Klinefelter syndrome is the most frequent chromosome disorder in men, but it is largely undiagnosed or receives a late diagnosis in adulthood. This condition is characterized by an extra X-chromosome: approximately 80%-90% of patients with Klinefelter syndrome have a 47,XXY karyotype, 10%-20% mosaicisms of two different genetic lines such as 47,XXY/46,XY, isochromosome X, and higher number of X chromosomes [Citation1,Citation2]. Although our knowledge on this syndrome substantially improved in last years, the diagnostic rate is still low. It has been estimated that only 25% to 40% of subjects with 47,XXY Klinefelter syndrome are ever diagnosed [Citation2,Citation3]. A prenatal diagnosis is made in 15–20% of these cases, 10% is diagnosed before puberty, 15% at puberty, and the remaining 50–60% of cases are diagnosed during adulthood, typically in the course of a fertility workup, with some cases diagnosed even after the age of 50 or 60 years [Citation2]. Variants with higher number of X chromosomes (48,XXXY and 49,XXXXY) have more severe phenotype and distinct clinical features, which leads to higher diagnostic rate than 47,XXY. This manuscript refers to the most common form 47,XXY syndrome and strategies to improve early and timely diagnosis.
This syndrome was described in 1942 in nine men affected by testicular hypotrophy, eunuchoidism, gynecomastia, elevated gonadotropins, and azoospermia [Citation4]. Many progresses have been made in basic and clinical aspects of this syndrome in the last 80 years [Citation2,Citation5,Citation6] and the ‘classic’ phenotype as described above and in earlier literature is actually now considered to affect a minority of patients. Importantly, the phenotype expression of patients with Klinefelter syndrome demonstrates substantial variability, both in terms of number and severity of symptoms and signs. As an example, even if testicular malfunction is a common feature, azoospermia is not obligated and 8–10% of patients have sperm in the ejaculate and the chance of sperm retrieval by testicular sperm extraction in azoospermic subjects is 45–50% [Citation7]. Furthermore, there is no single typical and specific clinical sign of this syndrome and many patients present a variety of nonspecific features [Citation1,Citation2,Citation8]. Finally, it is now recognized that patients with Klinefelter syndrome are at risk for increased co-morbidities, such as cardiovascular and metabolic diseases, osteoporosis, neurocognitive and psychosocial manifestations, that might be present in variable association [Citation1,Citation2,Citation8].
As said, not only Klinefelter syndrome is under-diagnosed, but also there is a marked delay in diagnosis. The variable expression of phenotype, the lack of stigmata and specific signs and the relatively low frequency of the ‘typical’ constellation of symptoms, in combination with a relatively high frequency of patients with mild symptoms and/or a variety of nonspecific symptoms, might explain these data. In addition, poor awareness, knowledge and consideration of Klinefelter syndrome among non-experts complicate the possibility for an early and timely diagnosis.
Advantages of early diagnosis are conceivable, as a dedicated and timely follow-up for prevention and early recognition of clinical signs, co-morbidities and consequences of the syndrome might change the natural history of the disease. For example, management of lifestyles, timely speech support, starting of testosterone treatment in cases of hypogonadism and fertility preservation might be optimally performed when diagnosis is done early and might be associated with better clinical progress of the syndrome and its possible clinical sequelae. For example, a recent study showed that the rate of osteoporosis and fractures in adult men with Klinefelter syndrome is correlated to age at diagnosis [Citation9].
An increase in diagnostic rate prenatally is only possible by implementation of prenatal genetic testing, whereas diagnostic rate postnatally could be improved only by better awareness and recognition of the symptoms and signs that could trigger clinical suspicion of Klinefelter syndrome at the different ages [Citation10] ( and ).
Figure 1. Schematic representation of the progression of phenotype expression in 47,XXY Klinefelter syndrome from birth to adulthood. On the left, the scale indicates the number of clinical signs from none to many. On the right, the scale indicates the specificity of clinical signs, from absent and nonspecific, to quite specific when combined. The scale on the bottom indicates the frequency of clinical signs, from none at right to frequent at left. Prenatal, birth, children and pre-puberty, adolescence and puberty, and adulthood ages are indicated, together with a red arrow showing the progression of clinical phenotype from clinically silent to clinical evident and full expression of the syndrome. The black arrow indicates possibilities for diagnosis at the different ages, from the fortuitous diagnosis during prenatal genetic analysis, to the late diagnosis made in adulthood
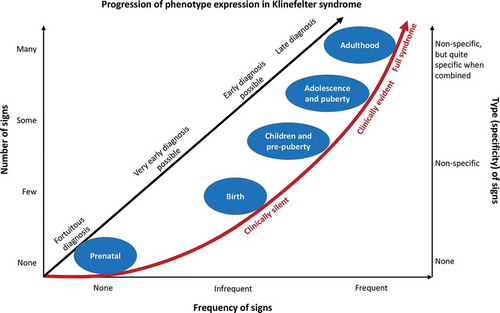
Table 1. General strategies for early diagnosis of 47,XXY Klinefelter syndrome
Prenatal diagnosis is generally the consequence of a fortuitous finding during prenatal karyotyping performed for other reasons, as male fetus with Klinefelter syndrome has no abnormalities detectable at ultrasonography and biochemical tests. It is presumable that the increasing use of prenatal tests (particularly noninvasive prenatal test – NIPT – on circulating cell-free fetal DNA in maternal plasma) will increase the diagnostic rate of Klinefelter syndrome. Similarly, the increased use of preimplantation genetic testing (PGT) during in-vitro fertilization techniques could give us more information on the prenatal prevalence of Klinefelter syndrome [Citation11].
At birth, Klinefelter syndrome is generally silent, as the majority of newborns do not present clinical signs and have a normal male phenotype. Signs of intrauterine hypogonadism such as cryptorchidism, reduced penile length and testicular volume might be present, but their actual prevalence is unclear [Citation12–14]. In a study on 600 newborns with isolated cryptorchidism we found a prevalence of Klinefelter syndrome of 1.3% that increased to 4.2% when considering only boys with persistent (no spontaneous testicular descent in 12–24 months) bilateral forms of cryptorchidism [Citation15]. However, the exact prevalence of cryptorchidism in patients with Klinefelter syndrome is unclear [Citation8,Citation14,Citation16], and only a general chromosomal screening at birth could help in defining this aspect. Newborn screening could be advocated as the best strategies to increase the diagnostic rate of Klinefelter syndrome [Citation17]. Nevertheless, as for prenatal screening, disadvantages (increase in parental anxiety, cost-effectiveness not known, risk of excessive medicalization, social and ethical problems) exceed the possible benefits (dedicated and timely clinical follow-up and better knowledge of the natural history of the disease). Prenatal diagnosis has also the possible risk of increasing the abortion rate. Therefore, the best way to increase the diagnostic rate at birth is to perform karyotype analysis in newborns with bilateral cryptorchidism (above all when spontaneous descent does not occur in the first year) and micro-penis (), as per guidelines [Citation18].
An increase in early diagnosis is better advisable in pre-pubertal children, or at least during adolescence. In these cases, the diagnosis has the least potential negative effects and provides the best opportunities for prevention, early detection and treatment of clinical conditions associated with Klinefelter syndrome. Support for speech and learning difficulties could be done only when the diagnosis is made in childhood, whereas opportunities for management of puberty and fertility, prevention of long-term consequences of hypogonadism, co-morbidities and complications are still available when the diagnosis is made in adolescence. Although the clinical signs are quite nonspecific in this period, some features (and above all their combination) might be useful for clinical suspect. The common feature of Klinefelter syndrome includes primary testicular failure starting from mid-puberty, with testicular hypotrophy and elevated gonadotropins, associated in about half of the cases with low testosterone and signs of hypogonadism [Citation18]. Therefore, diagnosis should be suspected in cases characterized by normal starting of puberty, followed by its arrest, lack of testicular growth and hypergonadotropic hypogonadism or compensated hypogonadism (elevated gonadotropins with still normal testosterone levels). In these cases karyotype analysis is recommended [Citation18]. Gynecomastia, increased fat/lean mass proportion and other signs of hypogonadism, such as long legs and tall stature or eunuchoid proportion and reduced virilization, as well as nonspecific behavioral, social and psychological difficulties might be present in some cases and further increase clinical suspect (). General pediatricians, pediatric endocrinologists, but also sport doctors or school doctors, could have a great opportunity for early diagnosing Klinefelter syndrome in this period of life.
An early diagnosis is possible and advisable pre-pubertally, but in children the clinical signs are actually few and often vague, and there is no consensus for recommendation of karyotype analysis based on clinical signs [Citation18]. Reduced testis volume, long legs and tall stature, tendency to overweight and increased fat/lean mass proportion, speech and learning difficulties, deficits in cognitive and executive functioning could be observed [Citation2] (). Although diagnosis in adolescence and puberty allows correct management from puberty onward, it is too late for neuropsychiatric intervention and speech therapy, and diagnosis in pre-pubertal children has all the advantages of prevention of co-morbidities and complications and timely clinical management, coupled with the least potential negative impact.
Diagnostic rate could be increased in adulthood, but obviously this is not by definition early diagnosis. Higher rate of diagnosis could be achieved if recommendations for karyotype analysis would be regularly followed [Citation18] (). Importantly, a better awareness of the suggestive signs () not only in specialists (endocrinologists/andrologists) but especially non-specialists (family, school, sport doctors) could increase diagnostic rate.
In conclusion, a higher number of patients could be diagnosed and the diagnosis could be made earlier and timely. This implies better knowledge and information of this syndrome among professionals, so that they are motivated to clinical suspicion even in the absence of specific signs. Besides endocrinologists and andrologists, also general practitioners, pediatricians, speech therapists, and school and sport medical officers should pay more attention to this syndrome. For example, an important and very simple step in this direction would be if all physicians would examine the testes of their patients routinely (), as small testicular volume is the most consistent symptom of KS [Citation6]. Education and increase awareness on this condition appear the best strategies [Citation19], possible with an ‘andrological screening and prevention program’ from birth onward promoted by national health systems [Citation20].
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Bonomi M, Rochira V, Pasquali D, et al. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest. 2017 Feb;40(2):123–134.
- Gravholt CH, Chang S, Wallentin M, et al. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocr Rev. 2018 Aug;39(4):389–423.
- Berglund A, Viuff MH, Skakkebæk A, et al. Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47,XXX and 47,XYY syndrome: a nationwide cohort study. Orphanet J Rare Dis. 2019 Jan;14(1):16.
- Klinefelter HJ, Reifenstein EJ, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without A‐Leydigism and increased excretion of follicle‐stimulating hormone. J Clin Endocrinol. 1942;2:615–627.
- Radicioni AF, Ferlin A, Balercia G, et al. Consensus statement on diagnosis and clinical management of Klinefelter syndrome. J Endocrinol Invest. 2010 Dec;33(11):839–850.
- Nieschlag E, Ferlin A, Gravholt CH, et al. The Klinefelter syndrome: current management and research challenges. Andrology. 2016 May;4(3):545–549.
- Corona G, Pizzocaro A, Lanfranco F, et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2017 May;23(3):265–275.
- Lanfranco F, Kamischke A, Zitzmann M, et al. Klinefelter’s syndrome. Lancet. 2004 Jul 17-23;364(9430):273–283.
- Vena W, Pizzocaro A, Indirli R, et al. Prevalence and determinants of radiological vertebral fractures in patients with Klinefelter syndrome. Andrology. 2020 Jun. DOI:10.1111/andr.12841
- Davis S, Howell S, Wilson R, et al. Advances in the interdisciplinary care of children with Klinefelter syndrome. Adv Pediatr. 2016 Aug; 63(1): 15–46.
- Mazzilli R, Cimadomo D, Rienzi L, et al. Prevalence of XXY karyotypes in human blastocysts: multicentre data from 7549 trophectoderm biopsies obtained during preimplantation genetic testing cycles in IVF. Hum Reprod. 2018 Jul;33(7):1355–1363.
- Lahlou N, Fennoy I, Ross JL, et al. Clinical and hormonal status of infants with nonmosaic XXY karyotype. Acta Paediatr. 2011 Jun;100(6):824–829.
- Ross JL, Samango-Sprouse C, Lahlou N, et al. Early androgen deficiency in infants and young boys with 47,XXY Klinefelter syndrome. Horm Res. 2005;64(1):39–45.
- Ratcliffe SG, Butler GE, Jones M. Edinburgh study of growth and development of children with sex chromosome abnormalities. IV. Birth Defects Orig Artic Ser. 1990;26(4):1–44.
- Ferlin A, Zuccarello D, Zuccarello B, et al. Genetic alterations associated with cryptorchidism. JAMA. 2008 Nov;300(19):2271–2276.
- Bojesen A, Juul S, Birkebaek NH, et al. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006 Apr;91(4):1254–1260.
- Herlihy AS, McLachlan RI. Screening for Klinefelter syndrome. Curr Opin Endocrinol Diabetes Obes. 2015 Jun;22(3):224–229.
- Zitzmann M, Aksglaede L, Corona G, et al. European academy of andrology (EAA) guidelines on Klinefelter syndrome. Andrology. 2020 Sep 22. DOI: 10.1111/andr.12909. Online ahead of print.
- Radicioni AF, De Marco E, Gianfrilli D, et al. Strategies and advantages of early diagnosis in Klinefelter’s syndrome. Mol Hum Reprod. 2010 Jun;16(6):434–440.
- Ferlin A. Prevention of male infertility: from childhood to adulthood. In: Aitken J, Mortimer D, Kovacs G, editors. Male and sperm factors that maximize IVF success. Cambridge: Cambridge University Press; 2020. p. 211–228.
