ABSTRACT
Introduction
Differences and disorders of sex development (DSD) are a diverse group of conditions, which often present in early childhood and may require input from a group of experts in a wide range of clinical fields. Clinical guidance in this field recommends that these experts function as a multidisciplinary team (MDT) within which each expert has a defined role, which ensures an integrated and streamlined approach to the care of affected individuals.
Areas covered
This review will focus on the benefits of multidisciplinary care for people with DSD, as well as the challenges that may be faced.
Expert opinion
Core members of the MDT for people with DSD include endocrinologists, surgeons, psychologists, geneticists, specialist nurses, radiologists, and gynecologists, although many other health-care professionals may also be pertinent, at different stages of the patient’s life. With greater acceptance of remote and digital health-care technology, there is a need to review the traditional concepts of the clinical MDT so that new care models can be explored for effective and efficient delivery of complex care.
KEYWORDS:
1. Introduction
Disorders of sex development (DSD) are a diverse group of congenital conditions in which genetic, chromosomal, gonadal, or anatomical sex is abnormal [Citation1]. Atypical genitalia at birth are the commonest manifestation of DSD occurring in approximately 1 in 300 births [Citation2]. However, genitalia that are sufficiently atypical to require investigations during early infancy are rare with a birth prevalence of about 1 in 1, 200 in term infants and those infants that require specialist input are even rarer at about 1 in 3, 300 term births [Citation3]. There are three main categories of DSD: 46,XX DSD, 46,XY DSD, and sex chromosome DSD. In early infancy, in over 80% of cases, the child will have a 46, XY karyotype and over 90% of these will be assigned a male sex [Citation3]. In a small number of cases ranging between 1 in 4, 500 births and 1 in 11, 000 infants, the genitalia may be so atypical that a decision regarding sex assignment may need to be delayed [Citation3]. There is a wide range of conditions that lead to XX DSD and XY DSD and its evaluation requires diagnostic input from a range of experts [Citation4]. Finally, sex chromosomal DSDs are the result of atypical sex chromosome pairings, leading to aneuploidy or absent chromosomes due to non-disjunction during meiosis. Conditions such as Turner syndrome (45,X), Klinefelter syndrome (47,XXY), and gonadal dysgenesis (45,X/46,XY and 46,XX/46,XY) are within this group. DSD is often associated with additional abnormalities, including neurodevelopmental problems, cardiac conditions and skeletal dysplasia [Citation5]. Parents of children affected with DSD also report the need for high levels of psychological support [Citation6]. Due to the complex and diverse nature of DSD, multidisciplinary teams (MDTs) are therefore recommended to optimize patient care [Citation4]. These teams consist of experienced clinicians of various specialty backgrounds, giving the patient simultaneous access to medical, surgical, and psychological care, depending on their individual needs.
2. The history of multidisciplinary teams
An MDT is a collection of health-care professionals from a range of specialist backgrounds, working together to provide holistic, patient-centered care to an individual presenting with a complex condition. The history of MDT working in DSD conditions has been extensively reviewed elsewhere [Citation7]. However, it has long been recognized that the management of DSD conditions requires the expertise of specialists in the field [Citation8], and in particular experienced psychologists [Citation9]. As early as the 1980s, clinicians sought a standardized MDT approach to DSD care [Citation10]. Finally, an international consensus statement, informally known as the Chicago consensus, was published in 2006, which specifically advised the establishment of MDT working in DSD [Citation1].
Certain characteristics of the MDT have been suggested to increase efficiency of the MDT (Supplementary Table 1) [Citation11–13]. Of note, although ‘multidisciplinary,’ ‘interdisciplinary,’ and ‘transdisciplinary’ teams each involve members of different disciplines of health care working together, there are some key differences between these models. For example, definitions of multidisciplinary care do not require that the treatment providers are all located in the same facility. Members of the MDT can also pursue treatments with separate goals that do not take into account the contributions of other disciplines [Citation14]. In comparison, the term ‘interdisciplinary care’ is supposed to convey a more integrated concept of care, with care being provided at the same facility and with a common goal, for example each member working together to improve the fertility of the patient. A transdisciplinary approach is one in which the members of the team have become so knowledgeable about the roles and responsibilities of the other team members that they can also perform these interchangeably [Citation15]. In practice, care is often delivered to a complex patient using a combination of all three of these models and the term MDT has often been used to cover all three approaches.
3. The role of individual members of a clinical DSD team
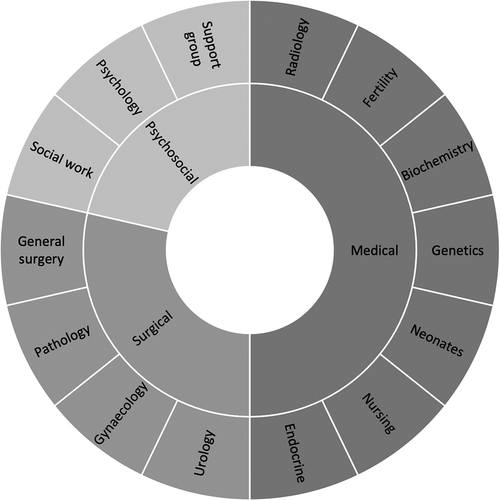
As per guidance from the British Society for Paediatric Endocrinology (BSPED) and the Society for Endocrinology, the DSD MDT should ideally include a pediatric endocrinologist, gynecologist and/or urologist, clinical geneticist, and psychologist [Citation4], as well as a specialist nurse, medical ethics advisor, and social worker where available [Citation1]. The core members of the team provide critical input into the initial diagnosis and management of the patient, but other team members may also be important in the care of a patient with DSD, as shown in .
Figure 1. Representative of the relative contributions of different professions to the management of a patient with DSD.
3.1. Neonatal care
Whether a DSD is prenatally suspected or not, the neonatologist will commonly be the first point of contact for the newborn. A nationwide review of cases of atypical genitalia in Scotland demonstrated that 93% of families of children with atypical genitalia met a neonatologist or general pediatrician working on a baby care unit within the first 3 months of life compared to 74% meeting a pediatric endocrinologist and 64% meeting a pediatric surgeon or urologist [Citation3]. As such neonatologists are clearly critical in the initial management of the infant in regard to both the DSD and any concurrent health conditions and should be integrated within the DSD MDT. Communication is an important aspect of this role, as not only must they communicate the potential presence of a complex condition to the neonate’s family in a sensitive, calm manner, they will also have to communicate with various pediatric subspecialties for guidance in first-line diagnosis and management paths. In this way, the neonatologist has a key role in establishing the initial MDT for a child with DSD.
3.2. Pediatric endocrinology
In 75% of cases, the pediatric endocrinologist is the lead clinician and overall care coordinator within the DSD MDT [Citation16]. This is in line with guidance issued by the Society for Endocrinology [Citation4]. The endocrinologist will be involved in the initial stages of diagnosis and management of the neonate suspected of having a DSD, supervising the first-line investigations. Hormone levels are analyzed using blood and urine samples, with results rapidly available for interpretation. Levels of testosterone, 17-hydroxprogesterone (17OHP), cortisol, and anti-Müllerian hormone (AMH) are among the most commonly investigated [Citation16]. Through comprehensive endocrine testing, potentially life-threatening disorders such as CAH, for example, can be identified and treatment rapidly administered. Input from the endocrinologist is also vital in later management of DSD where hormonal replacement therapy and hormonal monitoring are required.
3.3. Clinical genetics
Within the MDT, the clinical geneticist is responsible for overseeing and interpreting genetic analyses. Genetic analysis can be conducted on a single gene or a wide panel of genes through high throughput sequencing [Citation17]. From preliminary DSD classification to establishing a final diagnosis, genetic testing and interpretation dictates subsequent management throughout the patient’s life. Not only is this significant from a clinical perspective, but having a definitive diagnosis often offers useful information regarding the future for both the patient and their family. Although techniques such as next-generation sequencing (NGS) have previously been limited by high costs and long turnover times, resulting in them typically being used as second-line diagnostic tools, recent studies have indicated that NGS could be an efficient, cost-effective option for first-line testing [Citation18]. However, large-scale studies and further technical developments are required to confirm this. The clinical geneticist also plays an important role in terms of the provision of prenatal counseling. A study of the outcome of prenatally detected sex chromosome abnormalities in Scotland demonstrated that where formal genetic counseling was received from a clinical geneticist, rates of termination of pregnancy reduced from 43% to 2% [Citation19].
3.4. Pediatric urology
Along with the pediatric endocrinologist, the urologist will be involved in the primary physical examination of the external anatomy, and review of diagnostic imaging regarding the internal genitalia. The urologist may also perform surgical diagnostic techniques such as laparoscopies, gonadal biopsies, cystoscopies, and vaginoscopies [Citation20]. Following these diagnostic tests, the urologist must discuss the findings with the MDT and the parents, presenting the pros and cons of various surgical management options if applicable. Individuals with DSD may need multiple surgical interventions, with individuals with comorbidities and genetic diagnoses being most likely to encounter complications of surgery [Citation21]. Working within an MDT, the urologist can seek appropriate advice and support from the endocrinologist, the geneticist, and the psychologist so that complications can be anticipated and addressed holistically. A recent international study into practice of gonadectomy highlighted that some individuals with DSD may be having gonadectomy performed prior to referral to a specialist center [Citation22], and this should also be prevented, with prior discussion in the MDT, as well as by training being provided by the MDT to other professionals to increase awareness of this issue. In addition, approximately 10% of adults who had childhood hypospadias repair report difficulties with erection and ejaculation [Citation4], and as such, the urologist should be involved in the MDT care of the boy with DSD into adulthood.
3.5. Specialist nursing care
Within the MDT, the pediatric specialist nurse is responsible for liaising with all other members of the MDT and passing this information onto the patients’ parents, providing support throughout the diagnostic and management process. The nurse may also be responsible for organizing the various diagnostic investigations selected for by the specialist doctors. Due to this role, they may often be the keyworker in the team, having the most contact with the patient and their family. Not only does this limit the confusion of having information coming from multiple sources, it means the family will form a relationship with the nurse, which will provide comfort at a potentially distressing and uncertain period of their child’s life. However, despite this, a pediatric specialist nurse is the most commonly missing member of an MDT, with 29% of international clinics reporting that they did not have access to one [Citation16]. The majority of these clinics also reported that they would want a nurse present, indicating that the standard of care could be improved by increasing accessibility to specialist nurses.
3.6. Psychosocial support
Immediate psychological care should be available to the patient and patient’s family if a DSD is suspected [Citation4]. Initially, this support is for the infant’s parents, helping them address the emotional distress and confusion that often arises with the diagnosis of a DSD. Promoting parental adaptation will play a significant role in establishing a healthy parent–child bond with studies indicating that early psychological management relates to improved parental behavior and mental health in chronic childhood conditions [Citation23]. DSD conditions are believed to represent significant risk factors for parental emotional distress and subsequent maladaptive psychosocial and psychosexual development of the child [Citation24]. As the child grows, the ability to talk openly about aspects such as gender identity, sexuality, and relationships is essential for healthy psychosocial development, and the team psychologist can be helpful in helping the child to formulate their own emotions and opinions on medical procedures they may undergo in order to uphold their sense of autonomy. That said, a recent study examining the role of clinical psychology in DSD found that psychologists were considered to be more important after medical intervention, suggesting that MDT teams need to rethink the balance of care in patients with DSD to ensure that the team is truly multi-professional at all stages [Citation25]. Currently, however, one study of international practice in DSD care demonstrated that only 11% of adults were offered psychological support [Citation26], despite the fact that it is known that individuals with, for example, partial androgen insensitivity syndrome continue to require psychological follow-up into adulthood [Citation27].
3.7. Gynecology and assisted reproduction
Gynecologists are specialists in genital and reproductive anatomy, hormonal function, fertility, sexuality, and obstetrics [Citation28]. Following diagnosis and early management, the gynecologist will initiate conversations regarding long-term treatment options with the parents of female patients. This can include surgical interventions, hormonal therapy, and vaginal dilator training. They will then take the lead in implementing these management options at suitable times in the patient’s life. Not only are they important in the physical management of the patient, the gynecologist will also be responsible for discussing sexual and reproductive aspects surrounding DSD, such as future fertility, with the child as they grow up. In some cases, the condition may not present until puberty. In these incidences, the gynecologist may be the first clinician to examine the patient and begin the diagnostic process.
3.8. Adult endocrinology
DSD conditions are lifelong, and inclusion of an adult endocrinologist in the MDT ensures smooth transition to adult services at the time of leaving pediatric care. Congenital adrenal hyperplasia, for example, requires prescription and monitoring of glucocorticoid therapy and other DSD conditions may require sex hormone replacement therapy into adulthood [Citation29]. Inclusion of the adult endocrinologist will also facilitate longer-term prospective outcome data to be collected on young people with DSD, to allow for improved clinical care throughout the lifespan. Liaison with specialists in the field of assisted conception will also be required for any adults with DSD conditions, which are likely to impair fertility, and the patient may not wish to engage in this process until into adulthood.
3.9. DSD diagnostics
The DSD Diagnostic Team will include pediatric radiologists, pathologists, and biochemists working with the common goal of identifying an underlying diagnosis for the affected child.
The pediatric radiologist is responsible for conducting and analyzing various diagnostic imaging techniques, which can be performed both pre- and postnatally. On rare occasions, a DSD may initially be suspected through antenatal ultrasound scan (US), indicating abnormal sex development, for example, the presence of a micropenis in males or clitoromegaly in females. US is also the most common form of postnatal imaging [Citation30] during first-line diagnosis. With recent advances in US imaging, this method alone commonly provides the radiologist with sufficient information to confirm a DSD. However, other techniques such as magnetic resonance imaging (MRI) can also be conducted where results are deemed unclear. A standardized systematic approach to imaging, customized to the patient, of the genital folds, inguinal areas, adrenals, and remaining abdominal cavity [Citation30], has the potential to improve the accuracy of results obtained thus leading to a faster diagnosis and less distress to the neonate and parents. The results interpreted by the radiologists will influence subsequent sex assignment and will be critical for the ongoing management of the neonate, thus highlighting the importance of the role. Experienced pathologists are important for reviewing histopathology and immunohistochemistry to identify the types of gonadal tissue present on biopsy. Some DSD conditions are at increased risk of malignant gonadal germ cell tumors and image review and appropriate staining of tissues with tumor markers may be required by pathologists with expertise in this field [Citation31]. Where these expertise are not available locally, the MDT could include specialists from other centers, improving clinical care as well as team education. Finally, much of the initial management of children with DSD is led by analysis of steroid hormones in the blood and urine. As such, having an experienced biochemist on the MDT will ensure that appropriate tests are ordered and interpreted accurately. Increasingly, biochemistry is being used in tandem with molecular genetics to confirm underlying DSD diagnoses and a standardized approach in international centers specializing in DSD care will allow for consistency of diagnostic pathways, reducing any differences in care provided depending on where the affected patient is born [Citation32].
3.10. Service coordination and data management
MDT clinics can result in significant workload, with one study demonstrating 11 hours per week of clinical trainee time and 13 hours per week of consultant-level medic time for preparation of the MDT and a further 11 hours per week of MDT delivery time in one tertiary oncology center [Citation33]. As such, without highly structured management and communication, the effectiveness and quality of care delivered by the team will be compromised, leading to poorer patient outcomes. An MDT Service Coordinator could therefore ease this burden with time dedicated to clinic organization and preparation. As previously discussed, there remains a paucity of long-term outcome data on individuals with DSD. Standardized core outcome data could therefore also be collected and regularly reviewed by the MDT data manager.
3.11. Social worker
DSD conditions can be associated with feelings of shame and stigmatization, poor self-esteem, and body dissatisfaction in adulthood [Citation34]. Studies have demonstrated that parents of young children with DSD report clinically significant levels of stress and difficulties with coping, irrespective of the appearance of the external genitalia [Citation35]. In infants with truly atypical genitalia, where it is difficult to assess the sex of the baby, this can result in challenges between balancing the medical needs of the child, the likelihood of future fertility and the cultural and societal expectations of the family. Decisions about the need for genital surgery, for example, can often be particularly challenging [Citation36]. As such, social workers within the DSD can provide an opportunity for early discussions regarding how best to support the families with these difficult discussions and decisions.
3.12. Ethicist
On occasion, there may be conflict within the MDT regarding respecting the affected young person’s right to choose about gonadectomy compared with the need to undertake the procedure to reduce the risk of harm and germ cell tumor. Clinicians have a duty of care to act in the best interests of their patients, but objective standards of ‘best interests’ may be difficult to determine on a case-by-case basis and a medical ethicist may therefore be particularly useful for conflict resolution in these cases [Citation7].
3.13. Patient support group
Patient advocacy groups represent an excellent source of peer support and experience, which can be invaluable for families. In particular, the provision of individualized peer support should be offered as routine after the initial diagnosis is made [Citation37]. Such support may include suggestions of topics to discuss at clinic consultations to empower the patient to discuss issues of concern to them freely. Alternatively, it may include acknowledgment that other families have been in the same situation previously, and discussion regarding strategies to overcome difficulties [Citation38].
3.14. Additional team members
The above list of potential members of the DSD specific MDT is not exhaustive. Indeed, in some health-care systems, other specialists should be involved. For example, in some countries, patients may be referred to an internist or an andrologist, or local primary care physicians may play a more significant role in coordinating care of the patient [Citation39]. In some settings, religious and cultural beliefs may influence decision-making in cases of DSD, and consideration of inclusion of a cultural or religious advisor may therefore also be pertinent [Citation40].
4. Communication
4.1. Communication within the MDT
Communication is a critical aspect of MDTs and will directly affect patient outcomes. Clear, open communication must exist between team members, the patient, and the patient’s family. The communication within the team will generally take place during scheduled meetings in which the treatment and management of the patient, such as which diagnostic investigations should be performed, are discussed. It should be noted that all team members may not be present in person if the MDT has been formed through an online network. Time zone differences, different primary languages, and technical issues also have the potential to negatively impact meeting quality in these cases. All of the MDT discussions, and in particular, which members of the MDT were present during the discussions should be formally documented.
4.2. Communication with the patient and family
Once results have been obtained and discussed and preliminary plans have been decided, the information must be passed on to the patient or patients’ parents, depending on the patient’s age. This should all be communicated by the lead clinician in the team, typically the endocrinologist, who should present the patient with the relevant information. By filtering the information through one team member, confusing and overwhelming the patient is avoided. Additionally, having multiple individual consultations with each clinician can be avoided, saving on the time of the patient, their caregivers and the MDT team members. While it is recognized that in person face-to-face communication provides several advantages, the use of remote consultation should not be discounted given that there is increasing acceptance and familiarity of this modality. However, this should be performed with care and through clinically approved communication systems such as ‘Attend Anywhere’ [Citation41].
As DSD conditions are, in general, poorly understood by the public, it is important that the lead clinician delivers information in a clear manner, answering any questions that may arise. This is of particular importance when communicating with the patient as they grow up. Age-appropriate language must be used, with details of their condition discussed at the correct time. For example, having a conversation about sexual function and relationships with the child would not be appropriate with a toddler and may lead to distress. In addition, patients and health-care professionals will have different priorities in terms of topics for discussion at clinic, at different stages in the life course [Citation42].
Communication between the MDT and patient/family through the lead clinician also ensures that each member of the team is fully aware of the patient’s status and can continue working with the MDT to plan the next stages of treatment. Of course, certain circumstances will call for direct communication via other members of the team. When discussing aspects such as complicated genital surgery for example, the urologist should also be present and may take the lead.
Lastly, in order for the team to continue to work together in an efficient and effective manner, it is important that all results and decisions made are documented. This prevents miscommunication and confusion within the team and will also permit a smooth transition from a pediatric to adult healthcare system when the time comes.
5. Benefits and challenges of MDT
The benefits of MDTs have been highlighted in multiple studies. Within DSD, MDTs have resulted in better clinical- and patient-reported outcomes [Citation43]. Through the teams, there is an increased capacity to treat conditions in a more systematic manner, factoring in the physical and psychological health of the patient [Citation44]. It may be viewed as a more holistic approach to the patient rather than a system-specific approach. MDTs allow information to be shared more quickly and easily about novel therapies, with improved communication between specialists. Clinicians can focus on their own specialties and not have to manage issues that are outside of their competence, resulting in increased professional satisfaction. The MDT meetings that are held can provide a continuous learning environment that improves the overall competence of the team and also helps with training as sharing of experience is especially helpful for difficult cases whereby team members can learn from the experience of others. These aspects become even more powerful when the MDT can lean on the experience and knowledge of a wider group beyond the geographical constraints of a single center. This shared experience also leads to the concept of shared responsibility, knowledge, and skills for the care of patients, which can give reassurance to the clinician as well as the patient. The MDT may also facilitate research and innovation more effectively than disparate groups within a center.
That said, challenges with MDTs do exist. First, constructing a full MDT can be difficult and is dependent on the financial resources and geographical location of the clinic. Clinics in resource-restricted settings may not have the resources available to recruit the various specialists required for a comprehensive team. Geographic location of the clinic may also be a limiting factor due to reduced accessibility to resources. However, the increased acceptability of remote consultation among patients as well as health-care professionals should overcome this hurdle of geography. The recent development and growth of online international collaborative networks, such as the International Disorders of Sex Development (I-DSD) Registry, may provide a solution for these specific limitations [Citation45]. Such networks allow health-care professionals from across the world to connect and form an MDT remotely. However, the advanced level of communication and management required to ensure the team is functioning at its optimal capacity can be another potential limitation. A study investigating methods to improve the effectiveness of multidisciplinary cancer teams stated that the increased work pressure many MDTs faced reduced the time available for discussing and planning patient care [Citation12]. It has been suggested that ‘streamlining’ discussions, described as focusing on the more complex cases and introducing smaller pre-MDT meetings to tackle the simpler ones, would enhance team efficiency [Citation4]. Sometimes changing one clinician’s ownership of the decision to a process of collective decision-making that relies on the opinion of multiple clinicians can prove to be challenging and, again, will need to rely on regular communication and feedback. A recent systematic review sought to ascertain whether MDTs represented cost-effective health care was unable to reach any conclusions due to a paucity of data, as none reported whether the potential savings of MDT working were offset by the time and costs spent administering, preparing for and attending the meetings [Citation46]. Advances in video conferencing have however been shown to be more cost-effective than traditional face-to-face MDT meetings, with an estimated cost saving of almost 50% over 2 years [Citation47]. As such, it may be that future MDT meetings will aim to have an increasingly virtual or a mix of virtual and face-to-face focus.
A number of different tools to assess the efficacy of MDTs exist including the MDT Quality Improvement Bundle [Citation48], the Team Evaluation and Assessment Measure [Citation49] and the MDT-Meeting Observational Tool [Citation50]. Peer observers have also been introduced to identify ways to improve the efficiency of MDT meetings [Citation51]. To date, most studies utilizing these tools have focussed on the MDT for cancer care, with no studies investigating their role in DSD. However, to sustain MDTs over the longer term, there is a need to explore how the effectiveness of these teams can be regularly assessed objectively.
6. Initiatives that have promoted MDT care in DSD
Despite a recent drive for more standardized pathways of care in the field of DSD, a survey using data from 124 clinics from 38 countries found that only 40% of clinics had complete MDTs including specialists in endocrinology, surgery or urology, clinical psychology, and nursing [Citation16]. Although the inclusion of team members can vary depending on geographical location and resources of the clinic, as well as the type of disorder present, this indicates that further efforts are required to enable optimal DSD care on an international level. A suggested minimum core group should include representatives from Endocrinology, Urology, Clinical Psychology, Nursing, Genetics and Biochemistry.
It is recognized that children with DSDs may be born in centers that do not have significant experience in the care of these conditions, and referral to the regional specialist center will be required [Citation52]. Advances in video technology are likely to make this easier, assuming access to the internet is available. Of note, a recent study of patient-reported outcomes from adolescents and adults with DSD conditions in Europe identified that patients reported higher rates of satisfaction where the center provided care that was perceived to be more multidisciplinary [Citation53]. As such, the aim for a patient-centered approach to DSD worldwide should include the inclusion of MDT models.
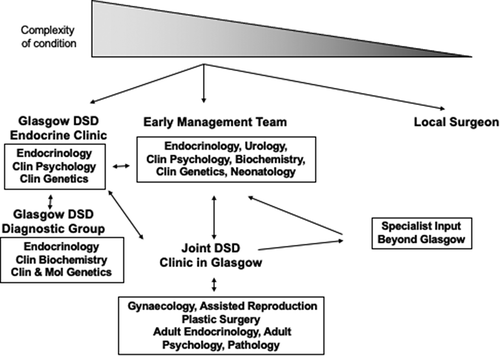
6.1. The Glasgow DSD Service
A standardized pathway for care for children with DSD in the West of Scotland is demonstrated in . Children with complex DSD conditions have access to the ‘Early Management Team,’ comprising Endocrinology, Urology, Clinical Psychology, Biochemistry, Clinical Genetics and Neonatology. They will then be followed up in the monthly DSD endocrine clinic where patients are seen jointly by Pediatric Endocrinology, Clinical Genetics, and Clinical Psychology. ‘Outreach’ clinics are also held regularly, where the clinical lead in DSD can attend clinics in person or remotely in other areas of the country. Prior to the monthly DSD endocrine, a monthly DSD Diagnostic Group Meeting is held on the same day and this reviews data on cases where the underlying diagnosis is unclear. This meeting is open to other hospitals in Scotland too through the secure NHS intranet, which extends across the whole of Scotland and can therefore serve the needs of the Scottish DSD Network. The DSD Diagnostic Group includes pediatric endocrinologists, clinical and steroid biochemists, and clinical and molecular geneticists. Cases are discussed and consensus is reached regarding any additional investigations required to confirm the cause of the DSD condition. The pathological significance of any biochemical or genetic findings is also discussed. This framework provides a structured approach to second- and third-line investigations for people with DSD conditions, with 364 individuals being discussed at this meeting since its inception in 2013. Children and young people with particularly complex DSD conditions may also be referred to the Joint DSD clinic, which is held 4 times a year and attended by a wider group of MDT professionals. This clinic was created in 2001 and an audit of the service from 2010 to 2021 demonstrated that 177 patients had been seen at this specialist MDT, with a median (range) of 1 (1, 7) attendances per person. The median (range) age at time of presentation at the SDSD clinic was 3.5 years (2 weeks, 52.5 years) years and the most common conditions referred to this clinic were nonspecific XY DSD (n = 86, 49%), disorders of gonadal development (n = 21, 12%) and congenital adrenal hyperplasia (n = 20, 11%). Summaries of the patients are discussed at the start of the clinic with all of the members of the MDT. Thereafter, patients and their families are seen in a separate room with only the key health-care professionals who are pertinent to their care at that time. The consultation in this room is however video linked, with consent from the patients to the first room so that all members of the MDT can stay involved, without intimidating the patient with the number of people involved in their care. The median (range) number of health-care professionals seen by the patient is 2 (1, 6), with the MDT members most commonly being seen face-to-face by the patient being pediatric endocrinology (n = 162, 92%), urology (n = 121, 68%) and psychology (n = 70, 40%). Other health-care professionals who have attended these clinics, however, have included pathologists, patient support group advocates and assisted conception specialists. More recently, these clinics are delivered in a hybrid format, with most of the team joining virtually, and only key members being present face-to-face. This model of MDT care has since been used successfully for other pediatric conditions in Glasgow. Through the Scottish DSD network, it was possible to organize prospective data collection on all infants born with atypical genitalia in Scotland from 2013 to 2019. This study noted that 57% of affected infants had specialist MDT input within the first 3 months of life [Citation3].
6.2. International DSD Networks
DSD is an area where a number of successful international collaborations have been implemented including multicenter registry-based networks (I-DSD and the DSD-TRN), educational initiatives (DSDNet) and clinical outcomes research (DSD-Life) [Citation54]. In addition to research, these activities are also now focusing on benchmarking of services [Citation29]. Audit of clinical activity, including patient-reported experience measures, participating in disease registries, building collaborative working partnerships, and attendance at joint clinics and education events are crucial if knowledge and information sharing is to be optimized across clinical teams. A clear example of an activity which has the potential to lead to an improvement in care quality is the model of the European Reference Networks (ERN) that have been funded by the EC for improving the care of people with rare conditions [Citation55]. Given that many specialist centers caring for patients with DSD do have not access to most of the members of the MDT [Citation16] and clear pathways and recommendations for MDT DSD care exist [Citation4], it is interesting to note that nearly 40% of children with atypical genitalia are not seen by the MDT in the first 3 months of life [Citation3]. As such, these pathways need to be regularly revised and reviewed to ensure a high quality of MDT care is available to all children with DSD and their families. These international networks have the potential to ease the access to expert input but implementing this in practice may be more challenging. One key goal of expert networks should be to provide information and training on the establishment of MDT working within the field of DSD. A study evaluating different approaches to the care of children with congenital adrenal hyperplasia and gonadal dysgenesis demonstrated that international clinical fellows had varying opinions on the role of the MDT [Citation56]. E-learning modules may therefore be useful in multiple languages to educate all professionals on the benefits and challenges of MDT working, with particular emphasis on the establishment of such teams in low-middle income (LMIC) countries, where specialists with experience in DSD care may not be easily accessible [Citation57].
7. Remote MDT consultations
Structures for providing clinical care are in constant flux currently. As such it is likely that all MDT clinics will need to utilize advances in technology and consider the use of remote consultations and platforms. Certain factors including the composition of the team, the infrastructure of the meetings and the governance of the team will influence the success of remote MDT working and should be optimized. Hybrid clinics offering virtual discussions followed by face-to-face consultations with the patient may represent the optimal conditions for MDT care in DSD, but further research is required to determine the cost-efficiency of these clinics, and whether they improve outcomes. When online patient interactions are recorded, care must be taken to ensure secure storage of the recordings and safe disposal of the footage thereafter, to maintain the highest standards of patient confidentiality [Citation58].
To date, however, video-conferencing of MDT clinics has been reported as being more efficient than in-person meetings with high levels of clinician-reported satisfaction with virtual models of MDT care [Citation59]. For virtual models to be successful, there is a reliance on provision of clinical information, which can be reviewed by the team prior to the meeting, as well as on decent technology being available and it is generally not considered acceptable that a first new patient consultation is done virtually [Citation60,Citation61]. The cost effectiveness of telemedicine MDT consultations, however, remains to be seen, with a randomized controlled trial of telemedicine for breast cancer care suggesting that a threshold of 40 meetings per year is required for telemedicine to be more cost-effective than face-to-face meetings [Citation62]. In an attempt to reduce health-care inequalities for patients with rare diseases in Europe, a Clinical Patient Management System (CPMS) has been developed by the European Union to provide expert specialized care remotely [Citation63]. This may be of particular advantage to patients who may otherwise have to travel long distances to reach their regional specialist DSD center. However, this facility is currently only available to health-care professionals within discipline-specific clinical networks and while they provide input to fellow colleagues, the platform needs further exploration for delivering true MDT care to the patient. Extension of these networks to include LMIC countries should also be facilitated [Citation64]. In some LMIC countries, for example, children may be more likely to be raised with atypical genitalia and secondary sexual characteristics into adolescence, representing unique management challenges, which are likely to benefit from experienced MDT discussions [Citation65].
8. Conclusions
The MDT is an essential requirement for the care of people with DSD conditions. It allows for a streamlined approach to the often complex psychological and medical needs of this population and reduces the need for multiple additional health-care appointments, improving the time and cost-efficiency of the clinic. Different models of MDT care exist and may include face-to-face or virtual components. Further research is required to determine whether MDT models are cost effective, but given they are perceived highly by both MDT members and patients, it is likely that they will continue to play an important role in DSD management.
9. Expert opinion
Given the heterogeneity of DSD conditions, high rates of associated comorbidities and psychological distress and the need for complex medical and surgical management, affected children and young people are required to see a number of different health-care professionals. Depending on the geographical location of the patient, this may result in a significant time and travel burden. These conditions are also relatively rare, but require the proficiency of health-care professionals, who have experience in the field. Multidisciplinary team working, therefore, represents the optimal way to ensure a high standard of care for individuals with these conditions and should be considered the gold standard for children and young people with DSD. The formulation of the MDT may vary according to the age and stage of the patient in question, but the general makeup will include pediatric endocrinology, urology, clinical psychology, genetics, specialist nursing, radiology, and gynecology. Additional team members may be required. MDT clinics will be responsible not only for the management of patients but also for the training of other health-care professionals who may encounter patients with DSD in their working practice. Regular review of MDT pathways and procedures is required to improve the quality of care offered by the teams and international efforts via reference networks and registries should be made to ensure that this quality of care is globally standardized. Increasingly, virtual MDT meetings may be utilized to improve the efficiency and cost-effectiveness of the MDT model. Given that the gold standard of DSD management is for care to take place in a specialist center with significant expertise in the field, advances in telemedicine would also allow for attendance at specialist MDT clinics, without patients having to travel large distances each time.
Article highlights
Given the heterogeneity of DSD conditions and the high likelihood of associated conditions, patients may require input from many different healthcare specialists.
Multidisciplinary care offers the opportunity for patients with DSD to see many specialists within a single coordinated service, reducing the need for multiple appointments.
Effective MDTs require leadership, resources and excellent communication.
Current research suggests MDT care is perceived highly by patients and their families.
Adaptations to the current MDT models will be required to accommodate advances in information technology and increased emphasis on remote consultations.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Supplymentary material
Supplymentary data for this article can be accessed online https://doi.org/10.1080/17446651.2022.2072829
Additional information
Funding
References
- Hughes IA, Houk C, Ahmed SF, et al. Consensus statement on management of intersex disorders. Arch Dis Child. 2006 July;91(7):554–563.
- Ahmed SF, Dobbie R, Finlayson AR, et al. Prevalence of hypospadias and other genital anomalies among singleton births, 1988–1997, in Scotland. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F149–F151.
- Rodie ME, Ali SR, Jayasena A, et al. A nationwide study of the prevalence and initial management of atypical genitalia in the newborn in Scotland. Sexual Dev. 2022;16(1):11–18.
- Ahmed SF, Achermann J, Alderson J, et al. Society for endocrinology UK guidance on the initial evaluation of a suspected difference or disorder of sex development (revised 2021). Clin Endocrinol (Oxf). 2021;95(6):818–840.
- Cox K, Bryce J, Jiang J, et al. Novel associations in disorders of sex development: findings from the i-dsd registry. J Clin Endocrinol Metab. 2014;99(2):E348–E355.
- Bennecke E, Werner-Rosen K, Thyen U, et al. Subjective need for psychological support (PsySupp) in parents of children and adolescents with disorders of sex development (dsd). Eur J Pediatr. 2015;174(10):1287–1297.
- Brain CE, Creighton SM, Mushtaq I, et al. Holistic management of DSD. Best Pract Res Clin Endocrinol Metab. 2010;24(2):335–354.
- Hendren WH, Crawford JD. The child with ambiguous genitalia. Curr Probl Surg. 1972;9(11):1–64.
- Wilkins L. The diagnosis and treatment of endocrine disorders in childhood and adolescence. London: Blackwell Scientific Publications; 1950. ISBN: 9780398058791.
- Drop S, Molenaar J, Scholtmeijer R, et al. A practical approach to the clinical evaluation and management of children with ambiguous genitalia. Zeitschrift für Kinderchirurgie. 1984;39(3):171–177.
- Ruhstaller T, Roe H, Thürlimann B, et al. The multidisciplinary meeting: an indispensable aid to communication between different specialities. Eur J Cancer. 2006 October 01;42(15):2459–2462.
- Hoinville L, Taylor C, Zasada M, et al. Improving the effectiveness of cancer multidisciplinary team meetings: analysis of a national survey of MDT members’ opinions about streamlining patient discussions. BMJ Open Qual. 2019;8(2):e000631.
- De Felice F, Tombolini V, de Vincentiis M, et al. Multidisciplinary team in head and neck cancer: a management model. Med Oncol. 2018 November 13;36(1):2.
- Gatchel RJ, McGeary DD, McGeary CA, et al. Interdisciplinary chronic pain management: past, present, and future. Am Psychologist. 2014;69(2):119.
- Cartmill C, Soklaridis S, Cassidy JD. Transdisciplinary teamwork: the experience of clinicians at a functional restoration program. J Occup Rehabil. 2011;21(1):1–8.
- Kyriakou A, Dessens A, Bryce J, et al. Current models of care for disorders of sex development – results from an international survey of specialist centres. Orphanet J Rare Dis. 2016 November 21;11(1):155.
- Audí L, Ahmed SF, Krone N, et al. Genetics in endocrinology: approaches to molecular genetic diagnosis in the management of differences/disorders of sex development (DSD): position paper of EU COST action BM 1303 ‘DSDnet.’ Eur J Endocrinol. 2018;179(4):R197–R206.
- Hughes LA, McKay-Bounford K, Webb EA, et al. Next generation sequencing (NGS) to improve the diagnosis and management of patients with disorders of sex development (DSD). Endocr Connect. 2019 Feb 01;8(2):100–110.
- Lucas-Herald AK, Cann F, Crawford L, et al. The outcome of prenatal identification of sex chromosome abnormalities. Arch Dis Childhood-Fetal Neonatal Ed. 2016;101(5):F423–F427.
- McNamara ER, Swartz JM, Diamond DA. Initial management of disorders of sex development in Newborns. Urology. 2017 October 01;101:1–8.
- Al-Juraibah F, Lucas-Herald A, Nixon R, et al. Association between extra-genital congenital anomalies and hypospadias outcome. Sexual Dev. 2019;13(2):67–73.
- Lucas-Herald AK, Rodie ME, Ahmed SF. Update on the management of a newborn with a suspected difference of sex development. Arch Dis Child. 2021;320872. doi: https://doi.org/10.1136/archdischild-2020-320872
- Law E, Fisher E, Eccleston C, et al. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev. 2019;3(3):CD009660.
- Sandberg DE, Gardner M, Callens N, et al. Interdisciplinary care in disorders/differences of sex development (DSD): the psychosocial component of the DSD—Translational research network. Am J Med Genet Part C. 2017;175(2):279–292.
- Liao L-M, Roen K. The role of psychologists in multi-disciplinary teams for intersex/diverse sex development: interviews with British and Swedish clinical specialists. Psychol Sexuality. 2021;12(3):202–216
- Dessens A, Guaragna-Filho G, Kyriakou A, et al. Understanding the needs of professionals who provide psychosocial care for children and adults with disorders of sex development. BMJ Paediatr Open. 2017;1(1):e000132.
- Lucas-Herald A, Bertelloni S, Juul A, et al. The long-term outcome of boys with partial androgen insensitivity syndrome and a mutation in the androgen receptor gene. J Clin Endocrinol Metab. 2016;101(11):3959–3967.
- Gomez-Lobo V, Oelschlager A-MA, FtNASf P, et al. Gynecological challenges in the diagnosis and care of patients with DSD: the role of the obstetrician gynecologist in the multidisciplinary approach to the patient. Am J Med Genet Part C. 2017;175(2):300–303.
- Cools M, Nordenström A, Robeva R, et al. Caring for individuals with a difference of sex development (DSD): a consensus statement. Nat Rev Endocrinol. 2018;14(7):415–429.
- Avni FE, Lerisson H, Lobo M-L, et al. Plea for a standardized imaging approach to disorders of sex development in neonates: consensus proposal from European society of paediatric radiology task force. Pediatr Radiol. 2019 August 01;49(9):1240–1247.
- Spoor JA, Oosterhuis JW, Hersmus R, et al. Histological assessment of gonads in DSD: relevance for clinical management. Sexual Dev. 2018;12(1–3):106–122.
- Kulle A, Krone N, Holterhus PM, et al. Steroid hormone analysis in diagnosis and treatment of DSD: position paper of EU COST Action BM 1303 ‘DSDnet.’ Eur J Endocrinol. 2017;176(5):1.
- Aherne E, Moriarty H, Egan M, et al. The impact of contemporary multidisciplinary meetings on workload at a tertiary level hospital. J Ir Coll Physicians Surg. 2017;186(2):359–362.
- de Vries AL, Roehle R, Marshall L, et al. Mental health of a large group of adults with disorders of sex development in six European countries. Psychosom Med. 2019;81(7):629.
- Duguid A, Morrison S, Robertson A, et al. The psychological impact of genital anomalies on the parents of affected children. Acta Paediatrica. 2007;96(3):348–352.
- Crissman HP, Warner L, Gardner M, et al. Children with disorders of sex development: a qualitative study of early parental experience. Int J Pediatr Endocrinol. 2011 October 12;2011(1):10.
- Moran ME, Karkazis K. Developing a multidisciplinary team for disorders of sex development: planning, implementation, and operation tools for care providers. Adv Urol. 2012;2012:604135.
- Magritte E. Working together in placing the long term interests of the child at the heart of the DSD evaluation. J Pediatr Urol. 2012;8(6):571–575.
- Indyk JA. Disorders/differences of sex development (DSDs) for primary care: the approach to the infant with ambiguous genitalia. Transl Pediatr. 2017;6(4):323.
- Deeb A, Khamis M, Al Sayed S, et al. Sex assignment practice in disorders of sexual differentiation: survey results from paediatric endocrinologists in the Arab region. J Pediatr Endocrinol Metab. 2019;32(1):75–82.
- NHSGGC. 2020. [2022 March 30] Available from: https://www.nhsggc.org.uk/your-health/health-services/specialist-childrens-services/attend-anywhere-near-me/#
- Sanders C, Edwards Z, Keegan K. Exploring stakeholder experiences of interprofessional teamwork in sex development outpatient clinics. J Interprof Care. 2017;31(3):376–385.
- Thyen U, Lux A, Jürgensen M, et al. Utilization of health care services and satisfaction with care in adults affected by disorders of sex development (DSD). J Gen Intern Med. 2014 August 01;29(S3):752–759.
- Palmer BW, Wisniewski AB, Schaeffer TL, et al. A model of delivering multi-disciplinary care to people with 46 XY DSD. J Pediatr Urol. 2012 Feb;8(1):7–16.
- Ali SR, Lucas-Herald A, Bryce J, et al. The role of international databases in understanding the aetiology and consequences of differences/disorders of sex development. Int J Mol Sci. 2019;20(18):4405.
- Ke KM, Blazeby JM, Strong S, et al. Are multidisciplinary teams in secondary care cost-effective? A systematic review of the literature. Cost Eff Resour Allocation. 2013 April 04;11(1):7.
- Dulai R, Shunmugam SR, Veasey RA, et al. An economic evaluation of an advanced video conferencing system for cardiac multidisciplinary team meetings. Int J Clin Pract. 2020 September 01;74(9):e13562.
- Lamb BW, Green JS, Benn J, et al. Improving decision making in multidisciplinary tumor boards: prospective longitudinal evaluation of a multicomponent intervention for 1,421 patients. J Am Coll Surg. 2013;217(3):412–420.
- Taylor C, Atkins L, Richardson A, et al. Measuring the quality of MDT working: an observational approach. BMC Cancer. 2012;12(1):1–10.
- Soukup T, Lamb BW, Arora S, et al. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J Multidiscip Healthc. 2018;11:49.
- Harris J, Green JS, Sevdalis N, et al. Using peer observers to assess the quality of cancer multidisciplinary team meetings: a qualitative proof of concept study. J Multidiscip Healthc. 2014;7:355–363.
- Van Bever Y, Brüggenwirth HT, Wolffenbuttel KP, et al. Under-reported aspects of diagnosis and treatment addressed in the Dutch-Flemish guideline for comprehensive diagnostics in disorders/differences of sex development. J Med Genet. 2020;57(9):581–589.
- Thyen U, Ittermann T, Flessa S, et al. Quality of health care in adolescents and adults with disorders/differences of sex development (DSD) in six European countries (dsd-LIFE). BMC Health Serv Res. 2018;18(1):527.
- Sandberg D, Callens N, Wisniewski A. Disorders of sex development (DSD): networking and standardization considerations. Hormone Metab Res. 2015;47(5):387–393.
- Pereira AM, Hiort O. Introduction to Endo-ERN–scope and mission. Endocrine. 2021;71(3):537–538.
- Kranenburg LJ, Reerds ST, Cools M, et al. Global application of the assessment of communication skills of paediatric endocrinology fellows in the management of differences in sex development using the ESPE e-learning. org portal. Hormone Res Paediatrics. 2017;88(2):127–139.
- Kalaitzoglou E, Majaliwa E, Zacharin M, et al. Multilingual global E-learning pediatric endocrinology and diabetes curriculum for front line health care providers in resource-limited countries: development study. JMIR Format Res. 2020;4(11):e18555.
- GMC. Making and using visual and audio recordings of patients 2011 [2022 March 30]. Available from: https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/making-and-using-visual-and-audio-recordings-of-patients
- Drop SL, Mure P-Y, Wood D, et al. E-consultation for DSD: a global platform for access to expert advice. J Pediatr Urol. 2012;8(6):629–632.
- Gallo G, Picciariello A, Di Tanna GL, et al. E-consensus on telemedicine in colorectal surgery: a RAND/UCLA-modified study. Updates Surg. 2021;26:1–8.
- Muscarella M, Kranenburg-van Koppen L, Grijpink-van den Biggelaar K, et al. Global application of disorders of sex development-related electronic resources: e-learning, e-consultation and e-information sharing. Understanding Differ Disord Sex Dev (DSD). 2014;27. 268–283.
- Kunkler IH, Prescott RJ, Lee RJ, et al. TELEMAM: a cluster randomised trial to assess the use of telemedicine in multi-disciplinary breast cancer decision making. Eur J Cancer. 2007 Nov;43(17):2506–2514.
- Mönig I, Steenvoorden D, de Graaf JP, et al. CPMS–improving patient care in Europe via virtual case discussions. Endocrine. 2021;7:549–554.
- Ionescu A, de Jong PG, Drop SL, et al. A scoping review of the use of e-learning and e-consultation for healthcare workers in low-and middle-income countries and their potential complementarity. J Am Med Inf Assoc. 2022;29(4):713–722.
- Ediati A, Juniarto AZ, Birnie E, et al. Gender development in Indonesian children, adolescents, and adults with disorders of sex development. Arch Sex Behav. 2015;44(5):1339–1361.