Lee , B.Y. and Burke , D.S. 2010 . Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles . Vaccine , 28 ( 16 ) : 2806 – 2809 .
- Lee , B.Y. and McGlone , S.M. 2010 . Pricing of new vaccines . Human Vaccines , 6 ( 8 ) : 619 – 626 .
- Mahoney , R.T. , Krattiger , A. , Clemens , J.D. and Curtiss , R. III . 2007 . The introduction of new vaccines into developing countries. IV: global access strategies . Vaccine , 25 ( 20 ) : 4003 – 4011 .
- Mangtani , P.K. , Mulholland , K. , Madhi , S.A. , Edmond , K. , O'Loughlin , R. and Hajjeh , R. 2010 . Haemophilus influenzae type b disease in HIV-infected children: a review of the disease epidemiology and effectiveness of Hib conjugate vaccines . Vaccine , 28 ( 7 ) : 1677 – 1683 .
- Milstien , J. , Cohen , J. , and Olsen I.T. , 2007 . An evaluation of the GAVI Alliance efforts to introduce new vaccines via the Accelerated Development and Introduction Plans (ADIPs) and the Hib Initiative (HI) [online]. Available from: http://www.norad.no/en/Tools + and + publications/Publications/Publication + Page?key=109787 [Accessed 26 May 2011] .
- Milstien , J. , Cárdenas , V. , Cheyne , J. and Brooks , A. 2010 . WHO policy development processes for a new vaccine: case study of malaria vaccines . Malaria Journal , 9 : 182
- Moon , S.S. , Wang , Y. , Shane , A.L. , Nguyen , T. , Ray , P. , Dennehy , P. , Baek , L.J. , Parashar , U. , Glass , R.I. and Jiang , B. 2010 . Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines . The Pediatric Infectious Disease Journal , 29 ( 10 ) : 919 – 923 .
- Moorthy , V. , Reed , Z. and Smith , P.G. 2007 . Measurement of malaria vaccine efficacy in phase III trials: report of a WHO consultation . Vaccine , 25 ( 28 ) : 5115 – 5123 .
- Moorthy , V. , Reed , Z. and Smith , P.G. 2009 . MALVAC 2008: measures of efficacy of malaria vaccines in phase 2b and phase 3 trials—scientific, regulatory and public health perspectives . Vaccine , 27 ( 5 ) : 624 – 628 .
- Obrist , B. , Iteba , N. , Lengeler , C. , Makemba , A. , Mshana , C. , Nathan , R. , Alba , S. , Dillip , A. , Hetzel , M.W. , Mayumana , I. , Schulze , A. and Mshinda , H. 2007 . Access to health care in contexts of livelihood insecurity: a framework for analysis and action . Public Library of Science Medicine , 4 ( 10 ) : 1584 – 1588 .
- Ojakaa , D.I. , Ofware , P. , Machira , Y.W. , Yamo , E. , Collymore , Y. , Ba-Nguz , A. , Vansadia , P. and Bingham , A. 2011 . Community perceptions of malaria and vaccines in South Coast and Busia regions of Kenya . Malaria Journal , 10 : 147
- PATH , 2007 . Vaccine presentation for pneumococcal vaccines: WHO consultation for the development of a TPP for pneumococcal vaccines [online]. Available from: http://sites.google.com/site/vppagp/Home/pneumo-tpp [Accessed 26 May 2011] .
- PATH , 2008 . Public-sector preferences for RTS,S/AS01 malaria vaccine formulation, presentation, and packaging [online]. Available from: http://www.technet21.org/index.php/documents/view-document/715-public-sector-preferences-for-rtss/as01-malaria-vaccine-formulation-presentation-and-packaging.html [Accessed 26 May 2011] .
- PATH Malaria Vaccine Initiative , 2011 . Malaria vaccine decision-making framework [online]. Available from: www.malvacdecision.net [Accessed 26 May 2011] .
- Raw , A.S. , Lionberger , R. , and Yu , L.X. , 2011 . Pharmaceutical equivalence by design for generic drugs: modified-release products. Pharmaceutical Research [online]. Available from: http://www.springerlink.com/content/0769h86828j7u76w/ [Accessed 26 May 2011] .
- Roth , A.H. , Jensen , H. , Garly , M.L. , Djana , Q. , Martins , C.L. , Sodemann , M. , Rodrigues , A. and Aaby , P. 2004 . Low birth weight infants and Calmette-Guerin bacillus vaccination at birth: community study from Guinea-Bissau . Pediatric Infectious Disease Journal , 23 ( 6 ) : 544 – 550 .
- RTS,S Clinical Trials Partnership , 2011 . First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children . New England Journal of Medicine , 365 20 , 1863 – 1875 .
- Sartori , A.M. 2004 . A review of the varicella vaccine in immunocompromised individuals . International Journal of Infectious Dieases , 8 ( 5 ) : 259 – 270 .
- Schreuder , B. , Arentsen , H. and Matosse , M. 1997 . How important are airfreight rates and vaccine packaging in cost-saving efforts for the Expanded Programme on Immunization? . Bulletin of the World Health Organisation , 75 ( 4 ) : 315 – 321 .
- Shann , F. , Nohynek , H. , Scott , J.A. , Hesseling , A. , Flanagan , K.L. and Working Group on Nonspecific Effects of Vaccines , 2010 . Randomized trials to study the nonspecific effects of vaccines in children in low-income countries . Pediatric Infectious Disease Journal , 29 5 , 457 – 461 .
- Steele , A.D. , Cunliffe , N. , Tumbo , J. , Madhi , S.A. , De Vos , B. and Bouckenoogghe , A. 2009 . A review of rotavirus infection in and vaccination of human immunodeficiency virus-infected children . Journal of Infectious Diseases , 200 ( Suppl. 1 ) : S57 – S62 .
- Tebbey , P.W. and Rink , C. 2009 . Target product profile: a renaissance for its definition and use . Journal of Medical Marketing , 9 ( 4 ) : 301 – 307 .
- Vaccine Presentation and Packaging Advisory Group , 2009 . Generic preferred product profile for vaccines, Version 2.1 [online]. Available from: http://sites.google.com/site/vppagp/gppp [Accessed 26 May 2011] .
- Vekemans , J. , Leach , A. and Cohen , J. 2009 . Development of the RTS,S/AS malaria candidate vaccine . Vaccine , 27 ( Suppl. 6 ) : G67 – G71 .
- WHO , 2000 . WHO policy statement: the use of opened multi-dose vials of vaccine in subsequent immunization session . WHO/V&B/00.0. 2000 .
- WHO , 2005 . Vaccine introduction guidelines: adding a vaccine to a national immunization programme: decision and implementation . WHO/IVB/05.18 .
- Yu , L.X. 2008 . Pharmaceutical quality by design: product and process development, understanding, and control . Pharmaceutical Research , 25 ( 4 ) : 781 – 791 .