ABSTRACT
Sex workers have been demonstrated to have increased vulnerabilities to HIV and a high population prevalence of the disease. Despite their increased risk, sex workers have been underrepresented in molecular epidemiology studies assessing HIV in Mesoamerica. This study aims to describe the sociodemographic characteristics and phylogenetic profile of HIV-1 within a cohort of HIV-positive female sex workers (FSW) situated at the Guatemala-Mexico border. HIV viral sequences were collected from a cohort of FSW ≥18 years of age from San Marcos, Guatemala (n = 6) and compared to viral sequences collected as part of the Mesoamerican Drug Resistance Monitoring Programme to assess HIV viral diversity in Mexico and Guatemala (n = 3956). All of the FSW sampled were determined to have genetically unrelated HIV infections, suggesting multiple introductions of the virus and/or the potential existence of populations not captured by current surveillance efforts. Many reported numerous vulnerabilities that may have heightened their risk of acquiring and transmitting HIV through sex work activities. Our phylogenetic analysis indicated that national surveillance programmes may not fully capture the viral diversity among FSW and their clients within this region. Additional research is needed to fully capture HIV diversity and transmission in Mesoamerica, especially in the Guatemala-Mexico border region.
Introduction
Guatemala has a higher proportion of individuals living with HIV than its surrounding nations with an estimated nationwide adult prevalence of 0.2% (UNAIDS, Citation2021). Substantial population shifts through Mesoamerica are thought to have facilitated viral diffusion across international boundaries and contributed to increased prevalence of HIV in Guatemala (Czaika & De Haas, Citation2014). The Guatemala-Mexico border is a critical region for migration, with around half a million undocumented migrants crossing through Guatemala annually and a negative net immigration rate (Migration Policy Institute, Citation2022; Villa et al., Citation2004). Additionally, Guatemala has a large number of sex workers and hosts a thriving sex industry, especially within the prominent border community of Tecún Umán (Goldenberg et al., Citation2015). The confluence of migrants in an environment of increased risk-taking behaviour has the potential to exacerbate the HIV transmission among this population.
Sex workers, especially those working within informal establishments, are at greater risk of acquiring and transmitting HIV (Morales-Miranda et al., Citation2014). The prevalence of HIV among FSW in Guatemala is estimated to be 1.0–2.6%, at least five times that of the general population (UNAIDS, Citation2021). The prevalence of HIV among this population may be higher as this number may not include FSW who are not registered as sex workers through the Guatemalan Public Health service and maintain a Sanitary Control Card. Those who work in informal settings often are not captured through public health sex worker registration programmes and could potentially experience higher incidence of HIV due to a lack of access to regular care or public health campaigns targeted to FSW. Additionally, individuals who are partaking in sex work may not be willing to participate in studies due to continued stigma surrounding HIV and sex workers.
Female sex workers in Guatemala face many vulnerabilities which may increase their risk of acquiring and transmitting HIV. For example, they often have large numbers of sex partners, concurrency of partners, and report infrequent or inconsistent condom use which can often be attributed to power imbalances or an inability to advocate for condom use with clients (Astemborski et al., Citation1994). While these may be contributing factors, the HIV epidemic among this population must be viewed within the context of social and structural factors which contribute to healthcare inequalities among sex workers. Vulnerabilities faced by these individuals are multifaceted and closely linked to factors including poverty, migration, substance abuse, physical abuse, coercion, and immigration (Platt et al., Citation2018).
Due to their increased risk, broad population reached through their clients, and high HIV prevalence among sex workers, efforts to understand the HIV epidemic among this population are critical to reducing transmission. Because of these factors, sex workers have often been considered a ‘key population’ in which the infection is endemic and from whom it can spread to a larger population (Plummer et al., Citation1991). Reducing HIV prevalence and transmission within this population can help reduce transmission within the region, and understanding the importance of core groups is key to developing effective prevention programmes. Public health programmes need to consider local distribution of behavioural and societal factors associated with HIV transmission and prevent further stigmatisation and discrimination of sex workers. Molecular epidemiology studies can help inform these interventions by assessing behavioural, societal, and structural factors associated with sex work and HIV transmission clusters.
While female sex workers (FSW) are known to have greater risk of acquiring and transmitting HIV, little is known about the molecular epidemiology of HIV among sex workers at a key transit site of the Americas – the Guatemala-Mexico Border. Because of challenges with accurately sampling FSW within this community, the role of sex work on HIV transmission throughout Mesoamerica is not yet fully understood. Molecular epidemiology studies allow for better understanding of disease transmission and resultant data can help inform interventions to contain HIV transmission on both a national and regional level. Specifically, identifying potential transmission links among high-risk populations, including FSW, allows for further evaluation of the role of sex work in the dynamics of the HIV epidemic and facilitates the development of effective public health programmes.
This study aims to assess and describe the molecular epidemiology of HIV-1 among a small group of geographically clustered female sex workers located at the Guatemala-Mexico border. The purpose of the study is to compare the phylogenetic diversity of sequences collected from a population of female sex workers to sequences captured as part of regional HIV surveillance efforts. We aim to describe the genetic diversity among a small subset of FSW to better understand the viral diversity within this region and assess if HIV among this population is captured in national surveillance efforts. Additionally, we intend to describe sociodemographic factors which may contribute to their risk of acquiring and transmitting HIV. We hypothesised that HIV among adult female sex workers at the Guatemala-Mexico Border will be related to cross-border transmission clusters consisting of heterosexual males, and possibly other sex workers.
Methods
Data and sequence collection
Female sex workers
We analysed data collected from a larger study exploring the context of rising drug use along the Mexico/Guatemala border (‘Cruzando Fronteras’, R01DA029899) (Chaillon et al., Citation2017). Female sex workers were recruited from the Guatemala-Mexico border community of Tecún Umán, Guatemala, a region which experiences dynamic population movement and hosts a large population of sex workers. Women were recruited from sex work venues such as bars, hotels, street corners, and truck stops. This analysis draws on ethnographic field work, survey data, and samples collected from participating individuals between November 2012 and February 2015. Women were invited to participate during outreach by a team of female workers from a well-established community-based organisation dedicated to HIV prevention and education among sex workers in Tecún Umán. We utilised purposive sampling (Strauss & Corbin, Citation1998; Straus & Corbin, Citation1990) to identify a cohort of FSW representative of diverse migration experiences, work environments, and age. Eligible participants were females ≥18 years old who exchanged sex for money, drugs, or other resources within the past 6 months and were willing to be tested for HIV and other infections. Eligible participants spoke Spanish and provided informed consent. Participants were provided compensation for their time.
All HIV-positive FSW participants (n = 6) were included in this sub-study. Surveys collected data on sociodemographics, substance use, sexual behaviours and experience, sex work history, knowledge of HIV/STI, medical history, healthcare access, incarceration/police experience, community and personal violence experience, and mental health. Surveys were written in Spanish and facilitated by those comfortable speaking the language. Those participating in the survey also provided a blood sample.
This research was guided by a Community Advisory Board of sex work advocates and conducted in close partnership with a local community-based organisation dedicated to HIV prevention and education for key populations, including sex workers. All procedures were approved by IRBs at the University of California, San Diego, Universidad del Valle de Guatemala, and the Guatemalan Ministry of Public Health and Social Assistance.
Mesoamerican Drug Resistance Monitoring Programme (MDRMP)
Sequences from Mexico and Guatemala were obtained from antiretroviral treatment (ART)-naïve HIV-infected individuals enrolled between 2012 and 2016 as part of the multicentre cross-sectional Mesoamerican Drug Resistance Monitoring Programme to assess transmitted drug resistance and viral diversity of HIV across Mexico and Central America (Ávila-Ríos et al., Citation2019). At contributing clinics, individuals were asked to participate in diagnosis or follow-up. The only exclusion criteria applied was known previous exposure to ART. All participating individuals provided written consent. Sociodemographic information, including age, sex, HIV risk factors (Heterosexual, Men who have sex with Men, people who inject drugs, Mother to Child Transmission, and other), and date of diagnosis were collected at enrolment. Each participant provided a blood sample (n = 3956) (UCSD#190121).
Sequencing and phylogenetic analysis
Mesoamerican samples were processed at the Centre for Research in Infectious Diseases (CIENI) of the National Institute of Respiratory Diseases (INER) in Mexico City. Partial HIVpol (HXB2 positions 2253–3554) was sequenced from free plasma virus (extraction from 1 mL of plasma, QIAmp Viral RNA Kit, QIAGEN, Valencia, CA), using an in-house protocol (Avila-Ríos et al., Citation2015) with a 3730×l Genetic Analyzer instrument (Thermo Fisher, Waltham, MA). Sequences were assembled using the web-based automated sequence analysis tool RECall (University of British Columbia, Vancouver, Canada) (Woods et al., Citation2012).
FSW samples were processed at the University of California San Diego using a previously described procedure (Park et al., Citation2021; Smith et al., Citation2009). Partial HIV-1 pol sequences for all samples were obtained by sequencing of the HIV-1 (prot/rt) from RNA or DNA extracted from blood samples. Sequences obtained from female sex workers were subtyped using both the REGA (Oliveira et al., Citation2005; Pineda-Peña et al., Citation2013) and SUDI subtyping tool (Robertson et al., Citation2000; Saber et al., Citation2016; Siepel et al., Citation1995).
All partial HIV pol sequences were briefly aligned to HXB2 reference sequence using MegaX Clustal Omega (Thompson et al., Citation1994). Cluster Picker 1.3 was used to identify clusters of related infections (Ragonnet-Cronin et al., Citation2013; Rose et al., Citation2017). Clusters were identified based on a genetic distance threshold of 1.5%. Cluster analysis was first conducted on the two cohorts independently. A subset was then obtained of the MDRMP cohort which included all Guatemalan districts and the following states in Mexico: Chiapas, Tabasco, Campeche, Quintana Roo, Yucatán, and Puebla. These states were selected as the Mexico-Guatemala border has been identified as a region with a high confluence of factors that could increase social vulnerability to HIV infection (Muñoz Martínez et al., Citation2020; Rocha-Jiménez et al., Citation2022). Cluster analysis was then completed using the cohort of female sex workers and subset of sequences from the MDRMP cohort. Phylogenetic trees were inferred using the Maximum Likelihood Method and Tamura-Nei Model (Tamura & Nei, Citation1993). Sequences were compared to reference sample of HIV-1 subtype B, as subtype B is the most prevalent subtype in Latin America (Hemelaar et al., Citation2011). Evolutionary analyses were conducted in MegaX (Mello, Citation2018).
Detailed phylogenetic and cluster analysis of the full cohort collected by the Mesoamerican Drug Resistance Monitoring Programme can be found in Chaillon et al. (Citation2017).
Results
Cohort of adult female sex workers
The cohort of female sex workers (n = 6) (see ) had an average age of 30 with a range from 25-36. Among the sampled FSW, four were born in Guatemala, one in Mexico, and one in Honduras. Of those who were not from Guatemala, both were undocumented. The education level of the FSW sample ranged from no formal education to incomplete diversificado (high school) education, with 83.0% having had at least some primary education.
Table 1. Demographics of Adult FSW at the Guatemala/Mexico Border (n = 6).
While all members of the cohort reported having used illicit drugs (See ), only one individual within the sample had ever injected drugs. Reports of physical and sexual abuse were common among the included FSW, with 66.7% reporting having been sexually assaulted. Half of the sample of FSW revealed that their first sexual encounter was non-consensual. One individual within the cohort indicated they began engaging in sex work due to being threatened. Fifty percent of the included individuals indicated they were very fearful of the police, with a smaller proportion stating they were only somewhat fearful of the police (16.7%). Some of the FSW were migrants (33.4%) and reported being undocumented.
Of the FSW included in the study, most indicated they initially engaged in sex work as it was the best way to make money (See ). Study participants listed reasons for continued engagement in sex work as they felt it was the best way to make money or due to a need to pay for drugs. On average, women in the sample engaged in sex work 34 days out of the past 6 months and saw an average of 33 clients, although this ranged to as many as 180 days and 150 clients. Included female sex workers were from indoor working establishments. Condom use was also assessed among the cohort with most individuals receiving condoms from their partner. While all the FSW in this sub-study indicated having access to condoms, most also noted not using condoms with clients or partners. When asked about HIV testing and their current status, the majority of the sampled FSW had been tested for HIV previously, however only 50.0% knew of their positive HIV status at the time of the interview.
Phylogenetic comparison of cohort of FSW
Subtyping using the REGA tool of sequences collected from FSW revealed that all sequences were HIV subtype B. However, when using the SUDI subtyping tool, the closest genetic neighbours to the study sequences included sequences from the US, Mexico, Honduras, Spain, Brazil, France and Kenya. The FSW in this study were sampled over a relatively short period of time from 2014 to 2016. Average genetic distance between the sequences from FSW compared to HXB2 reference sequence was 0.053 () and the average genetic distance of pairwise comparison between FSW was found to be 0.090 (suggesting again multiple diverse introductions). Average genetic distance of pairwise comparison between FSW and other sequences from the Tecún Umán region was 0.072 (). Pair-wise distances were found to be greater than differences from the HXB2 reference sequence or other sequences from the area. Out of the six study FSW, six unrelated infections were identified, demonstrating the large viral diversity among female sex workers.
Figure 1. Phylogenetic Analysis of Female Sex Workers. Phylogenetic characterisation of the included FSW (n = 6). All sequences were identified as HIV type 1 and aligned. The phylogenetic tree includes HIV type 1 reference sequence B.FR.83.HXB2.
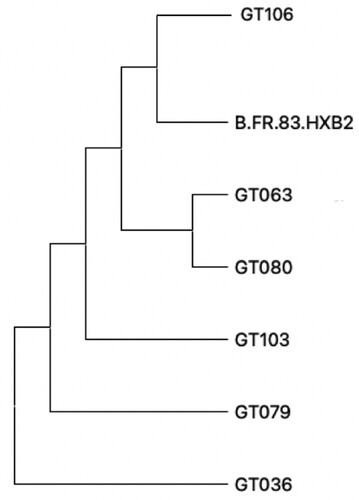
Figure 2. Phylogenetic analysis of Female Sex Workers (GT) cohort compared to sequences collected from men who have sex with men (GH) and migrants (GM) in the Tecún Umán region. Sequences highlighted with a green box indicate Female Sex Workers included in the study (n = 6) which were compared to samples from other high-risk populations within the same area of Tecún Umán. The prefix ‘GH’ indicates men who have sex with men (MSM) while ‘GM’ indicates individuals who are migrants. One additional FSW was included, GT064, however this individual is not part of the study cohort. The phylogenetic tree includes HIV type 1 reference sequence B.FR.83.HXB2. FSW were most closely related to sequences from MSM rather than other female sex workers.
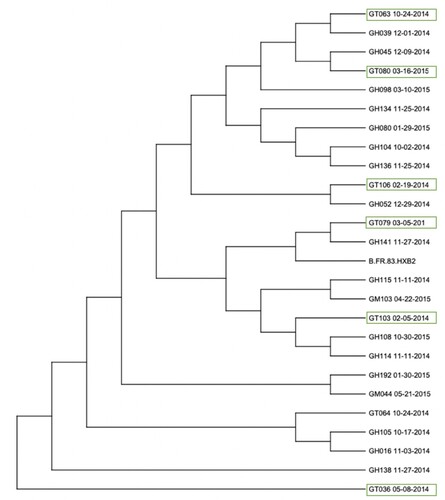
No clusters were identified among the cohort of FSW. When the HIV sequences from FSW were analysed with the subset of sequences collected by the Mesoamerican Drug Resistance Monitoring Programme, none of the female sex workers clustered with any other individual from the Mexico-Guatemala border region. The FSW within the cohort did not have any other closely related infection identified within the study population.
Discussion
The female sex workers in this study faced numerous factors that may have heightened their risk of acquiring HIV, including sexual and physical violence, police violence, and socioeconomic burdens. We found that some FSW within the study were migrants and faced fear of social and police discrimination. Many had financial obligations such as dependents, whom they supported, or owed money to other people, which motivated their engagement in sex work. Additionally, the need for money to pay for drugs may also motivate participation in sex work. The findings of this study build on previous evidence documenting the social and structural factors that influence sex worker’s safety and vulnerability to HIV (Muñoz Martínez et al., Citation2020; Rocha-Jiménez et al., Citation2022, Citation2020; Cusick, Citation2006; Basnyat, Citation2017; Martinez et al., 2020; Rhodes et al., Citation2005).
Previous research has demonstrated the effect of work setting on HIV risk and found that sex workers within formal establishments are much more likely to have had HIV testing (p < 0.001) and less likely to be positive for HIV (p < 0.039) than sex workers who work informally (Goldenberg et al., Citation2018; Sirotin et al., Citation2010). Additionally, a meta-analysis evaluating associations between sex work laws and sex worker’s health found repressive policing was associated with higher odds of any kind of violence (Platt et al., Citation2018). Similarly, physical or sexual violence from clients, which may contribute to HIV risk, was higher among those who had been exposed to repressive police activity (Platt et al., Citation2018). Many women within the cohort had been previously arrested and expressed fear of police abuse or harassment. Migrant women included described experiencing physical violence and coercion by police officers. Furthermore, barriers related to migration status can be common among migrant women which may limit access to conventional employment and has led to the over-representation of migrant women in the sex industry (Rocha-Jiménez et al., Citation2016). Lastly, sex workers face considerable health inequalities including access to HIV testing (Rocha-Jiménez et al., Citation2018). Particularly, migrant sex workers have been found to have serious gaps in healthcare related to increased stigma of this population (Rocha-Jiménez et al., Citation2018).
Additionally, many of the women included described risk factors for transmitting HIV. At the time of the study, only half of the sampled FSW were aware of their HIV-positive status, despite reporting previous HIV testing, and condom use with clientele was either inconsistent or absent for many of the included women. Given inconsistent condom use and number of clients, this could reflect a substantial population at risk of HIV. Efforts to increase prevention and treatment programmes among this population could be critical to reducing transmission within the region.
The Mesoamerican Drug Resistance Monitoring Programme previously conducted phylogenetic analysis of HIV in Mesoamerica and identified transmission clusters consisting largely of males, with several large clusters consisting of men reporting to be heterosexual (Chaillon et al., Citation2017). Several of the large transmission clusters identified consisted of both Mexicans and Guatemalans, including those from the Mexico-Guatemala border region. The findings in Chaillon et al. (Citation2017) indicated dynamic cross-border transmission and given the thriving sex industry in this region (Goldenberg et al., Citation2015) and evidence of cross-border transmission, we had hypothesised that sex workers in this region could have infections related to these large clusters. However, despite seeing transmission clusters at the Guatemala-Mexico border region in the previously conducted analysis (Chaillon et al., Citation2017), none of the FSW within the study clustered with sequences included from the Mesoamerican Drug Resistance Monitoring Programme. The FSW did not meet the threshold to cluster amongst each other or sequences from the Tecún Umán region, although they were related. Analysis indicated that these individuals may have been infected over a relatively similar period of time, within the span of 2013–2016.
We also identified a large amount of viral diversity among this small sample of geographically clustered sex workers, indicating that we sampled a genetically diverse sub-population. The viral diversity seen among the sex workers within the cohort could suggest multiple introductions of HIV into this high-risk population. However, lack of similar sequences within the data collected from the Mesoamerica Drug Resistance Monitoring Programme could also indicate that FSW who acquired HIV from transactional sex are not being fully captured through national surveillance programmes within this region. The sequences from the Mesoamerican Drug Resistance Monitoring Programme were collected from large reference hospitals in Guatemala City and may not include individuals with more limited access to care. Many of the participating FSW were migrants which may contribute to these individuals struggling to access routine care, especially in regard to HIV. A qualitative analysis assessing healthcare access among FSW in this region found that migrant sex workers faced enhanced barriers to healthcare including financial resources and legal status (Rocha-Jiménez et al., Citation2018). Privacy and experiences of stigma had also been found to be contributing factors in shaping access to care. Sex workers who test positive for HIV may face increased discrimination or fear of having their registration revoked. Often individuals were found to pay for care at private clinics to maintain the increased privacy (Rocha-Jiménez et al., Citation2018). Individuals within our study faced many obstacles that may limit their access to healthcare including migratory status, fear of police, drug use, and continued stigma over HIV and sex work. The lack of clustering demonstrated may provide evidence that these individuals might not be receiving regular access to HIV care in the national treatment programmes. Additionally, the lack of clustering may also indicate that the clientele of the included sex workers may not be receiving access to HIV care. Many efforts have carried out during the last decade to improve access to HIV testing for key populations, including MSM and FSW, through specific HIV diagnosis establishments developed for these populations (Morales-Miranda et al., Citation2014). However, access to health services and HIV testing may not be as prominent among heterosexual men who be the probable clients of the FSW that we have in the study. Given the volume of clientele of some of the sampled FSW, this may indicate a substantial population of individuals not represented within national treatment programme datasets.
Another reason transmission networks that include the FSW within this study may not be captured in national databases is the distance of the study location to the main participating hospital in Guatemala. Roosevelt hospital, located in Guatemala City, is approximately 285 km away from Tecún Umán, where the sequences from FSWs were obtained. Individuals who are receiving local care may not be captured in national systems. Only twenty-two individuals were included from the department of San Marcos, where Tecún Umán is located, with only 31% of those individuals clustering. Nevertheless, a small proportion of background sequences from Tecún Umán were included in the analysis and still no clusters were observed among the FSW. It is possible that localised transmission networks that include these sex workers occur within Tecún Umán and may not be fully represented in national data collection projects.
The findings of this study indicate potential gaps in national surveillance programmes among high-risk populations, such as the female sex workers located at the Guatemala-Mexico border. The lack of clustering observed may indicate populations not reached by national surveillance efforts and could suggest opportunities for further expansion of HIV treatment and prevention programmes.
Limitations
There are a number of limitations to this study. First, the small sample size may not adequately capture local transmission. The comparative cohort from the Mesoamerican Drug Monitoring project is a convenience sample that collects data from government health centres and may not capture transmission networks in more remote regions such as Tecún Umán. For example, there are few individuals from some Mexican states including Chiapas, located across the border from the community of Tecún Umán, where the sequences from FSW were obtained. Due to the convenience sampling, demographic characteristics and geographical distribution of the study cohort may not reflect the population living with HIV in Guatemala and Mexico. While transmission information can be inferred from this data, HIV transmission networks may not be fully understood due to current sampling methods.
Secondly, the individuals who are captured through the MDRMP may have different sociodemographics than the female sex workers within this study or the general population living with HIV within this region. The MDRMP collects samples from participating hospitals which are often located in large cities. Individuals within other areas of Mesoamerica, where there may be no participating locations, may not be captured within these national data sets. For example, sex workers and their clients may not have similar healthcare access, resources, or sociodemographics as the individuals from the MDRMP cohort, which may explain the lack of clustering seen.
Finally, the study is limited due to the number of FSW included. Extensive effort was needed to include this population and these individuals were only involved through the use of a dedicated outreach team. While the sample size is limited, demographic variables were consistent with risk factors identified in previous research (Goldenberg et al., Citation2015; Lyons et al., Citation2014; Muñoz Martínez et al., Citation2020; Shannon et al., Citation2008). A literature review revealed the limited number of sex workers included in molecular epidemiology studies (Baral et al., Citation2012; Bungay et al., Citation2016) and this cohort, while small, represents an extensive effort undertaken to ensure representation of this important population.
Conclusion
This study represents one of the few studies which specifically assesses molecular epidemiology of HIV among sex workers at the Mexico-Guatemala border region and the limited clustering observed highlights the need for increased representation of these individuals within larger HIV molecular epidemiology research. The sociodemographic variables assessed are consistent with conceptualised risk models of HIV risk and transmission. Our study is consistent with the increased vulnerabilities faced by sex workers which may affect their access to healthcare and thus representation in HIV-1 genetic surveillance. Further research is necessary to more clearly understand the molecular epidemiology of HIV among sex workers and their clients in this border region.
Acknowledgements
The authors gratefully acknowledge the participants, community collaborators, and study staff in both Guatemala and Mexico.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Astemborski, J., Vlahov, D., Warren, D., Solomon, L., & Nelson, K. E. (1994). The trading of sex for drugs or money and HIV seropositivity among female intravenous drug users. American Journal of Public Health, 84(3), 382–387. https://doi.org/10.2105/AJPH.84.3.382
- Avila-Ríos, S., García-Morales, C., Garrido-Rodríguez, D., Tapia-Trejo, D., Girón-Callejas, A. C., Mendizábal-Burastero, R., Escobar-Urias, I. Y., García-González, B. L., Navas-Castillo, S., Pinzón-Meza, R., Mejía-Villatoro, C. R., & Reyes-Terán, G. (2015). HIV-1 drug resistance surveillance in antiretroviral treatment-naive individuals from a reference hospital in Guatemala, 2010–2013. AIDS Research and Human Retroviruses, 31(4), 401–411. https://doi.org/10.1089/aid.2014.0057
- Ávila-Ríos, S., García-Morales, C., Valenzuela-Lara, M., Chaillon, A., Tapia-Trejo, D., Pérez-García, M., López-Sánchez, D. M., Maza-Sánchez, L., del Arenal-Sánchez, S. J., Paz-Juárez, H. E., Quiroz-Morales, V. S., Mehta, S. R., Smith, D. M., León-Juárez, E. A., Magis-Rodríguez, C., Reyes-Terán, G., Gamboa-Marroquín, J. A., Espinoza-Fernández, A. F., … Reyes-Terán, G. (2019). HIV-1 drug resistance before initiation or re-initiation of first-line ART in eight regions of Mexico: A sub-nationally representative survey. Journal of Antimicrobial Chemotherapy, 74(4), 1044–1055. https://doi.org/10.1093/jac/dky512
- Baral, S., Beyrer, C., Muessig, K., Poteat, T., Wirtz, A. L., Decker, M. R., Sherman, S. G., & Kerrigan, D. (2012). Burden of HIV among female sex workers in low-income and middle-income countries: A systematic review and meta-analysis. The Lancet Infectious Diseases, 12(7), 538–549. https://doi.org/10.1016/S1473-3099(12)70066-X
- Basnyat, I. (2017). Theorizing the relationship between gender and health through a case study of Nepalese street-based female sex workers. Communication Theory, 27(4), 388–406. https://doi.org/10.1111/comt.12114
- Bungay, V., Oliffe, J., & Atchison, C. (2016). Addressing underrepresentation in Sex work research. Qualitative Health Research, 26(7), 966–978. https://doi.org/10.1177/1049732315613042
- Chaillon, A., Avila-Ríos, S., Wertheim, J. O., Dennis, A., García-Morales, C., & Tapia-Trejo, D. (2017). Identification of major routes of HIV transmission throughout Mesoamerica. Infection, Genetics and Evolution, 54, 98–107. https://doi.org/10.1016/j.meegid.2017.06.021
- Cusick, L. (2006). Widening the harm reduction agenda: From drug use to sex work. International Journal of Drug Policy, 17(1), 3–11. https://doi.org/10.1016/j.drugpo.2005.12.002
- Czaika, M., & De Haas, H. (2014). The globalization of migration: Has the world become more migratory? International Migration Review, 48(2), 283–323. https://doi.org/10.1111/imre.12095
- Goldenberg, S. M., Rivera Mindt, M., Rocha Jimenez, T., Brouwer, K., Morales Miranda, S., & Fisher, C. B. (2015). Structural and interpersonal benefits and risks of participation in HIV research: Perspectives of female sex workers in Guatemala. Ethics & Behavior, 25(2), 97–114. https://doi.org/10.1080/10508422.2014.950270
- Goldenberg, S. M., Rocha Jiménez, T., Brouwer, K. C., Morales Miranda, S., & Silverman, J. G. (2018). Influence of indoor work environments on health, safety, and human rights among migrant sex workers at the Guatemala-Mexico border: A call for occupational health and safety interventions. BMC International Health and Human Rights, 18(1), 1–13. https://doi.org/10.1186/s12914-018-0149-3
- Hemelaar, J., Gouws, E., Ghys, P. D., & Osmanov, S. (2011). Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS (London, England), 25(5), 679. https://doi.org/10.1097/QAD.0b013e328342ff93
- Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA x: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547. https://doi.org/10.1093/molbev/msy096
- Lyons, T., Kerr, T., Duff, P., Feng, C., & Shannon, K. (2014). Youth, violence and non-injection drug use: Nexus of vulnerabilities among lesbian and bisexual sex workers. AIDS Care, 26(9), 1090–1094. https://doi.org/10.1080/09540121.2013.869542
- Martínez, R. M., Casanueva, C. F., & Morales, S. Border spaces: Stigma and social vulnerability to HIV/AIDS among Central American male migrants at the Mexico–Guatemala border.
- Mello, B. (2018). Estimating timetrees with MEGA and the TimeTree resource. Molecular Biology and Evolution, 35(9), 2334–2342. https://doi.org/10.1093/molbev/msy133
- Migration Policy Institute. Country Resources: Guatemala. Migration Information Source. (2022). Available from: https://www.migrationpolicy.org/country-resource/guatemala.
- Miura, S., Tamura, K., Tao, Q., Huuki, L. A., Kosakovsky Pond, S. L., Priest, J., Deng, J., & Kumar, S. (2020). A new method for inferring timetrees from temporally sampled molecular sequences. PLoS Computational Biology, 16(1), e1007046. https://doi.org/10.1371/journal.pcbi.1007046
- Morales-Miranda, S., Jacobson, J. O., Loya-Montiel, I., Mendizabal-Burastero, R., Galindo-Arandi, C., Flores, C., & Chen, S. Y. (2014). Scale-up, retention and HIV/STI prevalence trends among female sex workers attending VICITS clinics in Guatemala. PLoS One, 9(8), e103455. https://doi.org/10.1371/journal.pone.0103455
- Muñoz Martínez, R., Fernández Casanueva, C., González, O., Morales Miranda, S., & Brouwer, K. C. (2020). Struggling bodies at the border: Migration, violence and HIV vulnerability in the Mexico/Guatemala border region. Anthropology & Medicine, 27(4), 363–379. https://doi.org/10.1080/13648470.2019.1676638
- Oliveira, D., Deforche, T., Cassol, K., Salminen, S., Paraskevis, M., Seebregts, D., Snoeck, C., Van Rensburg, J., Wensing, E. J., Van De Vijver, A. M., Boucher, D. A., & A, C. (2005). An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics, 21(19), 3797–3800. https://doi.org/10.1093/bioinformatics/bti607
- Park, S. Y., Faraci, G., Murphy, G., Pilcher, C., Busch, M. P., & Lee, H. Y. (2021). Microdrop human immunodeficiency virus sequencing for incidence and drug resistance surveillance. The Journal of Infectious Diseases, 224(6), 1048–1059. https://doi.org/10.1093/infdis/jiab060
- Pineda-Peña, A. C., Faria, N. R., Imbrechts, S., Libin, P., Abecasis, A. B., Deforche, K., Gómez-López, A., Camacho, R. J., de Oliveira, T., & Vandamme, A. M. (2013). Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: Performance evaluation of the new REGA version 3 and seven other tools. Infection, Genetics and Evolution, 19, 337–348. https://doi.org/10.1016/j.meegid.2013.04.032
- Platt, L., Grenfell, P., Meiksin, R., Elmes, J., Sherman, S. G., Sanders, T., Mwangi, P., & Crago, A. L. (2018). Associations between sex work laws and sex workers’ health: A systematic review and meta-analysis of quantitative and qualitative studies. PLoS Medicine, 15(12), e1002680. https://doi.org/10.1371/journal.pmed.1002680
- Plummer, F. A., Nagelkerke, N. J., Moses, S., Ndinya-Achola, J. O., Bwayo, J., & Ngugi, E. (1991). The importance of core groups in the epidemiology and control of HIV-1 infection. AIDS (London, England), 5, S169–S176.
- Ragonnet-Cronin, M., Hodcroft, E., Hué, S., Fearnhill, E., Delpech, V., Brown, A. J. L., & Lycett, S. (2013). Automated analysis of phylogenetic clusters. BMC Bioinformatics, 14(1), 1–10. https://doi.org/10.1186/1471-2105-14-317
- Rhodes, T., Singer, M., Bourgois, P., Friedman, S. R., & Strathdee, S. A. (2005). The social structural production of HIV risk among injecting drug users. Social Science & Medicine, 61(5), 1026–1044. https://doi.org/10.1016/j.socscimed.2004.12.024
- Robertson, D. L., Anderson, J. P., Bradac, J. A., Carr, J. K., Foley, B., Funkhouser, R. K., Gao, F., Hahn, B. H., Kalish, M. L., Kuiken, C., Learn, G. H., Leitner, T., McCutchan, F., Osmanov, S., Peeters, M., Pieniazek, D., Salminen, M., Sharp, P. M., … Korber, B. (2000). HIV-1 nomenclature proposal. Science, 288(5463), 55–55. https://doi.org/10.1126/science.288.5463.55d
- Rocha-Jiménez, T., Brouwer, K. C., Silverman, J. G., Morales-Miranda, S., & Goldenberg, S. M. (2016). Migration, violence, and safety among migrant sex workers: A qualitative study in two Guatemalan communities. Culture, Health & Sexuality, 18(9), 965–979. https://doi.org/10.1080/13691058.2015.1122229
- Rocha-Jiménez, T., Morales-Miranda, S., Fernández-Casanueva, C., & Brouwer, K. C. (2020). The influence of migration in substance use practices and HIV/STI–related risks of female sex workers at a dynamic border crossing. Journal of Ethnicity in Substance Abuse, 19(4), 503–520. https://doi.org/10.1080/15332640.2018.1556763
- Rocha-Jiménez, T., Morales-Miranda, S., Fernández-Casanueva, C., Brouwer, K. C., & Goldenberg, S. M. (2018). Stigma and unmet sexual and reproductive health needs among international migrant sex workers at the Mexico–Guatemala border. International Journal of Gynecology & Obstetrics, 143(1), 37–43. https://doi.org/10.1002/ijgo.12441
- Rocha-Jiménez, T., Morales-Miranda, S., Fernández-Casanueva, C., Silverman, J. G., Zúñiga, M. L., Goldenberg, S. M., … Brouwer, K. C. (2022). Migration and mobility: Correlates of recent HIV testing among substance using female sex workers at the Mexico–Guatemala border. AIDS and Behavior, 1–10.
- Rose, R., Lamers, S. L., Dollar, J. J., Grabowski, M. K., Hodcroft, E. B., Ragonnet-Cronin, M., Gao, F., Hahn, B. H., Kalish, M. L., Kuiken, C., Learn, G. H., Leitner, T., McCutchan, F., Osmanov, S., Peeters, M., Pieniazek, D., Salminen, M., Sharp, P. M., … Laeyendecker, O. (2017). Identifying transmission clusters with cluster picker and HIV-TRACE. AIDS Research and Human Retroviruses, 33(3), 211–218. https://doi.org/10.1089/aid.2016.0205
- Saber, B. J., Torimiro, J. N., Barbara, A. T., Laure, T. A., Fokam, J., Takou, D., & Mbacham, W. F. (2016). Performance of rapid subtyping tools used for the classification of HIV type 1 recombinants isolated from selected countries in west and Central Africa. African Journal of Biotechnology, 15(50), 2744–2750. https://doi.org/10.5897/AJB2016.15554
- Shannon, K., Kerr, T., Allinott, S., Chettiar, J., Shoveller, J., & Tyndall, M. W. (2008). Social and structural violence and power relations in mitigating HIV risk of drug-using women in survival sex work. Social Science & Medicine, 66(4), 911–921. https://doi.org/10.1016/j.socscimed.2007.11.008
- Siepel, A. C., Halpern, A. L., Macken, C., & Korber, B. T. (1995). A computer program designed to screen rapidly for HIV type 1 intersubtype recombinant sequences. AIDS Research and Human Retroviruses, 11(11), 1413–1416. https://doi.org/10.1089/aid.1995.11.1413
- Sirotin, N., Strathdee, S. A., Lozada, R., Nguyen, L., Gallardo, M., Vera, A., & Patterson, T. L. (2010). A comparison of registered and unregistered female sex workers in Tijuana, Mexico. Public Health Reports, 125(4_suppl), 101–109. https://doi.org/10.1177/00333549101250S414
- Smith, D. M., May, S. J., Tweeten, S., Drumright, L., Pacold, M. E., Pond, S. L. K., Pesano, R. L., Lie, Y. S., Richman, D. D, Frost, S. D. W., Woelk, C. H., & Little, S. J. (2009). A public health model for the molecular surveillance of HIV transmission in San Diego, California. Aids (London, England), 23(2), 225–232. https://doi.org/10.1097/QAD.0b013e32831d2a81
- Strauss, A., & Corbin, J. (1990). Basics of qualitative research. Sage publications.
- Strauss, A., & Corbin, J. (1998). Basics of qualitative research: Techniques and procedures for developing grounded theory (2nd ed.). Sage Publications, Inc.
- Tamura, K., & Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10(3), 512–526.
- Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL w: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22(22), 4673–4680. https://doi.org/10.1093/nar/22.22.4673
- UNAIDS. Geneva: Joint united nations programme on HIV/AIDS. Geneva. (2021); Licence: CC BY-NC-SA 3.0 IGO. Available from: https://www.unaids.org/en/resources/documents/2021/2021_unaids_data.
- Villa, B., Tapia, A., Caballero, M., Dreser, A., Cuadra, S., González, T., … Bronfman, M. (2004). México, Ciudad Hidalgo, Chiapas. M. Bronfman, R. Leyva, & M. Nigroni. coord. Movilidad poblacional y VIH/Sida, 275-302.
- Woods, C. K., Brumme, C. J., Liu, T. F., Chui, C. K., Chu, A. L., Wynhoven, B., Hall, T.A., Trevino, C., Shafer, R. W., & Harrigan, P. R. (2012). Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. Journal of Clinical Microbiology, 50(6), 1936–1942. https://doi.org/10.1128/JCM.06689-11
