 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Communities’ knowledge and management strategies are crucial for mitigating and controlling the threat of existing and emerging diseases. In this study, we conducted randomised control trials (RCT) to examine the impact of health education on households’ knowledge and management of three Arboviral Diseases (ADs); Rift Valley fever, Chikungunya fever, and Dengue fever in Kenya. The study was based on a sample of 629 households drawn from the three of Kenya’s AD hotspot counties; Baringo, Kwale, and Kilifi. Employing a difference-in-difference method, our findings indicate that health education intervention significantly improved households’ understanding of ADs transmission modes, causes, and prevention strategies. However, this intervention did not sufficiently influence households’ disease management behaviour. We recommend the implementation of community engagement and outreach initiatives which have the potential to drive behavioural changes at the household level, thus enhancing the management and control of ADs in Kenya.
1. Introduction
Arboviral diseases (ADs) present a significant global health and socioeconomic burden, causing profound human and animal casualties (Ahmed et al., Citation2020; Medone & Hernández-Suárez, Citation2019). For instance, Dengue fever affects about 390 million people worldwide (Bhatt et al., Citation2013), with an estimated economic burden of US$8.9 billion annually (Shepard et al., Citation2016). ADs notably diminish patients’ quality of life, with communities lacking sufficient resources often disproportionately affected (LaBeaud et al., Citation2008). The risk of ADs has increased worldwide due to the expansion of vector habitats driven by climate change (Marchi et al., Citation2018; Paixão et al., Citation2018). However, a scarcity of evidence about the risk of ADs and health system’s capacity in Africa constrains the development of strategic interventions to manage these diseases (Ngoi et al., Citation2016).
Despite concerted efforts to identify potential vaccine-candidate antigens, only dengue fever currently has viable human vaccines (Thomas, Citation2023; Torres-Flores et al., Citation2022). In Kenya, periodic data collection on mosquito infestation is undertaken to comprehend the risk factors associated with the ADs (Forsyth et al., Citation2020; Heath et al., Citation2020; Kamau et al., Citation2023; Karungu et al., Citation2019; Ngugi et al., Citation2020). However, preventive interventions rely on vector control such as surveillance, applying synthetic pesticides, and insect growth regulators (Karungu et al., Citation2019; Mutero et al., Citation2020).
Inadequate awareness of the diseases and limited understanding of the infection pathways have hindered their effective uptake (Abdi et al., Citation2015; Bellini et al., Citation2014; Paixão et al., Citation2018). Previous studies have emphasised the importance of information in increasing awareness and curbing the costly interventions linked to community-based vector control (Abel Mangueira et al., Citation2019; Hermida et al., Citation2021; Santos et al., Citation2022; Sukesi et al., Citation2021; Usman et al., Citation2018). Therefore, health information campaigns are considered as the most effective long-term strategy for preventing Ads (Boonchutima et al., Citation2017; Medone & Hernández-Suárez, Citation2019).
This paper evaluates the impact of health education on rural households’ knowledge and management of three prevalent ADs (Rift Valley Fever (RVF), chikungunya, and dengue fevers) in Kenya using Randomised Controlled Trials (RCT). Although research on the effect of health education on ADs control in the developing world is scant, a few studies have explored the influence of health education on knowledge and practice related to a single AD (AhbiRami & Zuharah, Citation2020; Arneliwati & Dewi, Citation2019; Sukesi et al., Citation2021; Usman et al., Citation2018). However, no studies have evaluated the effect of health education on the three prevalent ADs in Kenya.
Grasping the impact of health education on understanding and managing multiple ADs is vital for devising evidence-based control measures and policies for human and animal health. Drawing from Santos et al. (Citation2022), this study is based on the behaviour change theories recommended by the World Health Organization (WHO) for delivering effective evidence at both individual and community levels (Michie & Johnston, Citation2012; Michie & West, Citation2013). For instance, the Health Belief Model suggests that behaviour and decision-making are shaped by the perceived susceptibility, diseases severity, barriers and benefits (Davis et al., Citation2015). Thus, managing ADs is likely to be hinged on certain beliefs or attitudes that favour preventive behaviour.
We evaluate the effect of health education training by comparing knowledge and management differences between trained and untrained households. We employ the difference-in-difference (DiD) method and a fixed effects regression to account for the unobserved heterogeneity (Muriithi et al., Citation2016). The proceeding section outlines the study’s methodology, and the subsequent sections present and discuss the results. The paper concludes with policy recommendations.
2. Materials and methods
2.1. Study design and sampling
This study was conducted in three counties of Kenya (Baringo, Kilifi, and Kwale), where ADs are prevalent (). Baringo County, located in Kenya’s Rift Valley, is endemic in dengue fever, yellow fever and RVF (Kamau et al., Citation2023). Kwale and Kilifi counties in the Kenyan coastal region are prone to dengue and chikungunya fevers (Khan et al., Citation2023; Lim et al., Citation2020; Nyamwaya et al., Citation2022). Malaria and lymphatic filariasis are other common mosquito-borne diseases in the study sites (Ndenga et al., Citation2017). To understand the most critical ADs in the study sites, focus group discussions (FGD) and key informant interviews (KII) were conducted. The community members indicated that RVF, chikungunya, and dengue fevers were the most prevalent ADs.
Figure 1. Health education training sites in Baringo, Kwale, and Kilifi Counties, Kenya. Source: icipe GIS unit.
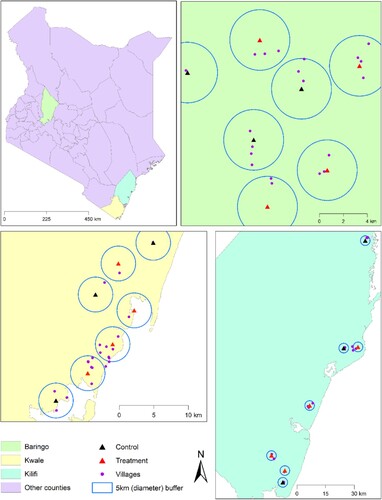
Between January and February 2021, a health education intervention was carried out by a research team from the International Centre of Insect Physiology and Ecology (icipe) in the study areas. The intervention involved two physical training days and the distribution of ADs awareness leaflets in the local language among communities in the intervention areas. The training covered ADs transmission modes, causes, and preventive measures and was delivered in the local language in each County (see details in Section 2.2).
2.2. Treatment description
In this study, 140 rural households were selected in each County and trained on ADs’ signs, symptoms, transmission methods, and prevention by the icipe team using standard training manuals. The training involved inviting randomly selected households to village meetings/workshops through their village guides or local community health workers. Although community members were encouraged to attend the training, the decision to attend was entirely voluntary. Two lecture training sessions were undertaken between January and February 2021, each lasting 5 hours with breaks in between.
During each session, the participants were taken through different training components covering how ADs are spread, what triggers their outbreak, the impact of the diseases on livestock, humans, and the economy, how one can become infected, who is at higher risk of getting infected, how one can we tell when the diseases are likely to happen, what to do when there are indications of high risk and how to prevent the diseases. After the indoor lecture, the participants visited the surrounding areas for practical lessons about identifying potential mosquito breeding sites and their control. Eventually, all participants were issued informative materials written in English but translated into Swahili for easy understanding. Finally, the participants were encouraged to eradicate mosquito breeding sites in their compounds once back home.
2.3. Data
The study used two waves of panel data gathered from a survey of 629 households using a semi-structured questionnaire. Throughout the research process, we diligently upheld all ethical considerations. The research protocol for this study was submitted and approved by the Scientific and Ethics Review Unit (SERU) of the Kenya Medical Research Institute (KEMRI)’s under reference number 3312.
The study commenced with acquiring verbal informed consent from respondents, underscoring the voluntary nature of their participation. The consent read,
Dear Sir/Madam, I work for icipe. We are conducting a survey to evaluate the effect of educational training on the knowledge and management of arboviral diseases in your village. Your responses will remain anonymous. Participation is voluntary, and if you choose not to participate without any consequences. Do you agree to participate in the interview?
The baseline survey was conducted between February and March 2020 to document the households’ socioeconomic status before the health education training intervention was introduced. For data collection, a semi-structured questionnaire programmed into the Census and Survey Processing System (CSPro) electronic data collection application was used. Our trained enumerators conducted face-to face interviews, gathering data on respondents’ knowledge of signs, symptoms, transmission, prevention, and management of various ADs. Respondents were asked:
Have you ever heard of RVF, chikungunya and dengue fevers? Do you consider these diseases a serious health problem in your area/village? Which ways do you believe a person can get infected with these diseases? Which insect is responsible for transmitting RVF, chikungunya and dengue fevers? Can you identify the signs and symptoms of RVF, chikungunya and dengue fever? How can a person be protected from contracting these diseases? What would your household do if you suspected an infection with these diseases within your family?
After implementing the health education training intervention, we conducted an endline survey of the same households between September and October 2021, in accordance with Kenya’s Ministry of Health COVID-19 guidelines. Due to some respondents’ unavailability, our sample was reduced to 574 households. The average attrition rate between the baseline and endline surveys was 9%, and between the treatment and control groups, it was 4% (Table A1 in the Appendix).
Non-random attrition may bias the results, thus, we analysed the association between attrition and baseline socioeconomic variables using the probit model (Ogutu et al., Citation2020). Only a few variables had significant associations with the attrition rate (Table A2 in the Appendix). Hence, we controlled for attrition bias by applying the inverse probability weighting approach in the DiD models (Wooldridge, Citation2002). The doubly robust DiD estimators for the average treatment effect command ‘drdid’ available in STATA software (Rios-Avila et al., Citation2021), were used to estimate the Average Treatment Effect on the Treated (ATT). The strength of this method is that the resulting estimates identify the ATT, even if either (but not both) the outcome regression models or the propensity score model are misspecified (Sant’Anna & Zhao, Citation2020).
2.4. Empirical estimation and identification
The effect of health education training on knowledge and management of the three ADs prevalent in the study counties was assessed using the following DiD specification (Ogutu et al., Citation2020):
(1)
(1) where yitc is the outcome variable of interest (knowledge and management) for respondent i at period t (where t = 0 and t = 1 for baseline and follow-up surveys, respectively), and c represents the study county (1 = Baringo, 2 = Kwale, 3 = Kilifi), Post is a period dummy variable taking a value of 1 for follow-up and 0 for baseline survey, Treat is the treatment dummy with values of 1 and 0 for treatment and control groups, respectively, while Wic and Cc captured individual household and county fixed effects, respectively. EquationEquation (1
(1)
(1) ) was estimated as a quasi-maximum likelihood fractional probit model (FPM) due to its flexibility and ability to generate more efficient and robust estimates than the full-information maximum likelihood methods (Papke & Wooldridge, Citation1996).
The causal effect of the treatment on knowledge and management scores in EquationEquation (1(1)
(1) ) was measured by β3 under the identifying assumption that Treat was orthogonal to ϵic following Shikuku et al. (Citation2019), the identifying assumption was satisfied by the random assignment of treatment to selected villages with minimal potential spill-over effects by allowing a 2.5 km distance between contiguous clusters. Knowledge spill-overs could occur between treatment and control groups, contaminating the treatment and leading to biased results and hence the random assignment of the treatments.
We employed two design features to reduce potential unintended effects. First, researchers assigned similar treatments to adjacent villages through cluster randomisation to reduce the likelihood of farmers knowing the treatments given to neighbours and therefore reduce potential spill-overs (Duflo et al., Citation2007). We used the baseline data to conduct a covariate balancing test to assess how effective the randomisation process was. The results in indicate that most variables were balanced between the control and treatment groups. Thus, the randomisation bias is not a major problem (Barrett & Carter, Citation2010). Secondly, no evaluation was mentioned either during the interviews or implementation of the treatments. Therefore, individuals in the treatment group were unaware of the evaluation, reducing the likelihood of their attempts to impress the evaluator. On the other hand, those in the control group could not feel disappointed for not receiving the training.
Table 1. Baseline household socio-demographic characteristics.
2.5. Measurement of variables
2.5.1. Dependent variables
Two outcome variables were used in this study: knowledge and management scores of prevalent ADs. The knowledge score was constructed from respondents’ understanding of AD transmission, signs and symptoms, and management practices. The actions taken by households to prevent the spread of ADs were used to compute the management score. To construct the knowledge score of each AD (RVF, chikungunya, and dengue fevers), 14 binary response questions were used, while seven binary response questions were used to compute the management score.
Following Nyangau et al. (Citation2021), the intensity of knowledge and that of management practices was computed as a fraction of the sum of correct answers divided by the total number of questions per dependent variable. The intensity of knowledge of RVF for instance was measured as a ratio of the number of correct responses out of the 14 knowledge questions. The intensity of the diseases management practices was measured as a share of correct responses on management out of the seven questions. Recall and self-reported data were used in measuring knowledge and management. These methods are commonly used when assessing individuals’ awareness and knowledge levels (Muthini et al., Citation2019). Even after long periods, no major biases have been attributed to recall data collected from self-reports (Beegle et al., Citation2012).
2.5.2. Independent variables
The literature informed the choice of covariates used in EquationEquation (1(1)
(1) ) (Abdi et al., Citation2015; Affognon et al., Citation2017; Dhimal et al., Citation2014; Harapan et al., Citation2018; Mallhi et al., Citation2018; Nyangau et al., Citation2021). The variables included household demographic characteristics, access to health information, social capital, and asset ownership. Household heads’ education, gender, and religion dummies were used to control for the heterogeneity of households.
Education was measured as the number of formal schooling years completed by the household head. In contrast, gender was measured as a dummy variable, with male household heads taking a value of one and zero otherwise. The expected sign for education on knowledge and management of ADs was positive (Dhimal et al., Citation2014; Nguyen et al., Citation2019). Religion was a categorical variable coded as 0, 1, and 2 to represent other religions, Christianity and Islam. Religion was hypothesised to have indeterminate effects on the respondents’ knowledge and management of the ADs (Chandren et al., Citation2015; Harapan et al., Citation2018).
The distance to the nearest health facility and experience of ADs were used as proxies for a household’s access to information. In this study, the distance was measured as the amount of time in walking minutes taken to reach the nearest health facility, while the experience was elicited as a dummy variable taking the value of one if a family member suffered from any AD in the past year preceding the survey and zero otherwise. Distance to nearest health facility was hypothesised to be negatively (Nyangau et al., Citation2021) related to knowledge and management of ADs, while experience would have a positive influence (Abdi et al., Citation2015; AhbiRami & Zuharah, Citation2020) on these outcome indicators.
Group membership was used as a proxy for a household’s social network and took a value of one if a household member belonged to a health promotion group and zero otherwise. Group membership was hypothesised to be positively associated with knowledge and management of ADs. Two variables were used to measure the household’s asset endowment: the number of tropical livestock units (TLU) and income earned by household members from all enterprises within the last 12 months (measured in Kenya shillings). Households with more livestock units and income were expected to have more knowledge and management skills (Castro et al., Citation2013; Nyangau et al., Citation2021; Selvarajoo et al., Citation2020).
3. Results
3.1. Descriptive statistics
3.1.1. Household socio-demographic profiles
presents the baseline socio-demographic characteristics of households disaggregated by treatment status. The t-test results of the difference of means showed no significant differences in education, gender, dengue fever experience, group membership, and income between the treatment and control groups, suggesting the two groups were balanced at baseline for these variables. However, the proportion of households affected by RVF and chikungunya fever was significantly higher in the treatment group than in the control group at baseline, indicating higher exposure levels to these diseases. Notably, the two groups had no significant difference regarding their prior experience with dengue fever. The average time taken by the control group to reach the nearest health facility was significantly longer than the treatment group. Furthermore, households in the treatment group owned more livestock than those in the control group.
3.1.2. Post-training knowledge and management scores
illustrates changes in knowledge and management scores among the treatment groups, pre- and post-health education training. The average knowledge score at baseline was below 0.4 across all three ADs but saw 43% increase to 0.6 post-training. This indicates that health education improved households’ knowledge of ADs. Of the three diseases, dengue fever exhibited the highest increase in knowledge. Similarly, the management scores of all ADs rose by at least 72% post-training, with RVF showing the highest increase. This suggests that the health education enhanced households’ capacity to manage these diseases.
The post-training knowledge and management scores in demonstrate a positive impact of health education intervention by County. After the training, there was a substantial increase in the understanding of ADs, with Kwale County reporting the highest increase in RVF knowledge scores, Kilifi and Baringo Counties showed the highest improvement in knowledge on Chikungunya and Dengue fevers. All three study sites reported a rise in their ADs management scores, indicating better disease control strategies. Kwale residents marked a noteworthy 168% improvement in RVF management scores, while Kilifi residents experienced 61% and 75% advancements in managing chikungunya and dengue fevers, respectively. While these findings are descriptive, they underscore the effectiveness of the health education intervention in enhancing the understanding and management capabilities of ADs.
Table 2. Changes in knowledge and management scores before and after training.
3.2. Econometric results
3.2.1. Effect of health education on knowledge and management of ADs
presents the estimates of the effect of health training on the knowledge and management of ADs in Kenya. On average, health educational training had a positive and significant influence on knowledge of ADs, at least at the 5% level, except for the understanding of Chikungunya fever. Respondents that attended the training were 9%, 10%, and 15% points more knowledgeable than their counterparts regarding RVF, chikungunya, and dengue fevers, respectively. However, the health education training did not affect the respondents’ disease management practices.
Table 3. Double robust DiD estimates of the effect of health education training on the intensity of knowledge and management of ADs in Kenya.
4. Discussion
This study evaluated the effect of health education training on the knowledge and management of ADs in Kenya. Our evaluation of the randomisation process’s effectiveness via a covariate balancing test showed that most variables were balanced across the treatment and control groups. We employed Difference-in-Difference (DiD) estimators to determine the effects of the training’s. Although, there was some attrition between the baseline and endline surveys, the attrition rate difference between the treatment and control groups was minimal. Nevertheless, to address the potential non-random attrition bias, we employed a doubly robust DiD approach (Sant’Anna & Zhao, Citation2020).
Overall, all study sites showed an increased awareness of the three ADs, aligning with prior studies suggesting that education positively influences households’ ADs knowledge (AhbiRami & Zuharah, Citation2020; Sreedevi et al., Citation2016; Suwanbamrung et al., Citation2015). A broader understanding of ADs, including transmission pathways, disease symptoms, and preventive measures, is crucial for households in mitigating these diseases. Thus, more frequent awareness campaigns targeting individuals in endemic regions should be pursued.
Contrary to initial expectations, the health education training did not have substantial impacts on respondents’ disease management strategies, potentially due to the limited duration of the Randomised Controlled Trial. This implies that the training did not markedly alter the household behaviour towards disease management, such as seeking hospital treatment, using treated bed nets, clearing bushes, draining stagnant water, and properly disposing of containers or covering water-holding containers.
Earlier studies have reported mixed results regarding the shifts in knowledge and behaviour following an intervention. Notably, the studies by Arneliwati and Dewi (Citation2019), Santos et al. (Citation2022), Sukesi et al. (Citation2021) and Usman et al. (Citation2018) reported increased knowledge and self-reported practices, whereas Abel Mangueira et al. (Citation2019) and AhbiRami and Zuharah (Citation2020) found improved knowledge but unchanged attitudes, beliefs, and practices regarding dengue fever. Additionally, Bodner et al. (Citation2016) noted decreased concern for mosquito-borne illnesses among treated households. Overall, the sample size of the reviewed studies ranged between 40 and 883 study participants with the treatments being delivered within two settings; the community (Abel Mangueira et al., Citation2019; Bodner et al., Citation2016) and within schools (AhbiRami & Zuharah, Citation2020; Santos et al., Citation2022; Usman et al., Citation2018).
Our study, employing a single instance of short-term health education training, may not significantly influence the management score behaviour of households. As AhbiRami and Zuharah (Citation2020), Lennon and Coombs (Citation2007) and Prochaska and Velicer (Citation1997) suggest, achieving a change in beliefs and disease management typically requires interventions over a more extended period. Given the limited effect of the training on ADs management, it is imperative to consider alternative measures such as enhancing the training offered or carrying out sustained community engagement campaigns to reinforce key training elements. This includes the use of community health workers to leverage the trust and influence that they have within their area, media campaigns by utilising various channels such as radio, television and print as well as social marketing campaigns that employ advertising and branding. In addition, frequent AD information reminders through text messages and use of nudges might also improve behaviour (Patel et al., Citation2023; Waughtal et al., Citation2021). Therefore, such approaches might be more effective in modifying actions towards ADs control.
5. Conclusions
In conclusion, effective strategies for mitigating and controlling the spread of ADs in sub-Saharan Africa depend on public knowledge changes and appropriate measures for successful disease control. Health education campaigns can serve as a long-term solution for controlling ADs, given their role in enhancing awareness and reducing the cost associated with vector control interventions.
This study contributes to the body of knowledge on impact assessment by analysing the effect of health education training on knowledge and management of three key ADs in Kenya: RVF, chikungunya fever, and dengue fever. Instead of adhering to the usual use of cross-sectional data, mainly focusing on a single arbovirus, this study employed a two-wave panel dataset from randomised control trials, thereby controlling for self-selection bias.
The results reveal that health education increased the respondents’ intensity of knowledge about ADs. However, it did not affect the management of ADs, probably because of the short period of the intervention. Education, experience, and asset ownership positively influenced respondents’ knowledge and management scores, while the distance to the nearest health facility had a negative effect.
The study recommends promoting health education campaigns to increase awareness and knowledge of the three ADs to stem their potential negative health impacts on the community. Such awareness campaigns could be directed towards school-going children since these diseases are prevalent among them. To harness the full benefits of health education campaigns, this study recommends peer learning on ADs management from community members who have previously experienced the three ADs. This could be delivered in a well-coordinated community outreach programme. Future research that includes larger sample size and repeated health education campaigns would be helpful gaining a more comprehensive understanding of the effect of education training on the management and control of ADs.
Acknowledgements
We gratefully acknowledge Dr. David Tchouassi, Edwin Ogola, Dorcus Omuga, Joseph Manono and Gilbert Rotich from icipe for designing the training materials used in this study. We appreciate the help accorded to us by the enumerators, health officers, community health workers, livestock officers, and local administration in all the study sites who facilitated the data collection exercise.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdi, I. H., Affognon, H. D., Wanjoya, A. K., Onyango-Ouma, W., & Sang, R. (2015). Knowledge, attitudes and practices (KAP) on rift valley fever among pastoralist communities of Ijara District, North Eastern Kenya. PLoS Neglected Tropical Diseases, 9(11), e0004239. https://doi.org/10.1371/journal.pntd.0004239
- Abel Mangueira, F. F., Smania-Marques, R., Dutra Fernandes, I., Alves Albino, V., Olinda, R., Acácia Santos-Silva, T., Traxler, J., Matheson, D., & Santos, S. (2019). The prevention of arboviral diseases using mobile devices: A preliminary study of the attitudes and behaviour change produced by educational interventions. Tropical Medicine & International Health, 24(12), 1411–1426. https://doi.org/10.1111/tmi.13316
- Affognon, H., Mburu, P., Hassan, O. A., Kingori, S., Ahlm, C., Sang, R., & Evander, M. (2017). Ethnic groups’ knowledge, attitude and practices and rift valley fever exposure in Isiolo County of Kenya. PLoS Neglected Tropical Diseases, 11(3), e0005405. https://doi.org/10.1371/journal.pntd.0005405
- AhbiRami, R., & Zuharah, W. F. (2020). School-based health education for dengue control in Kelantan, Malaysia: Impact on knowledge, attitude and practice. PLoS Neglected Tropical Diseases, 14(3), e0008075. https://doi.org/10.1371/journal.pntd.0008075
- Ahmed, A., Dietrich, I., LaBeaud, A. D., Lindsay, S. W., Musa, A., & Weaver, S. C. (2020). Risks and challenges of arboviral diseases in Sudan: The urgent need for actions. Viruses, 12(1), 81. https://doi.org/10.3390/v12010081
- Arneliwati, A., & Dewi, A. P. (2019). The effectiveness of health education using audiovisual media on increasing family behavior in preventing dengue hemorrhagic fever (DHF). Enfermería Clínica, 29, 30–33. https://doi.org/10.1016/j.enfcli.2018.11.013
- Barrett, C. B., & Carter, M. R. (2010). The power and pitfalls of experiments in development economics: Some non-random reflections. Applied Economic Perspectives and Policy, 32(4), 515–548. https://doi.org/10.1093/aepp/ppq023
- Beegle, K., Carletto, C., & Himelein, K. (2012). Reliability of recall in agricultural data. Journal of Development Economics, 98(1), 34–41. https://doi.org/10.1016/j.jdeveco.2011.09.005
- Bellini, R., Zeller, H., & Van Bortel, W. (2014). A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasites & Vectors, 7(1), 323. https://doi.org/10.1186/1756-3305-7-323
- Bhatt, S., Gething, P. W., Brady, O. J., Messina, J. P., Farlow, A. W., Moyes, C. L., Drake, J. M., Brownstein, J. S., Hoen, A. G., Sankoh, O., Myers, M. F., George, D. B., Jaenisch, T., Wint, G. R. W., Simmons, C. P., Scott, T. W., Farrar, J. J., & Hay, S. I. (2013). The global distribution and burden of dengue. Nature, 496(7446), 504–507. https://doi.org/10.1038/nature12060
- Bodner, D., LaDeau, S. L., Biehler, D., Kirchoff, N., & Leisnham, P. T. (2016). Effectiveness of print education at reducing urban mosquito infestation through improved resident-based management. PLoS ONE, 11(5), e0155011. https://doi.org/10.1371/journal.pone.0155011
- Boonchutima, S., Kachentawa, K., Limpavithayakul, M., & Prachansri, A. (2017). Longitudinal study of Thai people media exposure, knowledge, and behavior on dengue fever prevention and control. Journal of Infection and Public Health, 10(6), 836–841. https://doi.org/10.1016/j.jiph.2017.01.016
- Castro, M., Sánchez, L., Pérez, D., Sebrango, C., Shkedy, Z., & der Stuyft, P. V. (2013). The relationship between economic status, knowledge on dengue, risk perceptions and practices. PLoS ONE, 8(12), e81875. https://doi.org/10.1371/journal.pone.0081875
- Chandren, J. R., Wong, L. P., & AbuBakar, S. (2015). Practices of dengue fever prevention and the associated factors among the Orang Asli in Peninsular Malaysia. PLOS Neglected Tropical Diseases, 9(8), e0003954. https://doi.org/10.1371/journal.pntd.0003954
- Davis, R., Campbell, R., Hildon, Z., Hobbs, L., & Michie, S. (2015). Theories of behaviour and behaviour change across the social and behavioural sciences: A scoping review. Health Psychology Review, 9(3), 323–344. https://doi.org/10.1080/17437199.2014.941722
- Dhimal, M., Aryal, K. K., Dhimal, M. L., Gautam, I., Singh, S. P., Bhusal, C. L., & Kuch, U. (2014). Knowledge, attitude and practice regarding dengue fever among the healthy population of highland and lowland communities in Central Nepal. PLoS ONE, 9(7), e102028. https://doi.org/10.1371/journal.pone.0102028
- Duflo, E., Glennerster, R., & Kremer, M. (2007). Using randomization in development economics research: A toolkit. Handbook of Development Economics, 4, 3895–3962. https://doi.org/10.1016/S1573-4471(07)04061-2
- Forsyth, J. E., Mutuku, F. M., Kibe, L., Mwashee, L., Bongo, J., Egemba, C., Ardoin, N. M., & LaBeaud, A. D. (2020). Source reduction with a purpose: Mosquito ecology and community perspectives offer insights for improving household mosquito management in coastal Kenya. PLOS Neglected Tropical Diseases, 14(5), e0008239. https://doi.org/10.1371/journal.pntd.0008239
- Harapan, H., Rajamoorthy, Y., Anwar, S., Bustamam, A., Radiansyah, A., Angraini, P., Fasli, R., Salwiyadi, S., Bastian, R. A., Oktiviyari, A., Akmal, I., Iqbalamin, M., Adil, J., Henrizal, F., Darmayanti, D., Pratama, R., Setiawan, A. M., Mudatsir, M., Hadisoemarto, P. F., … Müller, R. (2018). Knowledge, attitude, and practice regarding dengue virus infection among inhabitants of Aceh, Indonesia: A cross-sectional study. BMC Infectious Diseases, 18(1), 96. https://doi.org/10.1186/s12879-018-3006-z
- Heath, C. J., Grossi-Soyster, E. N., Ndenga, B. A., Mutuku, F. M., Sahoo, M. K., Ngugi, H. N., Mbakaya, J. O., Siema, P., Kitron, U., Zahiri, N., Hortion, J., Waggoner, J. J., King, C. H., Pinsky, B. A., & LaBeaud, A. D. (2020). Evidence of transovarial transmission of chikungunya and dengue viruses in field-caught mosquitoes in Kenya. PLOS Neglected Tropical Diseases, 14(6), e0008362. https://doi.org/10.1371/journal.pntd.0008362
- Hermida, M. J., Santangelo, A. P., Calero, C. I., Goizueta, C., Espinosa, M., & Sigman, M. (2021). Learning-by-teaching approach improves dengue knowledge in children and parents. The American Journal of Tropical Medicine and Hygiene, 105(6), 1536–1543. https://doi.org/10.4269/ajtmh.21-0253
- Kamau, W. W., Sang, R., Rotich, G., Agha, S. B., Menza, N., Torto, B., & Tchouassi, D. P. (2023). Patterns of Aedes aegypti abundance, survival, human-blood feeding and relationship with dengue risk, Kenya. Frontiers in Tropical Diseases, 4, 1113531. https://doi.org/10.3389/fitd.2023.1113531
- Karungu, S., Atoni, E., Ogalo, J., Mwaliko, C., Agwanda, B., Yuan, Z., & Hu, X. (2019). Mosquitoes of etiological concern in Kenya and possible control strategies. Insects, 10(6), 173. https://doi.org/10.3390/insects10060173
- Khan, A., Bisanzio, D., Mutuku, F., Ndenga, B., Grossi-Soyster, E. N., Jembe, Z., Maina, P. W., Chebii, P. K., Ronga, C. O., Okuta, V., & LaBeaud, A. D. (2023). Spatiotemporal overlapping of dengue, chikungunya, and malaria infections in children in Kenya. BMC Infectious Diseases, 23(1), 183. https://doi.org/10.1186/s12879-023-08157-4
- LaBeaud, A. D., Muchiri, E. M., Ndzovu, M., Mwanje, M. T., Muiruri, S., Peters, C. J., & King, C. H. (2008). Interepidemic rift valley fever virus seropositivity, Northeastern Kenya. Emerging Infectious Diseases, 14(8), 1240–1246. https://doi.org/10.3201/eid1408.080082
- Lennon, J. L., & Coombs, D. W. (2007). The utility of a board game for dengue haemorrhagic fever health education. Health Education, 107(3), 290–306. https://doi.org/10.1108/09654280710742582
- Lim, J. K., Matendechero, S. H., Alexander, N., Lee, J.-S., Lee, K. S., Namkung, S., Andia, E., Oyembo, N., Lim, S.-K., Kanyi, H., Bae, S. H., Yang, J. S., Ochola, M. A., Edwards, T., Yoon, I.-K., & Njenga, S. M. (2020). Clinical and epidemiologic characteristics associated with dengue fever in Mombasa, Kenya. International Journal of Infectious Diseases, 100, 207–215. https://doi.org/10.1016/j.ijid.2020.08.074
- Mallhi, T. H., Khan, Y. H., Tanveer, N., Bukhsh, A., Khan, A. H., Aftab, R. A., Khan, O. H., & Khan, T. M. (2018). Awareness and knowledge of chikungunya infection following its outbreak in Pakistan among health care students and professionals: A nationwide survey. PeerJ, 6, e5481. https://doi.org/10.7717/peerj.5481
- Marchi, S., Trombetta, C. M., & Montomoli, E. (2018). Emerging and re-emerging arboviral diseases as a global health problem. In Anwarul Azim Majumder, Russell Kabir, & Sayeeda Rahman (Eds.), Public health – Emerging and re-emerging issues. (pp. 25–46). United Kingdom: IntechOpen.
- Medone, P., & Hernández-Suárez, C. M. (2019). ‘Swimming mosquitoes’: A key stepping stone to prevent dengue, Zika and chikungunya: An educative experience in Colima, Mexico. Health Education Research, 34(4), 389–399. https://doi.org/10.1093/her/cyz012
- Michie, S., & Johnston, M. (2012). Theories and techniques of behaviour change: Developing a cumulative science of behaviour change. Health Psychology Review, 6(1), 1–6. https://doi.org/10.1080/17437199.2012.654964
- Michie, S., & West, R. (2013). Behaviour change theory and evidence: A presentation to government. Health Psychology Review, 7(1), 1–22. https://doi.org/10.1080/17437199.2011.649445
- Muriithi, B. W., Affognon, H. D., Diiro, G. M., Kingori, S. W., Tanga, C. M., Nderitu, P. W., Mohamed, S. A., & Ekesi, S. (2016). Impact assessment of integrated pest management (IPM) strategy for suppression of mango-infesting fruit flies in Kenya. Crop Protection, 81, 20–29. https://doi.org/10.1016/j.cropro.2015.11.014
- Mutero, C. M., Okoyo, C., Girma, M., Mwangangi, J., Kibe, L., Ng’ang’a, P., Kussa, D., Diiro, G., Affognon, H., & Mbogo, C. M. (2020). Evaluating the impact of larviciding with Bti and community education and mobilization as supplementary integrated vector management interventions for malaria control in Kenya and Ethiopia. Malaria Journal, 19(1), 390. https://doi.org/10.1186/s12936-020-03464-6
- Muthini, D. N., Nzuma, J. M., & Nyikal, R. A. (2019). Variety awareness, nutrition knowledge and adoption of nutritionally enhanced crop varieties: Evidence from Kenya. African Journal of Agricultural and Resource Economics, 14(4), 225–237.
- Ndenga, B. A., Mutuku, F. M., Ngugi, H. N., Mbakaya, J. O., Aswani, P., Musunzaji, P. S., Vulule, J., Mukoko, D., Kitron, U., & LaBeaud, A. D. (2017). Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS ONE, 12(12), e0189971. https://doi.org/10.1371/journal.pone.0189971
- Ngoi, C. N., Price, M. A., Fields, B., Bonventure, J., Ochieng, C., Mwashigadi, G., Hassan, A. S., Thiong’o, A. N., Micheni, M., Mugo, P., Graham, S., & Sanders, E. J. (2016). Dengue and chikungunya virus infections among young febrile adults evaluated for acute HIV-1 infection in Coastal Kenya. PLoS ONE, 11, e0167508. https://doi.org/10.1371/journal.pone.0167508
- Ngugi, H. N., Nyathi, S., Krystosik, A., Ndenga, B., Mbakaya, J. O., Aswani, P., Musunzaji, P. S., Irungu, L. W., Bisanzio, D., Kitron, U., Desiree LaBeaud, A., & Mutuku, F. (2020). Risk factors for Aedes aegypti household pupal persistence in longitudinal entomological household surveys in urban and rural Kenya. Parasites & Vectors, 13(1), 499. https://doi.org/10.1186/s13071-020-04378-7
- Nguyen, H. V., Than, P. Q. T., Nguyen, T. H., Vu, G. T., Hoang, C. L., Tran, T. T., Truong, N. T., Nguyen, S. H., Do, H. P., Ha, G. H., Nguyen, H. L. T., Dang, A. K., Do, C. D., Tran, T. H., Tran, B. X., Latkin, C. A., Ho, C. S. H., & Ho, R. C. M. (2019). Knowledge, attitude and practice about dengue fever among patients experiencing the 2017 outbreak in Vietnam. International Journal of Environmental Research and Public Health, 16(6), 976. https://doi.org/10.3390/ijerph16060976
- Nyamwaya, D. K., Otiende, M., Mwango, L., Kariuki, S. M., Otieno, B., Omuoyo, D. O., Githinji, G., Kitsao, B. S., Karanja, H. K., Gitonga, J. N., de Laurent, Z. R., Davies, A., Mwarumba, S., Agoti, C. N., Thumbi, S. M., Hamaluba, M. M., Newton, C. R., Bejon, P., & Warimwe, G. M. (2022). Incidence of chikungunya virus infections among Kenyan children with neurological disease, 2014–2018: A cohort study. PLOS Medicine, 19(5), e1003994. https://doi.org/10.1371/journal.pmed.1003994
- Nyangau, P. N., Nzuma, J. M., Irungu, P., & Kassie, M. (2021). Evaluating livestock farmers knowledge, beliefs, and management of arboviral diseases in Kenya: A multivariate fractional probit approach. PLoS Neglected Tropical Diseases, 15(9), e0009786. https://doi.org/10.1371/journal.pntd.0009786
- Ogutu, S. O., Fongar, A., Gödecke, T., Jäckering, L., Mwololo, H., Njuguna, M., Wollni, M., & Qaim, M. (2020). How to make farming and agricultural extension more nutrition-sensitive: Evidence from a randomised controlled trial in Kenya. European Review of Agricultural Economics, 47, 95–118.
- Paixão, E. S., Teixeira, M. G., & Rodrigues, L. C. (2018). Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Global Health, 3(Suppl 1), e000530. https://doi.org/10.1136/bmjgh-2017-000530
- Papke, L. E., & Wooldridge, J. M. (1996). Econometric methods for fractional response variables with an application to 401(k) plan participation rates. Journal of Applied Econometrics, 11(6), 619–632. https://doi.org/10.1002/(SICI)1099-1255(199611)11:6<619::AID-JAE418>3.0.CO;2-1
- Patel, M. S., Milkman, K. L., Gandhi, L., Graci, H. N., Gromet, D., Ho, H., Kay, J. S., Lee, T. W., Rothschild, J., Akinola, M., Beshears, J., Bogard, J. E., Buttenheim, A., Chabris, C., Chapman, G. B., Choi, J. J., Dai, H., Fox, C. R., Goren, A., … Duckworth, A. L. (2023). A randomized trial of behavioral nudges delivered through text messages to increase influenza vaccination among patients with an upcoming primary care visit. American Journal of Health Promotion, 37(3), 324–332. https://doi.org/10.1177/08901171221131021
- Prochaska, J. O., & Velicer, W. F. (1997). The transtheoretical model of health behavior change. American Journal of Health Promotion, 12(1), 38–48. https://doi.org/10.4278/0890-1171-12.1.38
- Rios-Avila, F., Pedro, H. C. S., & Asjad, N. (2021). Drdid: Doubly robust difference-in-differences estimators for Stata. STATA.
- Sant’Anna, P. H., & Zhao, J. (2020). Doubly robust difference-in-differences estimators. Journal of Econometrics, 219(1), 101–122. https://doi.org/10.1016/j.jeconom.2020.06.003
- Santos, S., Smania-Marques, R., Albino, V. A., Fernandes, I. D., Mangueira, F. F. A., Altafim, R. A. P., Olinda, R., Smith, M., & Traxler, J. (2022). Prevention and control of mosquito-borne arboviral diseases: Lessons learned from a school-based intervention in Brazil (Zikamob). BMC Public Health, 22(1), 255. https://doi.org/10.1186/s12889-022-12554-w
- Selvarajoo, S., Liew, J. W. K., Tan, W., Lim, X. Y., Refai, W. F., Zaki, R. A., Sethi, N., Sulaiman, W. Y. W., Lim, Y. A. L., & Vadivelu, J. (2020). Knowledge, attitude and practice on dengue prevention and dengue seroprevalence in a dengue hotspot in Malaysia: A cross-sectional study. Scientific Reports, 10(1), 1–13. https://doi.org/10.1038/s41598-020-66212-5
- Shepard, D. S., Undurraga, E. A., Halasa, Y. A., & Stanaway, J. D. (2016). The global economic burden of dengue: A systematic analysis. The Lancet Infectious Diseases, 16(8), 935–941. https://doi.org/10.1016/S1473-3099(16)00146-8
- Shikuku, K. M., Pieters, J., Bulte, E., & Läderach, P. (2019). Incentives and the diffusion of agricultural knowledge: Experimental evidence from Northern Uganda. American Journal of Agricultural Economics, 101(4), 1164–1180. https://doi.org/10.1093/ajae/aaz010
- Sreedevi, A., Burru, R. V., Rao, G. V., Yalamanchili, P., Subhaprada, C., Kumari, V., Kala, S., & Aruna, M. (2016). Study on awareness about vector borne diseases and education about preventive measures in rural field practice areas of Kurnool Medical College, Kurnool. International Journal of Medical Science and Public Health, 5(9), 1803–1807. https://doi.org/10.5455/ijmsph.2016.25122015325
- Sukesi, T. W., Satoto, T. B., Murhandarwati, E. H., & Padmawati, R. S. (2021). Effects of health education based intervention on community’s perception, healthy house, and social capital of dengue in endemic area of Sleman Regency Indonesia. Open Access Macedonian Journal of Medical Sciences, 9(E), 428–436. https://doi.org/10.3889/oamjms.2021.6087
- Suwanbamrung, C., Kusol, K., Tantraseneerate, K., Promsupa, S., Doungsin, T., Thongchan, S., & Laupsa, M. (2015). Developing the participatory education program for dengue prevention and control in the primary school, southern region, Thailand. Health, 07(10), 1255. https://doi.org/10.4236/health.2015.710140
- Thomas, S. J. (2023). Is new dengue vaccine efficacy data a relief or cause for concern? Npj Vaccines, 8(1), 55. https://doi.org/10.1038/s41541-023-00658-2
- Torres-Flores, J. M., Reyes-Sandoval, A., & Salazar, M. I. (2022). Dengue vaccines: An update. Biodrugs, 36(3), 325–336. https://doi.org/10.1007/s40259-022-00531-z
- Usman, H. B., AlSahafi, A., Abdulrashid, O., Mandoura, N., Sharif, K. A., Ibrahim, A., Ahmed, L., Shamrani, E., Shamia, M., Usman, H. B., AlSahafi, A., Abdulrashid, O., Mandoura, N., Sharif, K. A., Ibrahim, A., Ahmed, L., Shamrani, E., & Shamia, M. (2018). Effect of health education on dengue fever: A comparison of knowledge, attitude, and practices in public and private high school children of Jeddah. Cureus, 10(12), e3809. https://doi.org/10.7759/cureus.3809
- Waughtal, J., Luong, P., Sandy, L., Chavez, C., Ho, P. M., & Bull, S. (2021). Nudge me: Tailoring text messages for prescription adherence through N-of-1 interviews. Translational Behavioral Medicine, 11(10), 1832–1838. https://doi.org/10.1093/tbm/ibab056
- Wooldridge, J. M. (2002). Econometric analysis of cross section and panel data. MIT Press.
Appendix A
Table A1. Attrition rates across treatment and control groups.
Table A2. Attrition probit regressions.

