ABSTRACT
Timely access to treatment is essential for women when they experience abortion complications. Out-of-pocket (OOP) expenditure is a known barrier to health care access. In 2018, we assessed the financial burden of accessing postabortion care (PAC) borne by women in Dakar, Senegal, where studies estimate that half of poor women with complications obtain PAC. We interviewed 729 women following discharge from PAC. Women reported expenditures on transportation, admission, treatment, family planning, hospitalisation, complementary tests, prescriptions, other medicines and materials. We compare women’s OOP on PAC by expenditure category, type of treatment and facility type, and use multiple generalised linear regression analysis to explain variation in overall OOP and forecast it under alternate scenarios. The average OOP was USD $93.84. At health centres it was $65.47 and at hospitals it was $120.47. The average cost of PAC using dilation and curettage was $112.37, manual vacuum aspiration was $99.84, and misoprostol $61.80. Overall OOP on PAC amounts, on average, to 15% of the average monthly salary for women living in Dakar. Strategies that emphasise timely access to misoprostol for treating complications in primary care settings will address the contribution of OOP costs to Senegal’s appreciable unmet need for PAC among the poor.
Introduction
In sub-Saharan Africa from 2010 to 2014, an estimated 8.2 million induced abortions occurred each year, representing approximately 15% of all pregnancies in the region, and a majority of women of reproductive age live in countries with restrictive abortion laws (Singh et al., Citation2018). It follows that, in these settings, safe abortion care services are usually unattainable. Indeed, during 2010–2014, an average of three out of every four abortions in sub-Saharan Africa were carried out by an untrained individual, helping to render the region highest in terms of abortion-related mortality. As of 2019, sub-Saharan Africa has the highest abortion case-fatality rate of any world region, approximately 185 deaths per 100,000 abortions, for a total of 15,000 preventable deaths per year (Bankole et al., Citation2020). Postabortion care (PAC) saves women from the fatal consequences of abortion complications and promotes access to contraceptive methods for women who wish to use them after receiving emergency care (Turner & Corbett, Citation2003). Access to PAC for women who need it, globally, has proven uneven. Studies estimate that between 2000 and 2008, about 60% of women who experienced complications from unsafe abortion obtained PAC in health facilities, while 40% did not (Singh & Maddow-Zimet, Citation2015).
In Senegal, the abortion law is both restrictive and unclear. Although the country’s criminal code completely prohibits the practice, the code of medical ethics permits pregnancy termination to save a pregnant woman’s life (Sedgh et al., Citation2015). In 1997, the Ministry of Health (MOH) of Senegal introduced PAC to the country (Bullough et al., Citation2005). To guide this process, the MOH commissioned studies that demonstrated PAC could be decentralised from tertiary hospitals and that midwives could effectively use manual vacuum aspiration (MVA) to treat abortion complications in primary care settings (CEFOREP, Citation1998), and that this led to increases in PAC utilisation and reductions in the need for more risky, expensive and painful treatment alternatives (Suh, Citation2019). Subsequent research has emphasised the use of provider and self-administration of misoprostol for treatment of abortion complications (Gaye et al., Citation2014). The MOH has since then called for the availability of both surgical aspiration and misoprostol for PAC provision at all levels of care throughout the Senegalese health system, garnering praise as a West African success story for PAC (Diadhiou et al., Citation2008; Suh, Citation2018).
Despite these successes, data from the previous decade raise concerns. During this period, between one-quarter and one-third of pregnancies in the country are unintended, and, on average, Senegalese women give birth to between one and two more children than they desire (Agence Nationale de la Statistique et de la Demographie & ICF International, Citation2019). In 2018–19, only 17.9% of women in Senegal were using modern contraception (Agence Nationale de la Statistique et de la Demographie & ICF International, Citation2019). Segdh et al. estimated the incidence of induced abortion and consequences of unsafe abortion in Senegal using data from 2012. 24% of all unintended pregnancies in the country end in abortions, 60% in unplanned births and 16% in miscarriages (Sedgh et al., Citation2015). 17 out of every 1000 women of reproductive age in Senegal of which two-thirds are carried out by untrained individuals, and virtually all are clandestine and unsafe (Sedgh et al., Citation2015). These conditions place women at a disproportionate risk of life-threatening complications from abortion. Whereas, the abortion complication rate in 2012 stood at 5.5 complications treated per 1000 women of reproductive age, access to treatment is uneven: only half of poor women with complications receive PAC (Sedgh et al., Citation2015).
A salient barrier to timely attainment of PAC are costs; yet health systems often lack data that can guide efforts to ameliorate economic barriers to access. A Nigerian study of the direct costs of unsafe abortion estimated that nearly three quarters of costs were shouldered by the woman and/or her household (Bankole et al., Citation2007). A study from Uganda estimated high direct costs for abortion-related care at US$62 per abortion (Babigumira et al., Citation2011). A study from Burkina Faso found that, excluding costs incurred prior to hospitalisation, the cost women incur on treatment for complications of induced abortion was considerably higher than spontaneous abortion (Ilboudo et al., Citation2015).
We present evidence on the costs borne by women who sought routine PAC services for the treatment of incomplete abortion in Dakar, Senegal. We compare clients’ expenditures on PAC obtained at hospitals with those incurred at lower levels of the health care system, as well as costs incurred on different treatment procedures. Furthermore, we compare the levels of expenditure incurred before and after facility admission for PAC across different categorisations of cost to examine the drivers of out-of-pocket expenditure (OOP) and illuminate opportunities to reduce economic barriers to PAC.
Materials and methods
Study environment and context
The study team included researchers from the international sexual and reproductive health NGO, EngenderHealth, and from Le Centre Régional de Formation, de Recherche et de Plaidoyer en Santé de la Reproduction (CEFOREP), a Senegalese reproductive health training, research, and advocacy NGO.
Although the Senegalese healthcare system includes both a public and a private sector, access to care in the private sector is limited, especially for PAC. According to research that was conducted in 2012, only 4% of facilities where PAC was available were private (Sedgh et al., Citation2015). Regardless of sector, OOP expenditures are typical, including for PAC (Leive & Xu, Citation2008; Lince-Deroche et al., Citation2020). For this study, research was conducted in eight public sector sites offering PAC in Dakar, and in the neighbouring town of Rufisque. We chose sites to reflect the three types of public sector facilities where PAC is available. We excluded from consideration for the study facilities that could not report a minimum of eight PAC clients per month for the previous year based on concerns about client recruitment as human subjects. Potentially eligible sites were visited by members of the research team who obtained information on PAC admissions from available registers. In the end, the sites selected were four tertiary hospitals, three intermediate-level health centres, and one health post. Among the hospitals, the mean number of PAC clients received in the past 12 months was, on average, 32 per month, while among the health centres and health post it was 22 and 19, respectively.
All the recruitment sites offered both surgical and medication PAC procedures. Surgical procedures are typically performed with MVA, and less often with electric vacuum aspiration (EVA). Relatively few procedures are performed with dilation and curettage, which has been recommended for removal from routine PAC protocols (World Health Organization, Citation2012; World Health Organization, Citation2015). Medication techniques are performed with a regimen of misoprostol alone. In Senegal, women typically remain at the facility where they receive misoprostol for up to two hours for observation and are discharged at providers’ discretion, with analgesics as needed. Women are eligible for all types of short-acting methods, e.g. oral contraceptive pills, injectable methods, condoms, immediately. As well, women can receive hormonal implants immediately following PAC, and intra-uterine devices as soon as a trained healthcare provider determines that emergency treatment is completed and that products of conception are completely evacuated from the uterus ( and ).
Table 1. Illustrate characteristics of the study participants in terms of their socio-demographic backgrounds and features of their PAC visits (client socio-demographic characteristics, care seeking info, e.g. gestational age, delays, etc.) (n = 729).
Table 2. Illustrate characteristics of the study participants in terms of their socio-demographic backgrounds and features of their PAC visits (components of PAC delivered) (n = 729).
Sample size
This study was the baseline component of pre–post evaluation of a PAC quality improvement intervention that would compare changes in the quality of PAC and uptake of postabortion contraception experienced in facilities assigned to a treatment and comparison study arm. Our overall sampling strategy was to determine the number of clients required for participation in each study arm at baseline and endline to detect an expected difference of 17% points in the proportion of PAC clients who accepted voluntary postabortion contraception in each arm with 80% power. To achieve this, the study enrolled 729 women in the baseline study, 461 in intervention sites and 268 in comparison sites between June and September 2018.
Study procedures
We conducted our study with a sample of 729 women who presented at the study sites with abortion complications that could be treated by routine provision of PAC. Women were eligible to participate in the study if they were 15 or older and were seeking care for complications in the first trimester of pregnancy. All data collection took place while women were at the sites for their initial PAC visit, not during follow up appointments. PAC providers who had been oriented to the study instrument and procedures screened clients to ensure that they were well enough, physically, and emotionally, to participate in data collection. Those deemed eligible were then introduced to female interviewers who recruited participants into the study.
Interviewers underwent a comprehensive, one-week training in the protocol and interviewing techniques prior to fieldwork. Interviewers remained onsite at study facilities during routine operating times and met with eligible PAC clients who had expressed a willingness to participate in the study in a private room within each facility. Interviewers read aloud to participants an informed consent form that explained the rationale for the research, steps of research participation, potential risks and benefits of participation, the measures the study would undertake to ensure their privacy and confidentiality, and how the information would be used. In addition, the informed consent form emphasised participants’ rights to withdrawal from the study and to request, and receive, additional information about the study at any time. Afterwards, women who agreed to participate signed or provided a thumbprint on the informed consent form. A staff member of the facility witnessed each informed consent procedure and signed the forms as well. Participants were given a copy of the consent form which contained contact information of study staff. At that point, the interviewer conducted an exit interview survey that took between 30 and 45 minutes to complete. All steps were conducted in Wolof or French. Informed consent documents were available in both languages.
Data collection included a 6-part questionnaire that included closed-answer questions on the following topics: clients’ socio-demographic background, reproductive health and family planning experiences, recognition of complications and care-seeking, recall of the elements of care received during PAC, out-of-pocket expenditures (OOP) on PAC and their perceptions of the quality of care received. For the data collected on OOP, data were recorded for all clients who could demonstrate that each payment was incurred by showing a receipt or invoice of payment (see ). An exception to this was made for OOP incurred on transportation to the facility. If receipts for this were not available, data collectors were permitted to record the amounts that participants reported based on recall only. The amounts recorded in the survey were the amounts recorded on each receipt. Data collectors, further, collected information on the number of distinct payments clients made for PAC. This variable was recorded as the number of payments made at the facility where PAC was obtained that could be confirmed by presentation of a receipt or invoice.
Table 3. Contains the results of our univariate analysis of the average OOP, by level of care, type of treatment and PAC treatment cost category.
Interviewers pre-tested the survey using the original version of the study questionnaire in electronic format using tablets programmed with ODK software. After two-days of pre-testing, the data collectors and study team re-convened to finalise the survey and software programming based on lessons from the pilot. During data collection, responses were entered into tablets, which interviewers uploaded into a central server managed by the research team at CEFOREP who monitored data completeness and quality daily. After collection, data were cleaned in the ODK database and then transposed into CSV files that were read into R software version 3.6.2 for analysis.
Study measures and analysis
The first step in our analysis was to estimate the mean OOP on PAC by level of care (health centre and hospital), type of treatment procedure (manual vacuum aspiration, electric vacuum aspiration, misoprostol, dilation and curettage or other) and PAC components that were itemised specifically on receipts that were maintained by participants (except transportation to facility). As the study was only able to identify one eligible health post, for our estimates that are subset by level of care, we grouped data from health centres and health posts together. These components are defined as follows:
Transportation: All payments incurred on public transportation, securing private transport (e.g. taxi) or other means of reaching the facility where clients received PAC and were enrolled in the interview.
Pre-admission care seeking: all payments incurred on care and treatment for the complication that were obtained at other locations (e.g. pharmacy or drug shop, health post) before reaching the facility where clients received PAC and were enrolled in the interview.
Admission fee: payments incurred by patients for admission to the facility, notwithstanding fees for the procedure, occupancy and other medications and materials received.
Treatment procedure: cost of receiving the evacuation procedure or medication (i.e. MVA, misoprostol, curettage)
Postabortion contraception: the amount paid for obtaining a contraceptive method after completing the treatment procedure.
Hospitalisation: additional fees that were for occupancy at the facility for the duration of time the client spent in care at the facility.
Supplementary tests: payments for echography, vaginal swab, blood tests and ‘other tests’.
Prescriptions: payments incurred for prescription medications obtained at facilities’ pharmacies before clients departed the facility.
Other medications and materials: other medications that were not prescribed or included with the treatment that were obtained after admission, most often pain medication, food, any other materials, (not specified) and ‘other expenditures’, including those for patients’ accompaniment, if any.
We used multivariate regression to identify variables that strongly influenced the total, overall, OOP for PAC. In this analysis, we used a generalised multivariate linear regression model with Gamma distribution and total OOP as our dependent variable, a continuous measure ranging initially from zero to 264,000 FCFA (Senegalese currency). To select independent variables for our analysis, we reviewed available literature to identify factors associated with care-seeking, financial and economic costs of PAC, abortion and emergency obstetric services and identified those that were also available in the exit interview. To determine the variables to include in the final model, we applied a ‘step-wise’ method of backward elimination, which involved starting with the above candidate variables, and testing the deletion of each in a model fit according to the above fit criterion. Variables were removed if their loss gave the most statistically insignificant deterioration of model fit. To determine the final model’s structure, we compared null and residual deviance and dispersion parameters derived from models that used a log- versus identity-link function for the generalised multivariate linear regression. When the identity link function was used, we log-transformed the dependent variable, total OOP, because its distribution was not normal. During this process, we also compared the Akaike Information Criteria (AIC) and Bayesian Information Criteria (BIC) and pseudo-R2 values of model alternatives which indicated which one better predicted the outcome. This analysis was performed as recommended by previous studies that used economic variables that did not comply with linear regression assumptions (Barrero et al., Citation2014; Vargas-Alzate et al., Citation2018). We carried out model assumption and diagnostic tests to examine the data for linear relationships, normal distribution, multi-collinearity, influential observations and outliers. Based on the outcomes of these tests, we arrived at our final model for estimating the OOP on PAC. We then developed a forecasting model for predicting the OOP for likely scenarios of PAC utilisation.
Ethical approval
Ethical clearance for this study was obtained from the National Council for Health Research (CNRS) of Senegal (Protocol SEN 17/53) and Western IRB in the United States (Protocol no. 20171739).
Results
Client and facility characteristics and the components of PAC
Overall, the mean age of women enrolled in the exit interview was 29 years. Although the protocol permitted enrolment of minors, in fact, the youngest participants in the study were 18 years-old. Most women had had at least two prior births and were married. Slightly more than one-third of women enrolled had completed primary school. The mean gestational age at the time of their complications was 9.5 weeks, and most women reported that their pregnancy had terminated five days prior to PAC. Most women received MVA (n = 310), followed by misoprostol (n = 158), EVA (n = 122), digital curettage (n = 51) and dilation and curettage (n = 5). A subset of women who could not recall procedure type reported that they had received an operation without offering more information on the nature of the procedure when prompted by the interviewer (n = 83). The majority of women did not face any waiting period upon admission to the facility. Less than one-fourth of women received counselling on the treatment procedure and one-third received pain relief medication (n = 241). Slightly more than this received information on when they could become pregnant again (n = 277). Fewer than half accepted a modern contraceptive method after treatment (n = 338), of whom a majority accepted a short-acting method (n = 300).
Estimation of out-of-pocket expenditures
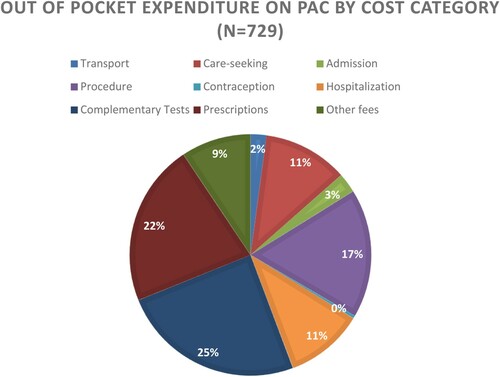
Among all women, average OOP on PAC was 51,427 FCFA (USD $93.84). Among clients who obtained care at hospitals it was 66,020 FCFA ($120.47) and among women who obtained care at health centres it was 35,880 FCFA ($65.47). The average cost of PAC administered using dilation and curettage 61,578 ($112.37), EVA 60,651 ($110.68), MVA was 54,710 FCFA ($99.84), digital curettage 47,814 ($87.25), and misoprostol 33,871 ($61.80). Among women for that could not recall the treatment method they received the average cost of PAC was 56,699 ($103.47). Among clients who utilised public or hired transport to access PAC, the average OOP on transport was 1501 FCFA ($2.74). Among those that sought care for abortion complications from an additional source before accessing PAC, the mean cost of additional care seeking was 15,518 FCFA ($28.32). The average cost of facility admission, treatment, and contraception, among women who received those services, respectively, was 2640, 13,086 and 678 FCFA ($4.82, $23.88, and $1.24). The average cost of hospitalisation, complementary tests, prescriptions and additional medications/materials/supplies, among women who received those services were 5669, 16,796, 13,146 and 9060 FCFA, respectively ($10.34, $30.65, $23.99 and $16.53). 4.8% women (n = 34) enrolled reported that they did not incur OOP expenditure on the PAC services they received.
shows the proportion of OOP expenditures incurred by women enrolled in the study that reported paying anything for PAC (n = 695) by cost category.
For the OOP expenditure model, all potential predictor variables used in this analysis are described in . We undertook steps to maximise the fit of the regression model to our data. In addition to comparing goodness of fit values, as discussed above, this further informed model selection by helping us to address how the presence of outliers in the data set and multi-collinearity was affecting model accuracy (Afifi, Citation1999; Cohen et al., Citation2003). In the end, we fit a multivariate log-linear regression model using cluster-robust standard errors to account for heteroskedasticity across facilities to a version of the dataset that removed extreme outliers (OOP ≤10,000 FCFA or ≥ 3-times the mean OOP for all observations, FCFA 154,281) (n = 648).
Table 4. Description of Predictor Variables of final fitted model, (n = 648).
The final fitted model included the following predictor variables: women’s parity, gestational age at time of abortion, whether they qualified for free treatment or had insurance that covered all or part of the cost of PAC, the type of facility where they obtained PAC, treatment type (reference MVA), uptake of a short-acting contraceptive method (SAM), uptake of a long acting reversible contraceptive method (LARC), whether the client used public or hired transportation, whether she received care before admission at the facility where she obtained PAC, supplementary tests, prescriptions, additional medications or materials, the number of payments incurred (reference ≤1 payment). Although our sample was confined to women who had obtained routine PAC for incomplete abortion, we still sought to identify variation in OOP that could be attributed to the severity of patients’ conditions. To do so, we also adjusted for length of hospitalisation (reference ≤ half day). The final fitted model for total OOP for PAC (i.e. ‘Y’) is below:
ln(Y) = 12.14 + 0.01parity + 0.01gest.age − 0.10rfree.insured – 0.31fac.type + 0.05evac.meth(EVA) − 0.36evac.meth(misoprostol) – 0.12.evac.meth(other) + 0.01stm.uptake + 0.01larc.uptake +0.14fac.stay(full.day) + 0.11fac.stay(over.night) + 0.16fac.stay(two.days) + 0.30fac.stay(three.days) + 0.37fac.stay(four.days) – 0.02transport – 0.113 additional.meds.mats – 0.26supp.tests – 0.10prescriptions – 0.30pre.pac.care– 0.02no.payments(two) + 0.16no.payments(three.more)
With the exceptions of women’s parity, use of EVA, postabortion contraceptive uptake (SAM and LARC), and whether women paid for PAC in two installments, all predictors were statistically significant at α = 0.05. Among the factors that were most appreciably associated with our outcome were the type of facility where PAC was received, whether the client was treated for her complications with misoprostol, whether the client received additional services and supplementary tests. After adjusting for all other covariates in our model, we found that women who received PAC at lower-level facilities, on average, incurred an OOP that was approximately 30.1% lower than that which was paid, on average, by PAC clients who received care at hospitals. Also, after adjusting for covariates in our model, women who received misoprostol for their complications paid, on average, 36% less than their counterparts that received MVA paid. In our analysis, women who did not receive additional services, such as food, pain medication and other medications, and supplementary tests and examinations, respectively, after covariate adjustment, were found to have paid, on average, 30% and 26% less than the OOP incurred, on average, than women who did receive those service components. Interestingly, relative to women who were only admitted for half of one day, or less, women who were admitted for one full day incurred an OOP that was, on average, 14% higher, again assuming all other covariates in the model are constant. This differential, when comparing those admitted for a half-day or less, with women who were admitted for 2-days is only slightly greater 16% ().
Table 5 . Shows the output of the final fitted model for OOP on PAC (n = 648).
We then tested our fitted model by predicting the cost of likely scenarios of future PAC utilisation based on possible cost category combinations observed in the original data. predictions assume mean values of parity, gestational age and length of stay at facility, that clients did not receive free care or have insurance that covers PAC, that clients did not receive contraception and that clients paid for PAC in one payment.
Table 6. Predicted OOP costs (FCFA) on PAC comparing clients who differ by duration of hospitalisation for PAC.
incorporates the same assumptions and structure of comparisons as the previous table; however, the predictions differ by cost category combinations where OOP is incurred.
Table 7. Predicted out-of-pocket expenditure on PAC from the fitted model (FCFA).* (USD = FCFA/548).
Discussion and conclusion
OOP expenditure, most often in the form of user fees, remains a principal means of financing healthcare across low-income countries, including Senegal (Chankova et al., Citation2008). Often, such payments result in decreased utilisation of health services, particularly among the poorest, and cumulatively OOP frequently become catastrophic and push households into poverty (Dagenais et al., Citation2013; Gilson, Citation1997; Palmer et al., Citation2004; van Doorslaer et al., Citation2006). Fear of this promotes delayed recourse for needed medical care or use of ineffective, often unprofessional care, in lieu of costly evidence-based services by appropriately trained service providers. When this occurs, care seeking for patients, who frequently obtain professional care late and after their complications have become more advanced, can become costly, and risks of long-term morbidity, and fatality, can increase (Lassi et al., Citation2019; Nahar et al., Citation2011).
This study is among the first attempts to identify the drivers of the expenditure that clients face when in need of care for abortion complications in a country where the demand for this service is high and largely unmet (Sedgh et al., Citation2015). By focusing our study on women who experience complications during their first trimester of pregnancy only, our results may only be generalisable to this subset of women who come to require PAC. However, the results of this are illuminating: the one-time cost that women incur to obtain treatment arising from first trimester abortions, of which the vast majority are non-severe and do not require advanced surgical techniques, is equal to, approximately 15% of the average monthly salary of women in Dakar.
The collection of detailed information regarding the treatment routes and all the associated costs permitted our analysis to disaggregate our findings across the spectrum of service delivery components that underlie high costs for women who, mostly, had not completed primary school, were low income and did not have insurance or were not eligible for PAC at a reduced cost. Our analysis projects that a woman that receives PAC for first trimester abortion complications at a hospital will pay, on average approximately 30% more for care that includes the same services than would a woman treated a lower-level facility. This is consistent with similar studies which report how PAC provided in tertiary hospitals is estimated to cost more to health systems as well (Baynes et al., Citation2019a; Grimes et al., Citation2006; Lince-Deroche et al., Citation2020). This study reports that within the same level of care, among women admitted for abortion complications in the first trimester that receive misoprostol will, on average, pay 36% less than a counterpart that receive MVA. Furthermore, a PAC client treated a lower-level site and discharged from care within a half day, will pay, on average, approximately half the mean cost of PAC services that were reported by clients in our study. These findings suggest a need to expand the use of misoprostol for first trimester PAC, at lower-level facilities, such as health centres and health posts. In other countries, increased awareness and training of pharmacists and pharmacy workers about medication treatment for abortion complications has been identified as key maternal health strategy (Sneeringer et al., Citation2012). Making misoprostol available for PAC in primary care settings, the first line of care before recourse to hospitals, will bring the service closer to homes and communities where women usually first recognise the onset of complications, make timely access of care more feasible for women and, as this analysis reports, help women avoid excessive costs.
Recourse to care at other sites before receiving PAC at study facilities was only associated with moderately higher OOP, on average 13% higher relative to that of those that did not. Such differences in the average cost of PAC were observed between clients who were hospitalised for two days or more relative to those that received treatment as outpatients and were discharged after less than half a day. Descriptive analyses of the survey data also demonstrated that among PAC clients who were hospitalised for two-days (n = 141) or more, a majority (n = 117) resorted to other sites for treatment before accessing PAC and endured higher than average delays in attaining PAC after their pregnancy had ended. In other words, recourse to ineffective treatment for complications, delays in obtaining professional appropriate care and long hospitalisations are deeply intertwined, and, it seems according to our data, ultimately result in high levels of OOP borne by clients themselves.
In our study, women who received ‘additional’ medications and materials, such as pain relief medication, on average, paid 30% more for PAC than did women who did not. Recent studies on PAC from other countries in sub-Saharan Africa have also documented the tendency of public health services to separate pain relief from the essential components of treatment for abortion complications (Baynes et al., Citation2019b). This raises ethical questions as to whether PAC clients who present with complications, but with fewer funds, are less entitled to a more comfortable procedure than other clients who can afford additional medications. In either case, this study calls for consideration to include such services within an essential service package to which all PAC clients are entitled.
Although this study did not capture information on the costs incurred by the health system to make PAC available to clients, it does observe that health facilities recover such costs through a fragmented process that includes multiple independent OOP payments from clients. Over one-fourth of all clients were required to make three or more independent payments for the care they received before discharge. Our model demonstrates that holding values for whether clients received specific components of PAC constant, that women who incurred more than two payments, on average, paid 16% more for PAC compared with those that incurred one payment only. This suggests that improving the affordability of PAC, without undermining facilities’ need to recover health systems costs, might include the bundling of payments into as few payments as possible. The fact that fewer than half of women accepted a contraceptive method after receiving PAC illuminates a crucial opportunity. In several of the hospitals and health centres where this study took place, in which PAC and contraceptive services are provided, and paid for, in separate areas of facilities, integrating services for the emergency treatment and family planning components of PAC may help to increase women’s opportunity to access postabortion contraception, meet their birth spacing needs and, thereby, reduce future unintended pregnancies and their negative sequelae. Helping women to manage the timing of their future fertility, by making postabortion contraception accessible at the same time in the same place as PAC itself, is shown to help women avoid future complications and the need to resort to PAC (Curtis et al., Citation2005). Although, the study could not ascertain whether the complications for which participants received PAC were due to induced or spontaneous abortions, the findings lend support to the economic argument for making safe abortion care more accessible. Evidence from other countries in sub-Saharan Africa indicate that safe abortion care is more cost-effective than PAC, due to the lower financial burden it imposes on clients and systems and the fact that it pre-empts expensive life-threatening complications from arising in the first place (Gebreselassie et al., Citation2010; Ilboudo et al., Citation2015; Leone et al., 2016; Parmar et al., Citation2017).
This study has limitations that are important to consider. Because they were considered the only convenient source of data on PAC service volume, researchers relied on register data for information used to select facilities for the study. It is important to acknowledge the evidence, also from Senegal, that indicates that register data often provide inaccurate counts of women treated for incomplete abortion (Suh, Citation2018). The study is based on data collected in Dakar, the capital city, which may not be representative of the national situation in Senegal where most of the population reside in less urban areas. One way in which this may have affected this study concerns findings regarding the evacuation methods performed on participants. For example, Suh (Citation2019) reveals that the practice of digital curettage persists throughout Senegal, particularly in rural settings, and how this is unrecognised owing to the primacy of MVA statistics in global and national monitoring and evaluation regimens on PAC. This study found that more than one out of five PAC clients received digital curettage, an amount that is not trivial, and, yet, likely lower than what it is, on average, throughout the rest of the country, outside Dakar, where facilities might experience more frequent stock outs of essential supplies and commodities for performing PAC with MVA or misoprostol. The data from the exit interview does not permit precise estimations of women’s socio-economic status, and from the information gathered, we are unable to determine how the OOP on PAC might have truly affected clients’ financial position and that of their families. The study only explored women’s OOP on healthcare and does not consider the wider economic costs they faced in terms of diminished productivity before and after PAC, the need to obtain additional care subsequent to discharge and the interview, and what the funds might have been used for but for the OOP expenditure. The study enrolled women in PAC settings of facilities where women are triaged for the management, typically, of relatively non-severe cases of incomplete abortion, rather than in obstetric and gynecological wards where women may be sent after admission for advanced complications. Accordingly, this analysis is largely limited to cases of incomplete abortion by routine provision of PAC. For some information used in the analysis, the study depended on women’s estimates, e.g. for gestational age and the number of days they had been suffering from complications for obtaining PAC, and these estimates may have been imprecise. Additional potential for inaccuracy arises from the possibilities that participants did not receive receipts for care received prior to admission despite incurring expense, and that participants paid bribes or unofficial payments to facilities’ staff that were not recorded. If either occurred, then the true OOP may be higher than our estimate. Finally, the study may have too narrowly focused on the financial aspects of PAC, limiting its capacity to pinpoint other influences on the quality of care. There are numerous studies on PAC that have described the tendency of providers to withhold evidence-based care, such as MVA and pain medication, for PAC clients based on suspicion that they had sought illicit abortions (Billings et al., Citation2007; Suh, Citation2019). In these situations, clients are discriminated against due to provider biases or fears that using stock of carefully monitored supplies might implicate them in an illegal practice. Although this study was not designed to identify it, as this tendency has been observed in Senegal, it is sensible to assume that, in addition to costs, discrimination is also a barrier to the quality of PAC services.
In conclusion, to maximally benefit its female population, the Government of Senegal should build on the widespread availability of PAC that it has established since the 1990s by directing concerted attention to barriers, including clients’ OOP. By making misoprostol available at lower-level facilities, promoting timely recourse to professional PAC, strengthening integration of emergency treatment and family planning services, and streamlining cost recovery schemes, the health system of Dakar can make large improvements in utilisation of PAC. Until the financial burden of PAC is improved in Senegal, the potentially catastrophic health and economic consequences of abortion complications for women and their families in the country will persist.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data from this study are not publically available due to IRB restrictions and regulations of the Government of Senegal. Materials such are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Afifi, A. (1999). Computer-aided multivariate analysis (3rd). Chapman & Hall/CRC.
- Agence Nationale de la Statistique et de la Demographie (ANSD) and ICF International. (2014). Senegal: Continuous demographic and health survey. ANSD.. and Rockville: ICF International.
- Babigumira, J. B., Stergachis, A., Veenstra, D. L., Gardner, J. S., Ngonzi, J., Mukasa-kivunike, P., & Garrison, L. P. (2011). Estimating the costs of induced abortion in Uganda : A model-based analysis. BMC Public Health, 11(1), 904. https://doi.org/10.1186/1471-2458-11-904
- Bankole, A., Remez, L., Owolabi, O., Philbin, J., & Williams, P. (2020). From unsafe to safe abortion in Sub-Saharan Africa: Slow but steady progress. Guttmacher Institute. https://www.guttmacher.org/report/from-unsafe-to-safe-abortion-in-subsaharan-africa. doi:10.1363/2020.32446.
- Bankole, A., Singh, S., Vlassof, M., & Woog, V. (2007). Estimating the cost of post-abortion care in Nigeria: A case study. (Fertility regulation behaviors and their costs). The World Bank.
- Barrero, L. I., Castillo, J. S., Leal, A. L., Sanchez, R., Cortes, J. A., Alvarez, C. A., & Gonzalez, A. L. (2014). Economic burden of methicillin-resistant staphylococcus aureus bacteremia in critical care patients in hospitals in Bogotá. Biomedica: Revista del Instituto Nacional de Salud, 34(3), 345–353. https://doi.org/10.7705/biomedica.v34i3.1692
- Baynes, C., Yegon, E., Kimaro, G., Lusiola, G., & Kahwa, J. (2019a). The unit and scale-up cost of postabortion care in Tanzania. Global Health Science and Practice, 7(Supplement 2), S327–S341. https://doi.org/10.9745/GHSP-D-19-00035
- Baynes, C., Yegon, E., Lusiola, G., Kahando, R., Ngadaya, E., & Kahwa, J. (2019b). Women’s satisfaction with and perceptions of the quality of postabortion care at public-sector facilities in mainland Tanzania and in Zanzibar. Global Health Science and Practice, 7(Supplement 2), S299–S314. https://doi.org/10.9745/GHSP-D-19-00026
- Billings, D., Crane, B., Benson, J., Solo, J., & Fetters, T. (2007). Scaling up a public health innovation: A A comparative study of postabortion care in Bolivia and Mexico. Social Science and Medicine, 64(11), 2210–2222. https://doi.org/10.1016/j.socscimed.2007.02.026
- Bullough, C., Meda, N., Makowiecka, K., Ronsmans, C., Achadi, E. L., & Hussein, J. (2005). Current strategies for the reduction of maternal mortality. BJOG: An International Journal of Obstetrics and Gynaecology, 112(9), 1180–1188. https://doi.org/10.1111/j.1471-0528.2005.00718.x
- Chankova, S., Sulzbach, S., & Diop, F. (2008). Impact of mutual health organizations: Evidence from West Africa. Health Policy and Planning, 23(4), 264–276. https://doi.org/10.1093/heapol/czn011
- Cohen, J. C. P., West, S. G., & Aiken, L. S. (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd). Lawrence Erlbaum Associates, Inc.
- Curtis, B. C., Curtis, C., & Moss-, T. (2005). Postabortion Family Planning : Addressing the Cycle Of Repeat Unintended Pregnancy and Abortion.
- Dagenais, C., Queuille, L., & Ridde, V. (2013). Evaluation of a knowledge transfer strategy from a user fee exemption program for vulnerable populations in Burkina Faso. Global Health Promotion, 20(SUPPL.1), 70–79. https://doi.org/10.1177/1757975912462416
- Diadhiou, M., Dieng, T., Diop, N., Faye, Y., Moreau, J. C., Thieba, B., Hijazy, Y., Traore, B., Nayama, M., & Akpadza, K. (2008). Les soins après avortement en Afrique de l’Ouest Francophone, 10 ans après: leçons apprises de leur introduction et de leur in- stitutionnalisation. J. Soc. Afr. Gynecologues Obstetriciens, 9(2), 38–46.
- Gaye, A., Diop, A., Shochet, T., & Winikoff, B. (2014). Decentralizing postabortion care in Senegal with misoprostol for incomplete abortion. International Journal of Gynecology and Obstetrics, 126(3), 223–226. https://doi.org/10.1016/j.ijgo.2014.03.028
- Gebreselassie, H., Fetters, T., Singh, S., Abdella, A., Gebrehiwot, Y., Geressu, T., & Kumbi, S. (2010). Caring for women with abortion complications in Ethiopia: National estimates and future implications. International Perspectives on Sexual and Reproductive Health, 36(1), 6–15. https://doi.org/10.1363/ipsrh.36.006.10
- Gilson, L. (1997). The lessons of user fee experience in Africa. Health Policy and Planning, 12(4): 273–285. https://doi.org/10.1093/oxfordjournals.heapol.a018882
- Grimes, D., Benson, J., Singh, S., Romero, M., Ganatra, B., Okonofua, F. E., & Shah, I. (2006). Unsafe abortion: The preventable pandemic. Lancet, 368(9550), 1908–1919. https://doi.org/10.1016/S0140-6736(06)69481-6
- Ilboudo, P., Greco, G., Sundby, J., & Torsvik, G. (2015). Costs and consequences of abortions to women and their households: A cross-sectional study in Ouagadougou, Burkina Faso. Health Policy and Planning, 30(4), 500–507. https://doi.org/10.1093/heapol/czu025
- Lassi, Z., Middleton, P., Bhutta, Z. A., & Crowther, C. (2019). Health care seeking for maternal and newborn illnesses in low- and middle-income countries: A systematic review of observational and qualitative studies: [version 1; peer review: 2 approved]. F1000 Research, 8, 1–14. https://doi.org/10.12688/f1000research.17828.1
- Le Centre de Formation, Recherche et de Plaidoyer en Santé de la Reproduction (CEFOREP). (1998). Introduction des soins obstétricaux d’urgence et de la planification familiale pour les patients présentant des complications liées à un avortment incomplete. Dakar, Senegal.
- Leive, A., & Xu, K. (2008). Coping with out-of-pocket health payments: Empirical evidence from 15 African countries. Bulletin of the World Health Organization, 86(11), 849–856. https://doi.org/10.2471/BLT.07.049403
- Lince-Deroche, N., Sene, I., Pliskin, E., Oluwadamilola, O., & Bankole, A. (2020). The health system costs of postabortion care in Senegal. International Perspectives on Sexual and Reproductive Health, 46, 99–112. https://doi.org/10.1363/46e9220
- Nahar, S., Banu, M., & Nasreen, H. (2011). Women-focused development intervention reduces delays in accessing emergency obstetric care in urban slums in Bangladesh: A cross-sectional study. BMC Pregnancy and Childbirth, 11(1), 1–10. https://doi.org/10.1186/1471-2393-11-11
- Palmer, N., Mueller, D., Gilson, L., Mills, A., & Haines, A. (2004). Health financing to promote access in low income settings - How much do we know? Lancet, 364(9442), 1365–1370. https://doi.org/10.1016/S0140-6736(04)17195-X
- Parmar, D., Leone, T., Coast, E., Farily Murray, S., Hukin, E., & Vwalika, B. (2017). Cost of abortions in Zambia: A comparison of safe abortion and post abortion care. Global Public Health, 12(2), 236–249. https://doi.org/10.1080/17441692.2015.1123747
- Sedgh, G., Hassane Sylla, A., Philbin, J., Keogh, S., & Ndiaye, S. (2015). Estimates of the incidence of induced abortion And consequences of unsafe abortion in Senegal. International Perspectives on Sexual and Reproductive Health, 41(1), 11–19. https://doi.org/10.1363/4101115
- Singh, S., & Maddow-Zimet, I. (2015). Facility-based treatment for medical complications resulting from unsafe pregnancy termination in the developing world, 2012 : A review of evidence from 26 countries. British Journal of Obstetrics and Gynaecology, 123(9), 1489–1498. https://doi.org/10.1111/1471-0528.13552
- Singh, S., Remez, L., Sedgh, G., Kwok, L., & Onda, T. (2018). Abortion worldwide 2017: Uneven progress and unequal access. Guttmacher Institute. https://www.guttmacher.org/report/abortion-worldwide-2017.
- Sneeringer, R., Billings, D., Ganatra, B., & Baird, T. (2012). Roles of pharmacists in expanding access to safe and effective medical abortion in developing countries: A review of the literature. Journal of Public Health Policy, 33(2), 218–229. https://doi.org/10.1057/jphp.2012.11
- Suh, S. (2018). Accounting for abortion: Accomplishing transnational reproductive governance through post-abortion care in Senegal. Global Public Health, 13(6), 662–679. https://doi.org/10.1080/17441692.2017.1301513
- Suh, S. (2019). What post-abortion care indicators don’t measure: Global abortion politics and obstetric practice in Senegal. Social Science and Medicine, 254(112248): 1–9. https://doi.org/10.1016/j.socscimed.2019.03.044
- Turner, K., & Corbett, M. (2003). Essential elements of postabortion care : Origins, evolution and future directions. International Family Planning Perspectives, 29(3), 106–111. https://doi.org/10.2307/3181075
- van Doorslaer, E., O'Donnell, O., Rannan-Eliya, R. P., Somanathan, A., Adhikari, S. R., Garg, C. C., Harbianto, D., Herrin, A. N., Nazmul Huq, M., Ibragimova, S., Karan, A., Wan Ng, C., Pande, B. R., Racelis, R., Tao, S., Tin, K., Tisayaticom, K., Trisnantoro, L., Vasavid, C., & Zhao, Y. (2006). Effect of payments for health care on poverty estimates in 11 countries in Asia: An analysis of household survey data. Lancet, 368(9544), 1357–1364. https://doi.org/10.1016/S0140-6736(06)69560-3
- Vargas-Alzate, C., Higuita-Gutiérrez, L. F., López-López, L., Cienfuegos-Gallet, A. V., & Jiménez Quiceno, J. N. (2018). High excess costs of infections caused by carbapenem-resistant gram-negative bacilli in an endemic region. International Journal of Antimicrobial Agents, 51(4), 601–607. https://doi.org/10.1016/j.ijantimicag.2017.12.012
- World Health Organization. (2012). Safe abortion: technical and policy guidance for health systems. Geneva. http://apps.who.int/iris/bitstream/handle/10665/70914/9789241548434_eng.pdf;jsessionid=73B891FF768069BD1F799094D1E1D35A?sequence=1.
- World Health Organization. (2015). Health worker roles in providing safe abortion care and postabortion contraception. Geneva, Switzerland.