ABSTRACT
We estimated the costs of Option B+ for HIV-infected pregnant women in 12 facilities in Morogoro Region, Tanzania, from a provider perspective. Costs of prevention of mother-to-child (PMTCT) HIV services were measured over 12 months to September 2017 to estimate the average costs per HIV testing episode, per HIV-positive case diagnosed, per patient-year on antiretroviral therapy (ART), and per neonatal HIV care. A one-way sensitivity analysis was undertaken to understand how staffing levels and other core resource inputs affected costs. The total number of HIV testing episodes was 25,593 with 279 HIV cases identified yielding a 1.1% positivity rate. The average cost per testing episode was US$5.49 (range US$2.13 to US$13.93), and the average cost per HIV case detected was US$503.29 (range US$230.61 to US$3330.38). The number of pregnant women initiated on ART was 278. The mean cost per patient-year on ART was US$159.89 (range US$100.91 to US$812.23). The average cost of neonatal HIV care was US$90.09 (range US$41.53 to US$180.26). PMTCT service costs varied widely across facilities due to variations in resource use, number of women testing, and HIV prevalence. The study provides further evidence against generalising cost estimates, and that budgeting and planning requires context specific cost information.
Introduction
The provision of anti-retroviral treatment (ART) to pregnant women based on CD4 cell count and clinical staging (e.g. Options A and B), contributed to a global decline in the number of infants becoming HIV-infected through their mothers from 400,000 to 240,000 between 2009 and 2013 (World Health Organization, Citation2014).
Global initiatives, including the World Health Organization’s (WHO) recommendation of Option B+, lifelong HIV treatment for all pregnant women living with HIV, as the favoured strategy in the prevention mother-to-child transmission (PMTCT) (WHO, Citation2012) have kept PMTCT high on the international agenda. Option B+ also addresses the 2018 UNAIDS target to reach 95% of pregnant women living with HIV with sustained and lifelong HIV treatment, and to reduce the annual number of newly infected children to less than 40,000 by the end of 2018 (UNAIDS, Citation2017). Option B+’s simplified approach to ART initiation reduces reliance on specialised laboratory services to perform CD4 counts, it also reduces the risk of resistance from multiple ART initiations among women in high-fertility settings (Kalua et al., Citation2017). Moreover, model-based studies, which are typically based on normative guidance, estimated that in the long-run Option B+ would be more cost-effective when compared to previous PMTCT mechanisms of Options A and B (Fasawe et al., Citation2013; Gopalappa et al., Citation2014; Ishikawa et al., Citation2014).
The combination of global targets, and the rapid evolution in WHO guidance, drove the widespread adoption of Option B+ for PMTCT. By 2015, 18 (of 21 countries in sub-Saharan Africa deemed priority for PMTCT by UNAIDS) had moved to roll-out Option B+ (UNAIDS, Citation2015).
However, lifelong ART provision requires financial commitment from donors and governments for both the drugs and the accompanying support services required to ensure treatment uptake and adherence. Empirical data on the per person costs of PMTCT services are therefore needed in order to estimate the level of commitment required, as well as to better utilise existing resources to ensure programme sustainability. While the costs of PMTCT have been documented in sub-Saharan African settings, most studies assess costs under previous ART strategies, with few empirical estimates relating to Option B+ (Siapka et al., Citation2014). In particular, as ART coverage increases and the undiagnosed population falls, service delivery will need to adapt to the issue that testing yields are falling and costs per case identified are going to rise.
In 2013, the government of Tanzania rolled out Option B+, and by 2015, 84% of pregnant women living with HIV were receiving lifelong ART (UNAIDS, Citation2017). Nine PMTCT service components are outlined within the national policy including counselling and testing of all pregnant women and their partners (unless they are already known to be HIV positive) during their first antenatal visit (Box 1) (Ministry of Health, Community Development, Gender, Elderly, and Children (MoHCDGEC), Citation2017). For pregnant women who are HIV negative, repeat testing should be conducted during the third trimester, or during labour and delivery. All breast-feeding mothers, unless known to be HIV-positive, should be counselled and tested during breast-feeding. For those who were tested during their third trimester, or at labour and delivery, a repeat HIV test should be offered at six months after the first test, and thereafter as per the general population. Pregnant women living with HIV and on ART should attend the antenatal care (ANC) clinic every month to be provided with adherence and medication support. Finally, infants born to HIV mothers receive Nevirapine prophylaxis, and undergo an HIV test at six weeks using deoxyribonucleic acid-polymerase chain reaction (DNA-PCR) (known as early infant diagnosis (EID)) (Bwana et al., Citation2018; MoHCDGEC, Citation2017).
The public health system in Tanzania is made up of dispensaries, health centres, and district hospitals and regional referral hospitals (Manzi et al., Citation2012). The majority of dispensaries and health centres are publicly owned (76% and 67% respectively) and the rest are split between faith-based and private ownership (MoHCDGEC, Citationn.d.). Forty-six percent of hospitals are publicly owned, 40% are faith-based and the remaining 14% are privately owned (MoHCDGEC, Citationn.d.). Over two-thirds of Tanzania’s population reside in rural areas and primarily rely on dispensaries and health centres, the most common type of facility, for health services (Manzi et al., Citation2012; MoHCDGEC, Citation2016). PMTCT was integrated into maternal and child health services such that PMTCT interventions are delivered within facilities providing antenatal care, labour and delivery, and postnatal care. This allows for the sharing of facility-level resources across different aspects of care and could have an important role to play in keeping PMTCT service delivery costs low. In 2015, there were approximately 2 million births in Tanzania (UNICEF, Citation2017). The vast majority (98%) of pregnant women attend ANC at least once (MoHCDGEC, Citation2016) although the majority attend in the second or third trimester, and among these, 3% were either already HIV positive or tested HIV positive during their ANC visit (Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), Citation2018).
The aims of this study was to use a case study approach to estimate the costs of Option B+ integrated into reproductive and sexual health services, and to explore cost variations across different levels of health facilities in Tanzania.
Methods
Study setting
This study estimates the costs of PMTCT, under Option B+, delivered within routine maternal and child health services, across12 health facilities serving the population of the Ifakara health and demographic surveillance system (HDSS) in Morogoro Region. The Ifakara HDSS was set up, among other research objectives, to describe health system aspects of maternal, neonatal and child health (Church et al., Citation2017; Geubbels et al., Citation2015). Since 2013, bi-annual health facility surveys have been conducted in all 12 facilities providing HIV services to the HDSS population and with ≥100 patients per month in order to assess HIV service delivery. The Ifakara HDSS covers a geographic area of 2400 km2 and monitors demographic events among a population of approximately 169,000 (125,000 in Ifakara Rural and 45,000 in Ifakara Urban), where HIV prevalence among adults measured 7% (Church et al., Citation2017). This cost study was nested in the third round of the health facility survey.
Health facility characteristics
Facilities were classified as dispensaries (n = 2) which only provide outpatient services; health centres (n = 7) which offer both outpatient and inpatient services; and hospitals (n = 3) (). Ten facilities were public (government-run), one was faith-based (hospital), and one was private-for-profit (hospital). Two health centres had a delivery ward within the ANC unit, and labour and delivery services were provided on site but in a separate room to other ANC services (but not within ANC services) in the other eight facilities. No facility charged user fees for PMTCT services.
Table 1. Facility characteristics.
Cost study
The PMTCT services costed in this study were: (1) routine HIV testing and counselling using HIV rapid diagnostic tests and including provision of both pre- and post-test counselling; (2) the provision of ART to pregnant women and mothers diagnosed with HIV according to national guidelines; and (3) neonatal HIV care which includes the provision of Nevirapine prophylaxis for exposed infants and EID at 6 weeks using the DNA-PCR method (shaded areas in Box 1).
This study was undertaken from the health service providers’ perspective, and included both financial cost estimates, resource inputs incurring actual expenditures, and economic cost estimates i.e. donated items which were valued at their market price. The costing methodology drew on costing guidelines for HIV prevention (UNAIDS, Citation2000) and followed standard principles (Drummond et al., Citation1997; Vassall et al., Citation2018). A micro costing approach was applied which used a combination of top-down and bottom up costing approaches to value resource use depending on the line item.
A standardised Excel-based instrument was used to record resource use and, where available, record price information from each facility. Data were collected retrospectively for the 12 months to September 2017. Fieldwork took place from October to December 2017. Resources were categorised into capital inputs e.g. buildings/room space, equipment and staff training; and recurrent inputs e.g. staff and personnel, HIV test kits and supplies, ART, other clinical and non-clinical supplies, and overheads.
Service delivery data were captured from two sources: (1) Health Management Information Systems (HMIS); and (2) service use data from facility records e.g. registers and summary reports that captured information on the delivery of HIV testing and counselling services, ANC services, and ART services to the HDSS population – some of which were captured in the facility survey. Service delivery data gathered from the HMIS included the number of pregnant or lactating women tested, and the number who tested HIV-positive, the number who were initiated on ART, the total number of PMTCT patients on ART, and the number of infants born to HIV-positive mothers who received Nevirapine prophylaxis and/or tested for HIV at 6 weeks.
ART data obtained from the HMIS were limited because of a lack of disaggregation by ART regimens which are priced differently. In order to estimate total ART drug costs, detailed ART data were collected from two facilities (health centre C and hospital L) which included for each month: the number of newly initiated patients, the total number of ART patients, ART regimen by patient, and the number of months the patient was enrolled under PMTCT. The vast majority of patients in health centre C (98%) and hospital L (91%) were recorded to be on the first-line ART regimen Tenofovir + Lamivudine + Efavirenz (TDF+3TC + EFV), with the remaining on the alternative first-line regimen Tenofovir + Emtricitabne + Efavarinz (TDF + FTC + EFV). In all other facilities, the costs of ART were based on apportioning ART patients to regimens observed in health centre C. This is because health centre C is more typical of health facilities in the region than hospital L which is unique because it also conducts HIV research.
Recurrent costs: Health care worker salaries were obtained from the facilities and the amount of time health workers spent on different HIV services was apportioned based on interviews with staff. The number of HIV test kits used over the study timeframe was based on the number of PMTCT patients tested. Prices for HIV test kits and ART supplies were obtained from the Global Fund ART price and quality reporting list for 2017 (Global Fund, Citation2017). Where possible, the cost of utilities and rent was gathered directly from the facilities, otherwise these costs were estimated using data gathered from the commercial property in Ifakara. These costs were allocated to the different HIV services based on the approximate proportion of staff time spent on those services. Prices of diagnostic or laboratory tests (e.g. DNA-PCR) were obtained from Hospital L where HIV laboratory services were provided to patients. Prices for other HIV supplies e.g. HIV testing and counselling record books; ART cohort registers etc. were obtained from the National AIDS Control Programme.
Capital costs: All space used for the delivery of PMTCT services were audited and subsequently valued using rental information (rent per square meter) from nearby commercial properties. Equipment and furniture in spaces used to deliver PMTCT services were audited, and prices attributed based on current replacement value, sourced through medical supplies catalogues and the National AIDS Control Programme procurement office, and were annualised over 25 years using a 3% discount rate. PMTCT training was provided by the Ministry of Health (MoH), the designated district hospital (Hospital L), and the Boresha Afya programme funded by the United States Presidents Emergency Plan For AIDS Relief. The cost per participant and per training schedule was obtained from the training provided by the designated district hospital and used to estimate Boresha Afya and MoH training costs. Training costs were annualised over 2 years using a 3% discount rate. Allocation of capital costs (equipment) across the different HIV services (HIV testing, ART, and neonatal HIV care) was based on interviews with staff on the approximate proportion of staff time spent on the different HIV services.
Cost analysis
Facility survey data were analysed using STATA v15 and facility-level cost and outcome calculations were undertaken using Microsoft Excel 2007. All costs were adjusted to 2017 United States dollars (US$) and costs collected in Tanzanian Shillings (TZS) were converted to US$ using the average exchange rate from October 2016 to September 2017: 1 US$ = 2250 TZS (Oanda, Citation2018). Total costs and average (unit) costs were calculated for each facility and for the following PMTCT services:
HIV testing: The cost per testing episode, based on the full testing algorithm for pregnant and lactating women (which may include confirmatory testing if carried out) and per patient diagnosed with HIV were calculated by dividing the total facility costs of running testing services in one year by the number of testing episodes and the number of HIV-positive individuals identified over the same timeframe, respectively.
ART: The annual cost per PMTCT patient in ART care was calculated by dividing the total annual costs of providing care and treatment services by the number of HIV patients receiving it.
Neonatal HIV care: The cost per exposed infant born receiving Nevirapine prophylaxis and/or tested for HIV at 6 weeks using DNA-PCR test was estimated by dividing the total costs of testing infants for HIV and provision of Nevirapine by the total number of exposed infants born.
A one-way sensitivity analysis was undertaken to understand how a +/- 10% variation in staffing levels, and prices of HIV test kits, ART, and DNA-PCR, affected the costs of each of the HIV services.
Ethics
Ethical clearance was provided by the National Institute of Medical Research, United Republic of Tanzania (NIMR/HQ/R.8a/Vol. IX /2579), and the London School of Hygiene and Tropical Medicine, United Kingdom (REF: 13536).
Results
PMTCT service delivery
PMTCT service delivery data are presented in . HIV testing and counselling services were provided at least five days per week in all 12 facilities. Across all facilities, 66 health workers provided PMTCT of whom 48.5% (n=32) staff members had received training on PMTCT in the two years prior to the data collection. An estimated 23 full time equivalent (FTE) staff members provided HIV testing, as in the integrated model of delivery staff time was spent on other activities as well as HIV services.
Table 2. Human resources and training input to PMTCT services, and service delivery data across 12 facilities in Tanzania (October 2016 to September 2017).
A total of 25,593 HIV testing episodes were reported over the 12 months averaging 2,133 testing episodes per facility; range702 (Hospital J) to 4452 (Health Centre I). This translated to approximately 4.6 tests per FTE testing staff member per day (assuming 240 working days per year); range 1.2 tests per FTE staff (Hospital L) to 18.6 tests per FTE staff (Health Centre I). The annual positivity rate averaged 1.1%; range 0.2% (Health Centre F) to 2.0% (Health Centre E).
The staff interviews found that the total number of FTE health workers providing HIV care and treatment was 13.9. At the end of the study period (September 2017), a total of 1,019 pregnant or lactating women were taking ART, of whom 278 (27.3%) had been newly initiated on ART during the 12-month study timeframe. ART initiation was reported to take place on the same day as a patient tested positive in eleven facilities, and the day after testing in one facility (Hospital L).
The number of HIV exposed infants born during the study period totalled 372; range 1 (Dispensary A) to 122 (Hospital L). Of these, 73% (n = 287) received neonatal HIV care (Nevirapine and/or HIV test at 6 weeks using DNA-PCR), and 2% (n = 6) were diagnosed to be infected with HIV.
PMTCT service delivery costs
The annual cost of HIV testing totalled US$140,419; range US$3,012 (Dispensary A) to US$27,392 (Hospital L) (). The average annual cost per PMTCT client testing episode was US$5.49; range US$2.13 (Health Centre I) to US$13.93 (Hospital L). The average cost per PMTCT client who tested HIV-positive was US$503.29; range US$230.61 (Health Centre G) to $3330.38 US (Hospital J). In each facility, staff time was the main cost driver of HIV testing services accounting for, on average, 79.2% of total HIV testing service costs, ranging from 55.1% (Health Centre I) to 88.9% (Hospital L) (). This was followed by HIV test kits which accounted for, on average, 15.3% of total costs, ranging from 6.0% (Hospital L) and 38.8% (Health Centre I).
Figure 1. Average cost per testing episode for PMTCT HIV testing and breakdown of costs by resource input in 2017 US$.
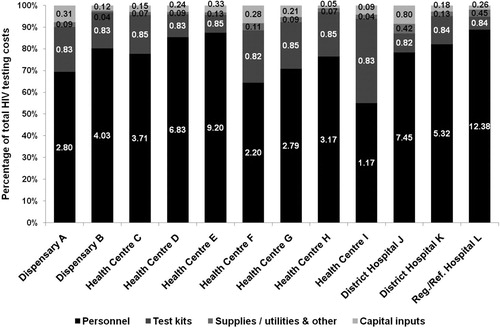
Table 3. Mean and range of annual facility level HIV service testing costs across 12 facilities in Tanzania, in 2017 US$.
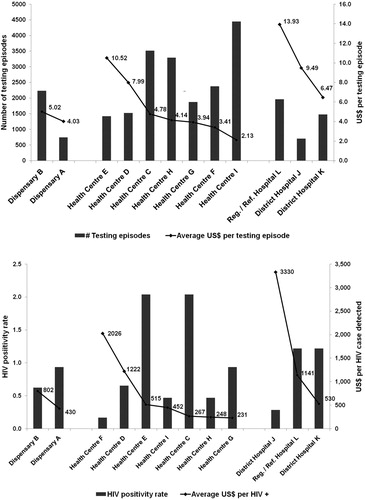
(a,b) indicates that there are likely to be inverse relationships between the cost per testing episode and the number of testing episodes conducted ((a)) as well as between the HIV positivity rate and the cost per HIV case diagnosed ((b)). Moreover, the cost per testing episode in hospitals is more than twice that in dispensaries and health centres: US$10,52; US$4.77; and US$4.47 respectively. The cost per HIV case diagnosed is also higher in hospitals compared with dispensaries and health centres: US$990.90; US$678.06; and US$385.89 respectively.
Figure 2. (a) Number of testing episodes and cost per testing episode for mothers within PMTCT services in 12 health care facilities in Tanzania (2017 US$). (b) HIV positivity rate and cost per HIV case diagnosed within PMTCT services in 12 health care facilities in Tanzania (2017 US$).
The total cost of delivering ART to pregnant and lactating women was US$162,930 averaging US$13,578 per facility; range US$3,249 (Hospital J) to US$70,563 (Hospital L) (). The average cost per PMTCT patient year was US$159.89, however there was variation in average cost across the 12 facilities; ranging from US$100.91 (Health centre C) to US$812.23 US (Hospital J). This variation in costs was driven by differences in personnel costs which, on average, accounted for 42% of total costs.
Table 4. The average annual facility cost of ART among pregnant and lactating women (mean and range) across 12 facilities in Tanzania, in 2017 US$.
The costs for the provision of neonatal HIV care are shown in . The total cost across all 12 facilities was US$25,857; range US$174 (Dispensary A) to US$11,176 (Hospital L). Recurrent costs contributed to the majority of total costs e.g. personnel, which accounted for 58% of total costs, and DNA-PCR tests, which accounted for 36% of total costs. The average cost of neonatal HIV care per infant was US$90.09; ranging from US$41.53 (Health Centre C) to US$180.26 (Dispensary B).
Table 5. Total facility costs of providing neonatal HIV care (Nevirapine and HIV test at 6 weeks using DNA-PCR) (mean and range) across 12 facilities in Tanzania, in 2017 US$.
Sensitivity analysis
When staff inputs were varied by ±10% across the three HIV services, the mean cost per person diagnosed with HIV changed by ±8% and ranged from $463.43 to $543.16 (). The mean cost per PMTCT patient-year resulted in a ±4% change; range $153.16 to $166.63, and the mean cost per neonatal care resulted in ±6% change; range $84.90 to $95.29. Varying the price of HIV test kits by ±10% resulted in a ±2% change in the mean cost per person diagnosed with HIV, and the mean cost per PMTCT patient-year changed by ±5% when ART prices where varied by ±10%. Finally, varying the price of DNA-PCR test resulted in a ±4% change in the mean cost per neonatal care.
Table 6. Sensitivity analysis results and change in average cost by HIV service, in 2017 US$.
Discussion
This study estimated the cost of PMTCT services in 12 facilities in Morogoro Region, Tanzania. The study is one of few to estimate costs along the PMTCT service cascade, with previous studies having focused on either testing or on care and treatment services. Our findings are also unique for sub-Saharan Africa as they include data from multiple sites with different characteristics allowing for an exploration of how size of the facility might drive costs. In addition, our analysis is based on all facilities, covering a large geographic area, which provide HIV services to a HDSS population, and which broadly reflects the health system nationally. The cost per HIV diagnosis ranged from US$230.61 to US$3330.38; the cost of care and treatment for positive pregnant and lactating women ranged from US$100.91 to US$812.23 and the cost of neonatal HIV care per HIV exposed infant ranged from US$41.53 to US$180.26. This contribution to the evidence base is invaluable for planning purposes and for showing how future budgets and programmes need to account for such cost variations and to optimise current funding.
The large variations in average costs for the different components of the care cascade across the facilities may suggest implementation inefficiencies across the different facilities, or that geographical or other factors require different levels of investment. For HIV testing and for neonatal HIV care, personnel was the main cost driver. For care and treatment of mothers, ART drugs were the main cost driver but not for all facilities. For HIV testing, we observed variation across the facilities in both the average cost per testing episode and the average cost per HIV case diagnosed. The average cost of an HIV testing episode amounted to $5.49 across all 12 facilities and ranged from $2.13 to $13.93. Estimates of PMTCT costs from earlier studies in east and southern Africa countries are higher than the costs presented here. For example, a four country study, conducted in 2012, estimated the average annual cost per facility-based PMTCT client tested for HIV ranged from US$18 (SD US$20) in Rwanda to US$89 (SD US$56) in South Africa (Bautista-Arredondo et al., Citation2016). This variation might be the result of cross-country variation but might also be a reflection of changing costs as PMTCT programmes have evolved over time.
The average cost per PMTCT patient diagnosed was US$503.29 (including pre- and post-test counselling) with large variation across the facilities, and likely driven by the HIV positivity rate. Despite, the wide range, this estimate is comparable with that found by Bautista-Arredondo et al. (Citation2016), who found the average cost per PMTCT client diagnosed as HIV-positive ranged from US$565 in Zambia to US$2021 in Rwanda. As found in this study, the authors identified that personnel was the key cost driver of PMTCT testing services, and, therefore, cross-country salary differences could explain the lower costs in Tanzania. For example, the average annual salary for a nurse in our sample was approximately US$5,250, slightly lower than documented in Rwanda (US$6,259) and considerably lower than that documented in Zambia (US$15,736) and South Africa ($33,976 US) (Bautista-Arredondo et al., Citation2016). In addition, in this study it was found that not all testing personnel spent 100% of their time on HIV services. This sharing of personnel across different services is an important element of how the integrated model has potential to increase efficiency without lower quality of services.
The average cost per patient year of providing ART for PMTCT was US$159.89 and ranged from US$100.91 to US$812.23. To date, few studies have empirically estimated the costs of PMTCT under Option B+, with existing studies using modelled-based approaches to demonstrate cost-effectiveness of Option B+ over prior PMTCT strategies (Karnon & Orji, Citation2016). To date, only two recently published studies, from Ethiopia and Eswatini, have estimated PMTCT treatment costs under Option B+. The study in Ethiopia collected data for 2013-2014, and found the unit cost (in 2014 US$) per pregnant woman-infant pair per year averaged US$701 US (SD US$324) in urban health facilities and US$302 (SD US$53) in rural health facilities (Zegeye et al., Citation2019). In Eswatini, cost data were collected from 2013 to 2015 and the researchers estimated the cost (in 2015 US$) per PMTCT patient treated per month was US$183 (Cunnama et al., Citation2018). These studies measure the costs of different units of PMTCT services to the study presented here so it is difficult to make direct comparisons. For example, the study in Ethiopia considers PMTCT as a whole (i.e. costed HIV testing, treatment and EID together), while the study in Eswatini was conducted during a period of transition to Option B+ and therefore, included Option B+ start-up and training costs. Finally, this study estimated the cost of neonatal HIV care per exposed infant was US$90.09 and ranged fromUS$41.53 to US$180.26. With few infants testing HIV positive at 6 weeks, this study did not seek to estimate the cost per infant diagnosed with HIV.
There are several limitations of this study that are important to note. Firstly, the study only captures data on a subset of core PMTCT services for pregnant women living with HIV, and critical steps following HIV diagnosis and enrolment in care, such as safer delivery practices and counselling for safer infant feeding practices, were not disaggregated. Linked with this point, the study estimates costs from a provider perspective and did not include direct costs to patients – a cost which might be crucial to consider in HIV treatment given the wide geographic and remote spread of these facilities which may hamper patient access. Thirdly, we relied on provider interviews, rather than direct observation, to measure and allocate staff time, and this may be subject to over-estimation of health worker contact time with patients (Bratt et al., Citation1999). Finally, because of data unavailability, this study did not capture information on essential above service delivery activities, that is activities coordinated at national and district levels which are essential for the delivery of HIV services, such as quality assurance and audits. Nevertheless, these findings are important because they are among the few studies to cost PMTCT along the service cascade which increases the evidence base for future cost projections, and provide us with a detailed understanding of the cost drivers in Option B+. Estimates of costs of PMTCT in Tanzania are scarce, and this study provides much needed guidance to policy makers on how to better target PMTCT services.
PMTCT costs in Tanzania vary across different types of health facilities and scale of operation. Hospitals, on average, had higher average costs than the health centres and dispensaries, and a weak inverse relationship between output and unit cost was observed per HIV case diagnosed. Personnel costs were confirmed as a key cost driver in Option B+, supporting the integrated care model that has been adopted in Tanzania. With a high birth rate alongside a declining HIV positivity rate, as the Option B+ programme matures and global efforts to achieve elimination of mother-to-child transmission, the average per case diagnosed within PMTCT services will likely increase. The integrated model provides a way to minimise these costs by allowing non-specific resources to be shared across a range of services. Careful planning in the allocation of resources, especially personnel, will be needed to ensure value for money when meeting this increased demand.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be available for the next five years upon request to the study PI: [email protected]
Additional information
Funding
References
- Bautista-Arredondo, S., Sosa-Rubı´, S. G., Opuni, M., Contreras-Loya, D., Kwan, A., Chaumont, C., Chompolola, A., Condo, J., Galarraga, O., Martinson, N., Masiye, F., Nsanzimana, S., Ochoa-Moreno, I., Wamai, R., & Wang’ombe, J. (2016). Costs along the service cascades for HIV testing and counselling and prevention of mother-to-child transmission. AIDS, 30(16), 2495–2504. https://doi.org/10.1097/QAD.0000000000001208
- Bratt, J. H., Foreit, J., Chen, P., West, C., Janowitz, B., & de Vargas, T. (1999). A comparison of four approaches for measuring clinician timeuse. Health Policy and Planning, 14(4), 374–381. https://doi.org/10.1093/heapol/14.4.374
- Bwana, V. M., Mfinanga, S. G., Simulundu, E., Mboera, L. E. G., & Michelo, C. (2018). Accessibility of early infant diagnostic services by under-5 years and HIV exposed children in Muheza district, North-East Tanzania. Public Health, 6, 139. https://doi.org/10.3389/fpubh.2018.00139
- Church, K., Machiyama, K., Todd, J., Njamwea, B., Mwangome, M., Hosegood, V., Michel, J., Oti, S., Nyamukapa, G., Crampin, A., Amek, N., Nakigozi, G., Michael, D., Gomez-Olive, F. X., Nakiyingi-Miiro, J., & Wringe, A. (2017). Identifying gaps in HIV service delivery across the diagnosis-to-treatment cascade: Findings from health facility surveys in six sub-Saharan countries. Journal of the International AIDS Society, 20(1), 21188. https://doi.org/10.7448/IAS.20.1.21188
- Cunnama, L., Abrams, E. J., Myer, L., Gachuhi, A., Dlamini, N., Hlophe, T., Kikuvi, J., Langwenya, N., Mthethwa, S., Mudonhi, D., Nhlabatsi, B., Nuwagaba-Biribonwoha, H., Okello, V., Sahabo, R., & Sinanovic, E. (2018). Cost and cost-effectiveness of transitioning to universal initiation of lifelong antiretroviral therapy for all HIV-positive pregnant and breastfeeding women in Swaziland. Tropical Medicine and International Health, 23(9), 950–959. https://doi.org/10.1111/tmi.13121
- Drummond, M. F., O’Brien, B., Stoddart, G. L., & Torrance, G. W. (1997). Methods for the economic evaluation of health programmes (2nd ed.). Oxford University Press.
- Fasawe, O., Avila, C., Shaffer, N., Schouten, E., Chimbwandira, F., Hoos, D., Nakakeeto, O., & De Lay, P. (2013). Cost-effectiveness analysis of option B+ for HIV prevention and treatment of mothers and children in Malawi. PLoS One, 8(3), e57778. https://doi.org/10.1371/journal.pone.0057778
- Geubbels, E., Amri, S., Levira, F., Schellenberg, J., & Masanja, H. (2015). Demographic surveillance system profile: The Ifakara rural and urban health and demographic surveillance system (Ifakara HDSS). International Journal of Epidemiology, 848–861. https://doi.org/10.1093/ije/dyv068
- Global Fund. (2017). Retrieved January 7, 2018 from: https://www.theglobalfund.org/en/; https://public.tableau.com/profile/the.global.fund#!/vizhome/PQRPricelist_English/PriceList
- Gopalappa, C., Stover, J., Shaffer, N., & Mahy, M. (2014). The costs and benefits of Option B+ for the prevention of mother-to-child transmission of HIV. AIDS, 28(Suppl. 1), S5–S14. https://doi.org/10.1097/QAD.0000000000000083
- Ishikawa, N., Shimbo, T., Miyano, S., Sikazwe, I., Mwango, A., Ghidinelli, M. N., & Syakantu, G. (2014). Health outcomes and cost impact of the new WHO 2013 guidelines on prevention of mother-to-child transmission of HIV in Zambia. PLoS One, 9(3), e90991. https://doi.org/10.1371/journal.pone.0090991
- Kalua, T., Tippett Barr, B. A., van Oosterhout, J. J., Mbori-Ngacha, D., Schouten, E. J., Gupta, S., Sande, A., Zomba, G., Tweya, H., Lungu, E., Kajoka, D., Tih, P., & Jahn, A. (2017). Lessons learned from option B+ in the evolution toward test and start from Malawi, Cameroon, and the United Republic of Tanzania. Journal of Acquired Immune Deficiency Syndromes, 75(Suppl. 1). https://doi.org/10.1097/QAI.0000000000001326
- Karnon, J., & Orji, N. (2016). Option B1 for the prevention of mother-to-child transmission of HIV infection in developing countries: A review of published cost-effectiveness analyses. Health Policy and Planning, 1–9. https://doi.org/10.1093/heapol/czw025
- Manzi, F., Armstrong Schellenberg, J., Hutton, G., Wyss, K., Mbuya, C., Shirima, K., Mashinda, H., Tanner, M., & Schellenberg, D. (2012). Human resources for health care delivery inTanzania: A multifaceted problem. Human Resources for Health, 10(1), 3. https://doi.org/10.1186/1478-4491-10-3
- Ministry of Health, Community Development, Gender, Elderly and Children. (2017). National Guidelines for the management of HIV and AIDS. 6th ed. Retrieved March 5, 2019 from: https://www.nacp.go.tz
- Ministry of Health, Community Development, Gender, Elderly and Children (MoHCDGEC). (n.d.). Retrieved May 23, 2020 from: http://opendata.go.tz/dataset/list-of-health-facilities-with-geographical-location
- Ministry of Health, Community Development, Gender, Elderly and Children (MoHCDGEC) [Tanzania Mainland], Ministry of Health (MoH) [Zanzibar], National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), and ICF. (2016). Tanzania demographic and health survey and malaria indicator survey (TDHS-MIS) 2015–16.
- OANDA. (2018). www.oanda.com/currency/average
- Siapka, M., Remme, M., Obure, C. D., Maier, C. B., Dehne, K. L., & Vassall, A. (2014). Is there scope for cost savings and efficiency gains in HIV services? A systematic review of the evidence from low- and middle-income countries. Bulletin of the World Health Organization, 92(7), 499–511. https://doi.org/10.2471/BLT.13.127639
- Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC). (2018). Tanzania HIV Impact Survey(THIS) 2016–2017: Final Report. http://phia.icap.columbia.edu
- UNAIDS. (2000). Costing guidelines for HIV prevention. http://data.unaids.org/publications/irc-pub05/jc412-costguidel_en.pdf
- UNAIDS. (2015). 2015 Progress report on the global plan: towards the elimination of new HIV infections among children and keeping their mothers alive. https://www.unaids.org
- UNAIDS. (2017). Start free, stay free, AIDS free: A super fast-track framework for ending AIDS among children, adolescents and young women by 2020. https://free.unaids.org/
- UNICEF. (2017). Maternal and newborn health disparities. https://data.unicef.org
- Vassall, A., Sweeney, S., Kahn, J. G., Gomez, G., Bollinger, L., Marseille, E., Herzel, B., DeCormier Plosky, D., Cunnama, L., Sinanovic, E., Bautista, S., Harris, K., & Levin, C. (2018). GHCC draft reference case_version 3 [Internet]. https://ghcosting.org/pages/standards/reference_case
- World Health Organization. (2012). Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants https://www.who.int/hiv/PMTCT_update.pdf
- World Health Organization. (2014). Global update on the health sector response to HIV. www.who.int
- Zegeye, E. A., Mbonigaba, J., Kaye, S., & Johns, B. (2019). Assessing the cost of providing a prevention of mother-to-child transmission of HIV/AIDS service in Ethiopia: Urban-rural health facilities setting. BMC Health Services Research, 19(1), 148. https://doi.org/10.1186/s12913-019-3978-4
