ABSTRACT
Introduction
Eating disorders (EDs) are common complications in people with type 1 diabetes (PwT1D), given the rigid focus on food and insulin dose adjustment. Dietary recommendations for T1D match those for the general population, yet many fail to achieve target HbA1c. Evidence suggests that lower carbohydrate meals and thus reduced insulin requirements may decrease inconsistencies in insulin absorption, maintain euglycemia and weight. Dietary restriction is a recognized risk factor for ED development, and Ketogenic Diets (KD) involve restriction of common family-based foods, thus impacting social normality and microbiome diversity. We reviewed the current literature on PwT1D following a KD to understand effects on ED risks.
Areas covered
Published data from MEDLINE, Embase, and PsycINFO were used. Search terms included: type 1 diabetes mellitus; or insulin dependent diabetes or T1D AND EDs or anorexia or bulimia or disordered eating AND low-carbohydrate diet or carbohydrate restricted diet or low carb diet or ketogenic diet.
Expert opinion
Research into the effects of KDs on ED outcomes in PwT1D are limited, given the concerns over risks of diabetic ketoacidosis, hypoglycemia, and dyslipidemia. Longer term studies on the participants’ experience and motivations of adhering or admonishing the diet are needed.
1. Introduction
The nature of type 1 diabetes management naturally predisposes many patients to eating disorder (ED) development. Given the emphasis placed on the strict maintenance of blood glucose control and attention to carbohydrate counting, portion control, and meal planning, disordered eating behaviors may go unnoticed by clinicians [Citation1]. While treatments for ED are generally aimed at removing the focus on food, in T1D this forms an integral part of managing the disease [Citation2]. As carbohydrates are the major contributor to post prandial hyperglycemia, increasing evidence highlights the benefits of very low-carbohydrate (ketogenic) diets to maintain euglycemia and weight, by improving HbA1C and satiety [Citation3], [Citation4], [Citation5]. Opponents of the ketogenic approach suggest that, depending on the degree of restriction, these diets may themselves lead to ED development, nutritional deficiencies, and increased cardiometabolic risks [Citation6], [Citation7], [Citation8].
1.1. Normal is an Illusion
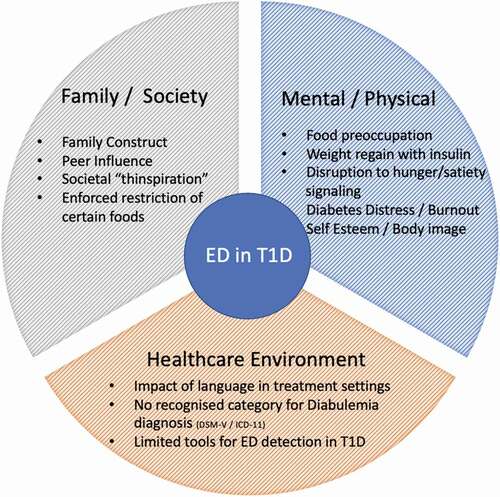
While the etiology of ED is complex, with psychological, biological, and sociocultural factors contributing to susceptibility, dietary restriction is believed to contribute to the development and maintenance () [Citation8]. T1D onset commonly occurs between the ages of 10 and 14 years which coincides with the onset of adolescence: a period of major emotional and hormonal upheaval and peak awareness of body image [Citation9], [Citation10], [Citation11]. Adolescents with chronic illnesses experience significantly higher body dissatisfaction compared to healthy peers [Citation12]. Adolescents who perceive themselves to have dysfunctional bodies characteristically develop body dissatisfaction and negative perceptions of self [Citation13]. For many young people with T1D, normality is an illusion, as they experience feelings of “being different” or “standing out.” While PwT1D appear to look exactly like their peers, the practicalities of managing their disease are highly visible and contribute to the stigma of diabetes [Citation11]. There is also a poor public awareness of the different etiologies of T1 and T2D, suggesting that clearer lexicon and more specific, differentiated naming of the two diseases would be helpful in removing some of the stigma for PwT1D. The concept of normality was greatly discussed by Davis (1995) and describes how being “normal” is universally desirable [Citation13]. Most people want to be normal, or if not “normal,” then selective about the reasons they stand out. T1D is not a self-selected lifestyle condition, and the burden of managing the disease can lead to burnout [Citation14].
Peterson et al. identified various medical pathways, which predispose disordered eating behaviors in PwT1D [Citation15]. Specifically, the effect of food preoccupation to match insulin dose adjustment and the emotional distress which results from the regained weight once insulin is introduced [Citation15–18], [Citation16]. Further studies expand on this, by highlighting the physiological aspects.
1.2. The Weight of Words
Numerous papers point to the negative impact of the language used in diabetes care, which is often described as judgmental and disempowering [Citation19], [Citation20]. Behavioral psychologists have demonstrated the impact of language on health outcomes and the effect of semantics on patients’ understanding and ownership of their disease [Citation16]. Time constraints in clinical appointments can result in a patient’s health being summarized to a single HbA1C result. Condensing the experience of life with diabetes into a single digit ignores the impact that factors beyond diet can have on blood glucose readings. Rhetorical phrases such as “being a good diabetic” or “controlling your diabetes” are frequently used in such settings and are perceived to imply that HbA1c is solely under the control of the patient, and any success or failure to achieve adequate HbA1C is due to their personal shortcomings [Citation19]. These phrases, often uttered in passing, contribute to the continual reinforcement and praise for practicing restraint and self-control around food. Conversations around diabetes management, which focus solely on numbers, are bound to fail as quantitative data cannot entirely capture the day-to-day complexities that encompass life with T1D [Citation20].
1.3. Diabetes Dietary Dilemma
Despite major medical and technological advances in T1D treatment, many patients fail to achieve or maintain target HbA1c levels [Citation2]. Dietary recommendations for PwT1D are the same as for the general population (). This includes “following a balanced diet from a variety of foods,” with ~50% of total dietary energy from starchy carbohydrates, opting for higher fiber or wholegrain versions where possible [Citation21].
Table 1. Comparisons of dietary approaches in the standard type 1 diabetes diet versus the low-carbohydrate diet and ED management.
While lower carbohydrate diets have gained increased interest, classification of these diets differ greatly within the literature. Differences are based on the proportion of total daily energy from carbohydrate and/or absolute carbohydrate intake, adding to the complexity of comparative study ().
Table 2. Suggested definitions of different carbohydrate diets by Feinman et al. (2015) and Seckold et al. (2017).
The definition provided by Feinman (2015) and Seckold (2019) recommends reducing carbohydrate intake to <10% (or 20–50 g per day, thereby inducing ketone production as an alternative source of energy) [Citation23], [Citation24]. Restricting carbohydrates to this degree induces glycogen depletion and ketone production from the mobilization of fat, resulting in nutritional ketosis. Differentiating Diabetic Ketoacidosis (DKA) from nutritional ketosis for PwT1D following a KD poses a challenge. While both conditions result from increased ketone production, DKA is caused by the additional shortage of insulin. Little has been published on how to adjust medications for DKA prevention in T1D following a KD, although prudent blood glucose management and insulin dose adjustment are advised especially as insulin requirements decrease following weight loss [Citation25].
There are also concerns that carbohydrate restricted diets (CRD), particularly KD may promote renal damage, due to their higher protein content [Citation26]. Diabetes is an established risk factor for chronic kidney disease (CKD), and hyperglycemia is central to disease progression, through its effect on increased blood pressure, hyperfiltration, and impaired protein metabolism, particularly in hyperinsulinemic T1D patients [Citation26], [Citation27], [Citation28], [Citation29]. Clinical trials on patients with CKD are challenging and highly restricted, due to the multiple barriers of conducting research on patients with often-complex comorbidities [Citation30].
2. Eating Disorder Diagnosis
The management of any medical condition starts with a diagnosis and the use of specific terminology to facilitate detection, monitoring, and treatment. Classification of psychiatric conditions is challenging given the lack of biological or pathophysiological markers that indicate the presence of “disease.”
Despite the high incidence of ED in PwT1D, there are no specific criteria to describe the unique ability of patients to manipulate insulin doses for weight loss. Diabulimia is an informal term used to describe deliberate insulin omission for the purpose of weight loss [Citation1]. While this behavior may be due to psychological, social, and cognitive factors, including diabetes burnout, it is not classed as a mental health condition in DSM-V or ICD-11. Instead, it is recognized as either an inappropriate compensatory feature of bulimia nervosa (BN) or a purging disorder and referred to formally as “Eating Disorders in Diabetes Mellitus Type 1.” The National Institute for Health and Care Excellence (NICE) guidelines (2017) in the UK admit to the increasing need for screening of ED in PwT1D; however, there is currently no consensus on an appropriate screening tool [Citation2]. Standard ED screening tools do not consider the effect that both hyper- and hypoglycemic states may trigger binge eating and promote inappropriate compensatory behaviors. This may result in patients incorrectly being labeled as bulimic or binge eaters [Citation31].
ED are classified in relation to dominant discourses about “health and wellbeing.” While T1D ED patients are encouraged to “eat a normal, healthy diet” and and follow a balanced approach to physical activity, they are a unique patient group who subsequently have their dietary choices analyzed for the degree to which they have stuck to the guidelines () [Citation32]. These shared traits of need for control and valuing diet adherence as a proof of self-control put patients at a high risk for developing this “disease disguised as a virtue” [Citation32].
Bratman first proposed the concept of Orthorexia Nervosa (ON), describing a pathological obsession for eating only (biologically) pure foods, with a rigid avoidance of food believed to be unhealthy [Citation33]. Unlike other ED, ON focuses on quality rather than quantity of food consumed. This fixation may develop into other psychological and physiological disorders, as sufferers start to limit social interactions or highly restrict their diets, leading to nutritional deficiencies that closely resemble the malnutrition status typical in Anorexia Nervosa (AN). It is still undecided whether ON is a unique disorder or a subtype of AN or obsessive-compulsive disorder (OCD). Within current DSM-V categories, orthorexia would be classed as “avoidant/restrictive food intake disorder” (ARFID), although it may represent a distinct subtype [Citation32]. Koven et al. compared the strongest commonalities among AN, OCD, and ON; and found that the most frequent overlapping traits to be anxiety, need for control, certain food avoidance, perfectionism, and ritualistic behaviors; and concluded that character traits of one may influence the development of the other [Citation34]. Both AN and ON sufferers exhibit an overwhelming need to analyze the origin and processing of food and to eat in a ritualized manner, although ON patients display a highly ego-syntonic perception. Whereas AN sufferers try to hide their habits, orthorexic individuals tend to show off their behavior [Citation32].
3. Literature Review
This review aims to investigate the current research on T1D patients following a Ketogenic Diet to understand its effect on ED risk.
3.1. Method
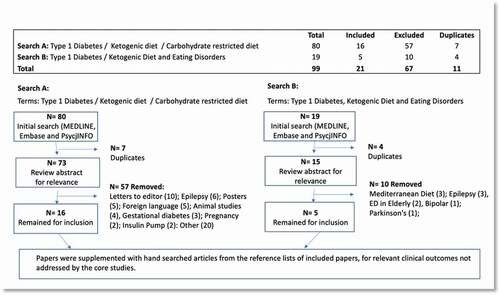
Published data were searched using the Medline National Library of Medicine, MEDLINE, Embase, and PsychINFO (). Only English language papers published between 2000 and 2022 were included. Search terms used included type 1 diabetes mellitus or insulin-dependent diabetes or T1D AND ED or anorexia or bulimia or disordered eating AND Low-carbohydrate diet or carbohydrate restricted diet or low carb diet or ketogenic diet. 99 papers were identified, and after initial review of titles and abstracts for relevance, 11 duplicates were removed. A secondary review removed all those with irrelevant demographics (including gestational diabetes, pregnancy; epilepsy, animal trials (rats); insulin pump research; conference posters, letters to editor, Parkinson’s, Mediterranean diet). 21 papers remained for inclusion, which were supplemented with hand searched articles from the reference lists of included articles, for relevant clinical outcomes not addressed by the core studies.
A narrative review, adopting a systematic synthesis of the available evidence, was conducted with all papers reviewed by authors. Thematic analysis was conducted to identify potential benefits and challenges of T1D patients following a ketogenic diet and the effect on ED development. These will be discussed here.
4. Potential Benefits Of The Ketogenic Diet In Patients With T1d
Research into Ketogenic Diet effects on PwT1D is challenging, given the assumed risks of DKA, hypoglycemia, and dyslipidemia. Current studies are mostly of shorter term duration (<2 years) with small sample sizes primarily consisting of motivated individuals, making population-based findings difficult ().
4.1. Weight Loss
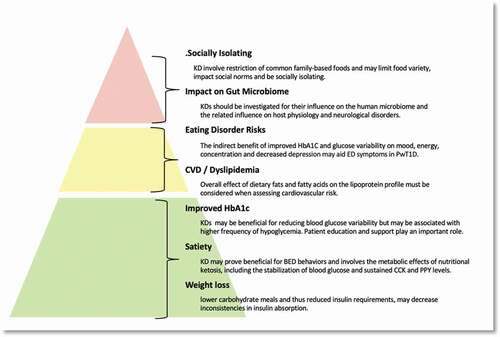
The phenotype of T1D has changed in the past two decades; with more patients exhibiting features of metabolic syndrome (MetS) or “Double Diabetes” [Citation35]. Chronic use of corrective insulin doses administered subcutaneously (following higher carbohydrate meals) results in reduced inhibition of hepatic lipolysis and increased levels of circulating non-esterified fatty acids (FEFA) [Citation36]. A mismatch typically exists when administering corrective insulin doses after a large carbohydrate meal, increasing the risk of hypoglycemia. This promotes greater lipid storage in skeletal muscle, increased oxidative stress, and ectopic fat accumulation [Citation3]. Moreover absorption rates of subcutaneous insulin vary greatly when insulin dosage levels are high [Citation3], [Citation37]. This suggests that lower carbohydrate meals and thus reduced insulin requirements may decrease inconsistencies in insulin absorption.
An RCT of 10 T1D participants compared the glycemic control, glucose variability, and daily insulin requirements of a CRD (50–75 g carbohydrate per day) with a standard diet (SD) [Citation38]. The group were randomly allocated (1:1) to either dietary approach. After 12 weeks, the CRD group had significant reductions in daily insulin use (64.4 to 44.2 units/day, p < 0.05), HbA1c (63 to 55 mmol/mol (8.9–8.2%), p < 0.05), and non-significant reductions in body weight (83.2 to 78.0 kg). While the weight change may be attributed to the reduced insulin doses, this study provides promising benefits in enabling weight loss in PwT1D. Further studies of longer duration and larger, more diverse participant demographics are required.
4.2. Improved Hba1c
A comprehensive review by Barber et al. summarized the beneficial short-term effects of KD on blood glucose control, particularly in patients with type 2 diabetes (38). Research on KD in PwT1D is limited, due in part to concerns about hypoglycemia and DKA [Citation39].
While HbA1C provides a standardized measurement of average blood glucose with low intra-person variation and fewer requirements for sample collection, it demonstrates neither the magnitude of blood glucose variability nor the length of time these fluctuations last. Frequent or large glucose fluctuations may independently contribute to diabete-related complications, and this variability has more detrimental micro-and macro vascular effects than sustained hyperglycemia [Citation37], [Citation39], [Citation40].
A short-term (7-day) observational study investigating the effect of carbohydrate restriction on glycemic variability in PwT1D found that participants spent a mean of 73.7% ± 0.1% time within blood glucose range and experienced 0.9 (0.0–2.0) episodes of hypoglycemia per day [Citation41]. This suggests that while CRD may be beneficial for reducing blood glucose variability, they may be associated with a higher frequency of hypoglycemia. While no comparative data for a standard diabetes diet (SDD) was taken, data from the T1D Exchange Registry show that ~80% of PwT1D following the SDD fail to meet the HbA1c targets set by the American Diabetes Association [Citation42].
A longer term, 4-year retrospective study by Nielsen et al. found that PwT1D who received training and support on implementing a CRD achieved mean HbA1c of 6.4 ± 0.8%, compared with the non-CRD group (52%) 7.4 ± 0.9% after 4 years [Citation43]. The results reported in this study highlight the importance of patient knowledge and motivation, the influence of a supportive environment, and the effects of socioeconomic ability in affecting lifestyle behavior change. The realistic application of this approach at a population level remains unanswered.
4.3. Satiety
The composition of the diet plays a vital role in satiety homeostasis, body weight, and adiposity. While there is no biomarker that measures satiety, and the specific effects of KD have mostly been assessed using self-reported measures, evidence supports the effectiveness of KD in weight loss. This is particularly true for individuals that struggle with calorie restricted diets, although the mechanisms underlying the physiology are still unclear [Citation44].
Diet induced weight loss is commonly followed by compensatory increases in appetite, which lead to weight regain. Weight loss induced via the KD seems to moderate the increased levels of circulating ghrelin and resulting increase in appetite [Citation4]. Appetite reduction experienced on the KD may result from the metabolic “cost” of protein digestion and its effects on appetite control hormones [Citation5]. KD naturally have a higher percentage of protein and fat to compensate for the reduced calories from carbohydrate. Ketosis has been demonstrated to exert an anorexigenic effect via cholecystokinin (CCK) release and reduced orexigenic signaling from ghrelin [Citation45]. Circulating levels of CCK are maintained at pre-weight loss levels, while patients remain ketotic [Citation46].
Evidence suggests there may be a neurometabolic role in the development of maladaptive eating behaviors such as binge ED (BED) [Citation47], [Citation48]. Psychiatric conditions such as epilepsy, depression and BED share several common mechanistic biopathologies, including glucose hypometabolism, neurotransmitter imbalances, and oxidative stress [Citation49]. The mechanisms by which the KD may prove beneficial for BED behaviors are complex and involve the metabolic effects of nutritional ketosis, including the stabilization of blood glucose and sustained CCK and PYY levels [Citation50].
A 2014 systematic review by Kendall found that dietary pulses (high in fiber and protein) contribute to acute satiety but not second meal intake volume [Citation51]. Results showed that dietary pulses produced a 31% greater satiety incremental area under the curve (ratio of means = 1.31, 95% CI: 1.09 to 1.58, P = 0.004), without affecting second meal intake. Increasing fiber content and decreasing the GI had a positive (decreasing) effect on plasma glucose, insulin, and ghrelin responses. Earlier findings by McCrory (2010) showed the beneficial effects of fiber in weight loss when consumption was coupled with energy restriction [Citation45]. This suggests that fiber content and lower GI is an important contributor to postprandial satiety.
4.4. Eating Disorders
Diagnosis of ED is difficult given that they occur along a continuum of body mass index (BMI). Literature on the effect of different macronutrients in ED research center mostly on restriction of dietary fats, with very little research found on the associations between carbohydrate restriction and the development of ED [Citation23], [Citation24]. While the incidence of AN is not much different in PwT1D compared to non-diabetes patients, BN and Binge Eating Disorder (BED) rates are significantly higher among PwT1D [Citation52], [Citation53]. There are concerns that highly restrictive KD may lead to the elimination of entire food groups, causing poor nutritional status and increased risk of ED development. There is however limited data on the potential psychological and behavioral effects of KDs in PwT1D, given the indirect benefits of improvements in glucose variability on mood, energy, and concentration, and the effect that higher HbA1c has on increased depression and anxiety [Citation54], [Citation55].
The global prevalence of obesity and ED have increased in the past 50 years, in parallel with industrialization and increased consumption of Ultra Processed Foods (UPF). Evidence suggests a metabolic link between the increased consumption of UPF and the development of maladaptive eating [Citation56], [Citation57]. Ayton et al. showed that the foods patients consumed in a binge eating pattern were entirely NOVA 4 category foods, regardless of the patient’s ED diagnosis [Citation58]. Foods containing both carbohydrates and fat (a combination rare in nature) produce greater brain activation of the circuits associated with reward and habit formation than either carbohydrates or fats alone [Citation59]. Food addiction is not currently recognized as a disorder in the DSM-V; however, UPFs trigger mediating neurochemical responses, altering signaling and activation of the mesolimbic dopamine reward pathways, causing addiction behavior [Citation48], [Citation60]. The degree of alteration is dependent on glucose oxidation.
Obesity and dysglycaemia are risk factors for inappropriate weight control practices such as BED. A comparative cross-sectional study by Al Hourani et al. aimed to determine the prevalence of ED among adolescents with and without dysglycaemia to determine the associated factors [Citation52]. 497 patients (aged 10–24 years) with dysglycaemia (T1D and pre-DM) and 504 age-matched nondysglycaemic participants were screened using the ED Diagnostic Scale to assess the presence of different types of ED. Dysglycaemic patients showed a significantly higher prevalence of binge EDs (11.9% vs 5.8%, p = 0.001) after adjusting for possible confounders. The limitations of this study were its non-distinction between T1D and pre-DM patients as the etiology of these diseases, and thus, their insulin responses to carbohydrate consumption may affect binge eating behaviors. Furthermore, no data on dietary intake were provided.
Comparisons of ED in PwT1D to the general population should be made with caution, as the causes of binge eating may differ, particularly considering hypoglycemic response. Additionally, ED measurement tools across various studies that are not specifically adapted to PwT1D are prone to delivering skewed results.
4.5. Dyslipidemia and CVD Risk
Studies indicate that binge eating is associated with reduced glycemic control, alterations in body fat distribution, and increased CVD risk [Citation52], [Citation61], [Citation62]. Hyperglycemia and glucose variation increase inflammatory markers in PwT1D, and the benefits of the KD on improved HbA1c have been discussed above.
KD inherently include an increased percentage of calories from fats and a higher proportion of saturated fat intake [Citation63]. Although the specific role of different macronutrients (particularly fat) on inflammation is unclear, evidence has shown that there are no significant benefits in the restriction of total fat intake and heart disease or CVD mortality [Citation63], [Citation64], [Citation65]. The quality of the fat rather than the total amount consumed seems more important [Citation64].
Studies demonstrated that KD increase total cholesterol, non-HDL cholesterol, and triglyceride, as well as vitamin D3 (cholecalciferol) [Citation47], [Citation66]. No studies reported on the composition of the LDL cholesterol profiles, which may be the most important indicator of risk [Citation67]. The composition and not the number of LDL cholesterol particles more accurately determine CVD risk [Citation64], [Citation65]. A clear link exists between LDL particle size, obesity, and insulin resistance [Citation68]. Patients with lower LDL cholesterol levels but higher particle counts therefore demonstrate a higher risk of CVD events. Macronutrient studies show that a high saturated fat intake increases LDL particle size as well as high-density lipoprotein cholesterol (HDL-C) [Citation69], [Citation70]. Therefore, the overall effect of dietary fats and fatty acids on the lipoprotein profile must be considered when assessing cardiovascular risk.
A cross-geographical location study suggests that vitamin D deficiency is more common in T1D compared to the general population, and ethnic background accounts for a large variation in vitamin D levels [Citation71], [Citation72]. Vitamin D is a fat-soluble vitamin that plays an important role in calcium and bone metabolism, cardiovascular, and metabolic function, including insulin synthesis and secretion [Citation67]. A study by Huynh et al. investigated the relationship between 25(OH)-vitamin D3 and the severity of DKA in children at diagnosis of T1D [Citation71]. Fourteen of the 64 children had low 25(OH)-vitamin D [Citation3] levels at presentation, and 12 had low bicarbonate levels (<18 mmol/L) (p = 0.001). Ethnic background accounted for 10% of the variation in vitamin D levels. The levels of 25(OH)-vitamin D3 increased in 10 of the 11 children in this study, with resolution of the acidosis. Low vitamin D status may therefore contribute to a child’s risk of presenting with DKA.
Vitamin D deficiency has a role in the pathogenesis of T1D, and there is an independent association between 25(OH)-vitamin D3 deficiency and risk of diabetes [Citation73]. A prospective, nonblinded controlled trial of 80 T1D found that those with vitamin D deficiency experienced marked improvement in HbA1C after receiving a 12-week course of 4000 IU 25(OH)-vitamin D3. The glycemic effect was sustained during the 12 week period, although further studies are required to understand the safety of high dose supplementation long term. The KD may therefore provide a safer vitamin D augmentation alternative for PwT1D.
5. Potential Risks of the Ketogenic Diet in Patients with T1d
5.1. The Gut Microbiome
T1D is an autoimmune disease, and pathogenesis is based in genetic susceptibility and environmental factors. The gut microbiota plays a pivotal role in host immunity and metabolic parameters, and there are strong associations between gut dysbiosis T1D pathogenesis and broader health [Citation74], [Citation75], [Citation76], [Citation77].
The microbiota engages in complex bidirectional communication, via the microbiota-gut-brain axis [Citation76]. While there is limited understanding of the biological mechanisms underlying EDs, there is growing recognition that the diversity of gut microbiota might play a causal role in AN development [Citation78]. A common metric of gut health is the diversity of microbial species that inhabit it [Citation78]. AN patients demonstrate lower microbial diversity compared with healthy controls, which coincides with increased levels of depression and ED psychopathology [Citation78], [Citation79].
Multiple factors, including genetics, microbial species acquired at birth, immunological factors, antibiotic use, and diet, influence the composition of the microbiome. Any acute changes can modify this composition within just 24 hours [Citation79]. Gut microbes reside mostly in the distal gut and contribute to host health through biosynthesis of vitamins and essential Short Chain Fatty Acids (SCFA). There are concerns that KD are potentially deficient in vitamins B1, B6, folate, and dietary fiber, as they limit intake of fiber-rich starchy vegetables including legumes and whole grains [Citation80]. Dietary fiber is associated with overall metabolic health, through pathways that influence insulin sensitivity [Citation76].
Fibrous, indigestible carbohydrates serve as a food source for many of the microbiota found in the gut, and a commensal partnership exists between the host and these bacteria. The human gastrointestinal (GI) tract does not produce the enzymes needed to degrade the structural polysaccharides found in plant material and relies on the bacteria of the large intestine to digest resistant starch, non-starch polysaccharides, and oligosaccharides left undigested by the small intestine [Citation81]. These indigestible substances serve as a primary energy source for microbiota, where they are degraded and fermented to produce SCFAs. Diets high in fiber enable a better microbial balance within the gut and a higher production of SCFAs, which contribute tryptophan metabolites and omega-3-fatty acids [Citation82]. Low-calorie diets (as in the case of AN) reduce the availability of circulating tryptophan for serotonin (5-HT) synthesis [Citation83]. Altered 5-HT may play a part in some of the psychological consequences of dieting, including the development of clinical EDs and depression, particularly in women [Citation83], [Citation84].
A high percentage of patients with diabetes have some form of GI tract symptoms. Diabetic Gastroparesis (DGP) is a serious complication of diabetes mellitus, defined as a delay in gastric emptying, associated with upper GI symptoms, in the absence of any mechanical obstruction [Citation85]. Population-based data are limited, although data from tertiary care centers report one third of diabetes patients (both type 1 and type 2) suffer from the condition, with higher incidence found in females [Citation86]. Delayed gastric emptying affects glycemic control and may contribute to poor nutritional status [Citation75]. Nutrient interactions in the small intestine help to regulate gastric emptying and fat solids generate the most potent effect on delayed emptying [Citation87]. High fat solid meals, as in the case of KD, are directly implicated with overall increased gastroparesis symptoms [Citation87]. KDs should therefore be investigated for their influence on the human microbiome and the related influence on host physiology and neurological disorders [Citation81].
5.2. Social Adaptation to Dietary Restrictions
One of the main aspirations of diabetes nutritional management is to encourage lifelong healthy eating habits, while preserving social, cultural, and psychological wellbeing. Food is central to the human experience of social belonging and helps to assert a sense of hierarchy and connection. Often even the smallest deviation from an established pattern of eating results in branding a person or an entire group as “different“ or “strange” [Citation88]. KDs involve restriction of common family-based foods and may lead to limitations in food variety, impact social normality, and be socially isolating [Citation89].
Studies on the lived experience of following KD in adults were conducted mainly on individuals who had self-subscribed to the diet. Despite the limited support from health care practitioners to provide guidance and risk assessment, these individuals independently source information and support elsewhere [Citation90]. There is however a lack of validated dietary data that demonstrates the true adherence rates, with the majority duration of studies covering between 3 and 18 months of observation data [Citation3], [Citation38], [Citation90].
Conversely, studies on adolescents with T1D whose parents enforce a CRD may experience behavioral responses to the restriction akin to food insecurity [Citation91]. Children with T1D who do not know when they will again get access to sweets, or other “treat” foods due to restrictions may be prone to “sneaking” or hoarding these foods [Citation92]. Growing up with consistent labeling of “good” and “bad” foods may contribute to ED cognitions and insulin restriction, which is likely in adults who believe they have broken food “rules.” Adolescents on an enforced CRD may also engage in maladaptive diabetes management behavior to have access to carbohydrate or sugary foods, by purposefully over-administering insulin to create a situation that will allow them to “treat” the resulting hypoglycemia with sweets [Citation93].
Family and social construct have consistently been shown to predict the development of maladaptive eating attitudes and behaviors, particularly in adolescent females with T1D [Citation17], [Citation94], [Citation95]. A qualitative study by Wong et al. showed that in adolescents, peer pressure and the stress of feeling different from peers at mealtimes could be a deterrent to following a restrictive diet long term [Citation90].
The current literature provides an insight into potential clinical outcomes and health benefits of the KD, but provides little perspective into the participants’ experience, motivations, and challenges of adhering to the diet or their reasons for discontinuation. The two major facets of diabetes management rely heavily on adherence to insulin administration and normalization of eating behaviors, which can only be achieved through patient-centered choice. A multidisciplinary team approach to treatment is considered the standard of care for both diabetes and ED management, and the value of patient experience should not be underestimated [Citation96]. Qualitative research highlighting these aspects is needed, to offer increased patient input and explanation as to how they safely transition and manage their diabetes day-to-day.
6. Expert Opinion
While the invention of modern insulin analogues has enabled more dietary flexibility for PwT1D, increasing the recommendations for carbohydrate intake, alongside multiple insulin doses contribute to weight gain and diminished glycemic control. This review highlighted potential benefits of the KD approach in PwT1D in the short term. Longer term studies are needed to establish the feasibility of social and lifestyle integration, and the effects of altered gut microbiome on ED outcomes ().
Diabetes and obesity are two major causes of CKD, and while there are some concerns about the impact of KD on renal outcomes, particularly at more advanced stages of CKD, there are currently no RCTs investigating this dietary approach in T1D [Citation97]. Given the major impact of hyperglycemia in the pathogenesis of CKD, it seems logical to suggest that a lower carbohydrate approach may be beneficial for some of these patients. Because there is currently no agreement on isocaloric comparisons recommending a specific carbohydrate intake for T1D, clinicians are challenged to provide risk assessments and guidance [Citation98]. Additionally, current clinical guidelines do not wholly address all areas of dietary management in CKD, due to existing controversies and gaps in knowledge about specific interventions such as dietary protein restriction [Citation99]. There is also unfortunately a poor understanding among health care professionals of the difference between nutritional (physiological) ketosis and insulin deficiency-induced (pathological) ketosis, which may deter further research studies being proposed [Citation100].
Clinical nutrition research has played a pivotal role in establishing causality between diet and disease. However, accurate comparative research on the effects of the KD on PwT1D is challenging, given that few studies investigated the baseline nutritional status of participants, and there was no means of blinding participants or researchers in a randomized approach. Data from all studies were derived from participant memory and relied on self-reporting and honesty. In a population who already feel highly scrutinized for their dietary behavior, there may be a tendency to mis-report actual meal (and particularly carbohydrate) intake, and therefore, any specific deductions on practicality of implementing this approach are challenging.
Across the studies, there was a notable limit in demographic variety, specifically minority ethnic groups and those from lower socioeconomic backgrounds. It is well known that lower income groups face large disparity in access to intensive insulin therapies, specialist diabetes care and education [Citation101]. Therefore, promoting a ketogenic approach would be challenging for some of these groups, as high- fat, high-protein foods generally cost more than the staple foods commonly consumed. Studies investigating the effect of family dynamics on body image also provided a restricted view, as the perspectives and influence of fathers were not included [Citation10], [Citation12], [Citation17].
Given the challenges in detecting ED in PwT1D, there is a need for more effective screening. By its very nature ED are secretive and sufferers may be reluctant to talk about their vulnerabilities, thus increasing the risks of missed diagnosis and long-term complications [Citation96]. Two standalone, brief measures frequently used in U.K. primary care include the Eating Disorder Screen for PrimaryCare (EDS-PC) and the SCOFF, neither of which adequately address ED detection in T1D [Citation102].
Current treatment protocols for ED do not make provision specifically for T1D and are failing dismally, as individuals are treated for the effects of diabulimia rather than the diabulimia itself [Citation31]. Two patients displaying the same pathology and behavior may receive a different diagnosis based solely on one factor, such as BMI. The challenge in primary health care is a lack of knowledge of ED treatment [Citation103–107]. A focus on diet will always be necessary in T1D; as insulin dose is based largely on meal composition. An alternative dietary approach, low in refined carbohydrates and higher in healthy fats, may be helpful in alleviating symptoms of (some) ED types in T1D patients, although longer term research is needed.
Article highlights
While not classed as an ED in its own right, “diabulemia” (an informal term used to describe deliberate insulin omission for the purpose of weight loss) is a common complication in PwT1D, especially in females.
Advances in T1D management have enabled more freedom around food choice but have conversely increased the need for corrective insulin dose adjustment. Chronic corrective insulin administration of subcutaneous insulin into the peripheral circulation results in reduced inhibition of hepatic lipolysis and may be the reason for increased incidence of metabolic syndrome in PwT1D.
There are currently limited data on the potential psychological and behavioral effects of KDs in PwT1D, given the indirect impact of glucose variability on mood, energy, and concentration and the effect that higher HbA1c has on increased depression and anxiety.
Evidence has shown that there are no significant benefits in the restriction of total fat intake and coronary heart disease or CVD mortality. The composition and not the number of LDL cholesterol particles more accurately determines CVD risk.
The mechanisms by which the KD may prove beneficial for binge eating behaviors are complex and involve the metabolic effects of nutritional ketosis, including the stabilization of blood glucose and sustained Cholecystokinin (CCK) and Peptide YY (PYY) levels.
Author’s orientation with the topic
Diagnosed with T1D at age 11, the lead author (SS) has lived experience of the challenges of managing chronic health through diet. As a young competitive athlete, she developed a deep awareness of the impact of food on performance and body image. Diagnosed with end stage renal disease and placed on a highly restrictive renal dialysis diet, she used this knowledge and experience to write her MSc thesis on the lived experience of the diet and lifestyle challenges of a T1D on a highly restrictive dialysis diet. Although some argue that researchers need to account for the fact that their presence has some influence on the research findings, it is acknowledged that, as members of a culture or group, these researchers are privy to information and experiences that may be withheld from outsiders and thus help to “make the lived experiences more visible and intelligible to others” (108). This insider knowledge has contributed to the detailed analysis and interpretation of the literature included in this review.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Van Heyningen CD, Mandharan KS. Diabulimia: an easily missed diagnosis? Br J Diabetes. 2018;18(4):167–170.
- NICE National Institute for Health and Care Excellence). (2015). Tighter blood glucose targets for people with type 1 diabetes. Accessed: 2022 Jan 10. Available at: https://www.nice.org.uk/news/article/stricter-blood-glucose-targets-for-people-with-diabetes
- Heinemann L. Variability of insulin absorption and insulin action. Diabetes Technol Ther. 2002;4(5):673–682.
- Sumithran P, Prendergast LA, Delbridge E. Ketosis and appetite‐mediating nutrients and hormones after weight loss. Eur J Clin Nutrition. 2013;67(7):759‐64.
- Veldhorst M, Smeets A, Soenen S, et al. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94(2):300–307.
- Watanabe M, Tuccinardi D, Ernesti I, et al. Scientific evidence underlying contraindications of the ketogenic diet: an update. Obesity Rev. 2020;21(10). doi:https://doi.org/10.1111/obr.13053
- Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276(11):875–881.
- Nichols D, Arcelu J. Making eating disorders classification work I ICD-11. Eur Eating Disorders Rev. 2010;18(4):247–250.
- Maahs DM, West NA, Lawrence JM, et al. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–497.
- Verbist LI, Condon L. Disordered eating behaviors, body image and social networking in type 1 diabetes population. J Health Psychol. 2019;26:1–12.
- Coleman SE, Caswell N. Diabetes and eating disorders: an exploration of ‘Diabulimia. BMJ Psychol. 2020;8:10.
- Vilhjalmsson R, Kristjansdottir G, Ward DS. Bodily deviations and body image in adolescence. Youth Soc. 2012;44(3):336–384.
- Davis LJ. The disability studies: constructing Normalcy. the bell curve, the novel, and the invention of the disabled body in the nineteenth century. New York and London: Routledge; 1997.
- Broadley MM, White M, Andrew B. Executive function is associated with diabetes-specific disordered eating in young adults with type 1 diabetes. J Psychosom Res. 2018;211:1–12.
- Peterson CM, Fischer S, Young-Hyman D. Topical review: a comprehensive risk model for disordered eating in youth with type 1 diabetes. J Pediatric Psychol. 2015;40(4):385–390.
- Amrapala A, Chowdhury TA. Severe oedema in a patient with diabetes. Clin Med J. 2019;19(4):325–6).
- Kichler JC, Crowther JH. Effects of maternal peer modelling and negative communications on young girls’ eating attitudes and body image. Behav Ther. 2003;32(3):443–457.
- Criego, Crow, Goebel-Fabbri AE. Eating disorders and diabetes: screening and detection diabetes care. Diabetes Spectr. 2009. 22(3):143–146.
- Dickenson J, Guzman S, Maryniuk MD. The use of language in diabetes care and education. Diabetes Care. 2017 Dec;40(12):1790–1799.
- Broom D, Whittaker A. Controlling diabetes: moral language in the management of diabetes type 2. Soc Sci Med . 2004;58(11):2371–2382.
- Scientific Advisory Committee for Nutrition (SACN); (2015). Carbohydrates and Health Report. Available at: https://www.gov.uk/government/publications/sacn-carbohydrates-and-health-report. Available to Jan 21, 2022.
- American Diabetes Association. Diabetes Care. 2021;42:1(S646–60).
- Feinman RD, Pogozelski WK, Astrup A, et al., Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 31(1): 1–13. 2015.
- Seckhold R, Fisher E, de Bock M. The ups and downs of low‐carbohydrate diets in the management of Type 1 diabetes: a review of clinical outcomes. Diabetic Medicine. (2019). Available at: https://onlinelibrary-wiley-com.libezproxy.bournemouth.ac.uk/doi/full/10.1111/dme.13845. Available to Dec 11, 2020.
- Buehler LA, Knapp S, Isaacs D, et al. Ketogenic Diets in the management of type 1 diabetes: safe or safety concern? Cleveland Clin Med. 2021;88:10.
- Malhotra R, Cavanaugh KL, Kahagambe EK. Higher protein intake is associated with increased risk for incident end stage renal disease among blacks with diabetes in southern community cohort study. Nutri Metab Cardiovasc Dis. 2016;12:1079–87.
- Lentine K, Wrone EM. New insights into protein intake and progression of renal disease. Curr Opin Nephrol Hypertens. 2004;13(3):333–336.
- Hebert SL, Nair KS. Protein, and energy metabolism in type 1 diabetes. Clin Nutr. 2010;29(1):13–17.
- Bellizzi V, Cupisti A, Locatelli F, et al. Low-protein diets for chronic kidney disease patients: the Italian experience. BMC Nephrol. 2016;17(1):1–17.
- Decker E, Kendrick J. Research in the CKD Clinic: highs and lows. Adv Chronic Kidney Dis. 2014;21(4):344–348.
- Allan J. Diabetes and eating disorders: update to the NICE guideline. J Diabetes Nursc. 2017;21(3):103–107.
- Volpe U, Atti AR, Cimino M. Beyond anorexia and bulimia nervosa: what’s “new” in eating disorders? J Psychopathol. 2015;21:415–423.
- Bratman S. Original essay on orthorexia. (2001). Available at: https://www.sciencedirect.com/science/article/abs/pii/S1471015315300362. Available to Apr 10, 2020.
- Koven NS, Abry AW. The clinical basis of orthorexia nervosa: emerging perspectives. Neuropsychiatr Dis Treat. 2015;11:385–394.
- Merger SR, Kerner W, Stadler M, et al. Prevalence and comorbidities of double diabetes. Diabetes Res Clin Pract. 2016;119:48–56.
- Lui HY, Yehuda-Shnaidman E, Hong T. Prolonged exposure to insulin suppresses mitochondrial production in primary hepatocytes. J Biol Chem. 2009;284(21):187–195.
- Ceriello A, Esposito K, Piconi L. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–1354.
- Krebs JD, Parry Strong A, Cresswell P. A randomized trial of the feasibility of low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac J Clin Nutr. 2016;9(4):78–84.
- Suh S, Hyeon-Kim J. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39(4):273–282.
- Xia J, Yin C. Glucose variability and coronary artery disease. Heart Lung Circ. 2019;28(4):553–559.
- Leow ZZ, Guelfi KJ, Davis EA. The glycemic benefits of a very‐low‐carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycemia risk and dyslipidemia. Diabetic Med. 2018;35(9):9.
- Foster N, W BR, M MK, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):2.
- Nielsen JV, Gando C, Jonsson E, et al. Low carbohydrate diet in type 1 diabetes, long-term improvement, and adherence: a clinical audit. Diabet Metab Syndr. 2012; 4:1–5.
- Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2009;359(3):229–241.
- McCrory MA, Hamaker BR, Lovejoy JC, et al. Pulse consumption, satiety, and weight management. Adv Nutr. 2010;1:1 17–30.
- Paoli A, Bosco G, Camporesi E. Ketosis, ketogenic diet, and food intake control: a complex relationship. Front Psychol. 2015;6:27.
- Norwitz N, Dalai SS, Palmer C. Ketogenic diet as a metabolic treatment for mental illness. Curr Opin Endocrinol Diabetes Obes. 2020;27(5):269–274.
- Dalai SS, Sinha A, Gearhardt AN. Low carbohydrate ketogenic therapy as a metabolic treatment for binge eating and ultraprocessed food addiction. Wolters Kluwer Health, Inc; 2020.
- Morris P, Puri BK, Caravaolho A. Induced ketosis as a treatment for neuroprogressive disorders: food for thought. Int J Neuropsychopharmacol. 2020;3:366–384.
- Huda MSB, Wilding JPH, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev. 2008;7(2):163–182.
- Kendall CW, Kendall CWC, Souza RJ. Dietary pulses, satiety, and food intake: a systematic review and meta-analysis of acute feeding trials. Obesity. 2014;22(8):1773–1780.
- Al Hourani H, Ababmeh R, Khawaja N, et al. Eating disorders among Jordanian adolescents with and without dysglycaemia: a comparative study. EMHJ. 2020;26:12.
- Rosmark B, Berne C, Holmgre S. Eating disorders in patients with insulin-dependent diabetes mellitus. J Clin Psychiatry. 1986;47(11):547–550.
- Hermanns N, Scheff C, Kulze B, et al. Association of glucose levels and glucose variability with mood in type 1 diabetic patients. Diabetologia. 2007;50(5):930–933.
- Buchberger B, Huppertz H, Krabbe L, et al. Symptoms of depression and anxiety in youth with type 1 diabetes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2016;70:70–84.
- Petrus RR, Do Amaral Sobral PJ, Tadini C, et al. The NOVA classification system: a critical perspective in food science. Trends Food Sci Technol. 2021;116:603–608.
- Shebanic SD, Anika S. 2020. Low carbohydrate ketogenic therapy as a metabolic treatment for binge eating and ultra-processed food addiction. Endocrinology, diabetes, and obesity. Curr Opin Endocrinol Diabetes Obes. 27:5.
- Ayton A, Ibrahim A, Dugan J, et al. Ultra-processed food and binge eating: a retrospective observational study. Nutrition. 2021;83:11023.
- DiFeliceantonio AG, Coppin G, Rigoux L, et al. Supra-addictive effects of combining fat and carbohydrate on food reward. Cell Metab. 2018;28(1):33–44.
- Small DM, Di Feliceantonio AG. Processed foods and food reward. Science. 2019;363(6425):346–347.
- Spitzer RI, Yanovski S, Wadden T, et al. Binge eating disorder: its further validation in a multisite study. Int J Eating Disorders. 1993;12:161–169.
- Moskovich AA, Dmitrieva NO, abvak MA, et al. Real time predictors and consequences of binge eating among adults with type 1 diabetes. J Eat Disord. 2019;7(1):7.
- Garonzi C, Forsander G, Maffeis C. Impact of fat intake on blood glucose control and cardiovascular risk factors in children and adolescents with type 1 diabetes. Nutrients. 2021;13(8):2625.
- Lawrence G. Dietary Fats and Inflammation. Handbook Lipids Human Func. 2016;13:635–665.
- Harcombe Z, S BJ, J DJ, et al., Evidence from randomized controlled trials does not support current dietary fat guidelines: a systematic review and meta-analysis. Open Heart. 3(2): e000409. 2016.
- Almsaid H, Muhsin H. The effect of Ketogenic diet on Vitamin D3 and testosterone hormone in patients with diabetes mellitus type 2. Pharm Med Sci. 2021;33(4):202–205.
- Savastio S, Cadario F, Beux S, et al. Vitamin D and type 1 diabetes. Open Rheumatol J. 2018;12(1):289–299.
- Kang HS, Gutin B, Barbeau P, et al. Low-density lipoprotein particle size, central obesity, cardiovascular fitness, and insulin resistance syndrome markers in obese youths. Int J Obes Relat Metab Disord. 2002;26(8):1030–1035.
- Dreon DM, Fernstrom HA, Campos H, et al. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am J Clin Nutr. 1998;67(5):828–836.
- Mensink RP, Zoc PL, Kester AD, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoprotein: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–1155.
- Scragnh T, Rm G, Nyunt O, et al. The association between ketoacidosis and 25 (OH) – vitamin D 3 levels at presentation in children with type 1 diabetes mellitus. Pediat Diabetes. 2009;10(1):38.
- Smith M. Seasonal, ethnic and gender variations in serum vitamin D3 levels in the local population of Peterborough. Biosci Horiz. 2010;3(3, Vol 5):124–131.
- Scragg R, Holdaway I, Singh V, et al. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27(3):181–188.
- Luu M, Visekruna A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2018;49(6):842–848.
- Zheng P, Li Z, Zhou Z. Gut microbiome in type 1 Diabetes: a comprehensive review. Diabetes Metab Res Rev. 2018;34(7):e3043.
- Barber TM, Kabisch S, Pfeiffer AFH, et al., The health benefits of dietary fibre. Nutrients. 12(10): 3209. 2020.
- Barber TM, Valsamakis G, Mastorakos G, et al. Dietary influences on the microbiota–gut–brain axis. Int J Mol Sci. 2021;22(7):3502.
- Kleiman SC, Watson HJ, Bulik-Sullivan EC, et al., The intestinal microbiota in acute anorexia nervosa and during renourishment. Psychosom Med. 77(9): 969–981. 2015.
- Butler MJ, L A PA, Eckel LA. The role of the gut microbiome, immunity and neuroinflammation in the pathophysiology of eating disorders. Nutrients. 2021;13(2):500.
- Freedman MR, King J, Kennedy E. Popular diets: a scientific review. Obes Res. 2001;9 Suppl 1:1S–40S.
- Paoli A, Mancin L, Bianco A, et al. Ketogenic diet and microbiota: friends or enemies? Genes (Basel). 2019;10(7):534.
- Calabrese CM, Valentini A, Calabrese G. Gut microbiota and type 1 diabetes mellitus: the effect of the Mediterranean diet. Frontiers. 2021;2021:329.
- Anderson MP, Newsholme EA, Fairburn CG, et al. Dieting reduces plasma tryptophan and alters brain %-HT function in women. Cambridge University Press; 2009.
- Eating Disorders Review. The effects of Tryptophan depletion. Reprinted Eating Disorders Rev. 2000;11:1.
- Krishnasamy S, Abell S. Diabetic gastroparesis: principles and current trends in management. Diabetes Therapy. 2018 Jul;9(Suppl 1):1–42.
- Vanormelingen C, Tack J, Andrews CN. Diabetic gastroparesis. Br Med Bull. 2013;105(1):213–230.
- Homko CJ, Duffy F, Friedenberg K, et al. Effect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesis. Neurogastroenterol Motility. 2015;27(4):501–508.
- Perianova I. The polyphony of food: food through the prism of maslow’s pyramid. Cambridge Scholars Publishing; 2012.
- Steinglass J, Foerde K, Kostro K, et al. Restrictive food intake as a choice – a paradigm for study. Int J Eating Disorders. 2015;48(1):59–66.
- Wong K, Raffray M, Roy-Fleming A, et al. Ketogenic diet as a normal way of eating in adults with type 1 and type 2 diabetes: a qualitative study. Can J Diabetes. 2021;45(2):137–143.
- Faith MS, S KS, Birch LL, et al. Parent-child feeding strategies and their relationships to child eating and weight status. Obesity. 2004;12(11):1711–1722.
- Becker CB, Middlemass K, Taylor B, et al. Food insecurity and eating disorder pathology. Int J Eating Disorders. 2017;50(9):1031–1040.
- Gallagher KA, DeSalvo D, Gregory J, et al. Medical and psychological considerations for carbohydrate restricted diets in youth with type 1 diabetes. Curr Diab Rep. 2009;19:19–27.
- Maharaj SJ, Rodin GM, Olmsted MP, et al. Eating disturbances in girls with diabetes: the contribution of adolescent self- concept, maternal weight and shape concerns and mother–daughter relationships. Psychol Med. 2003;33(3):525–539.
- Neumark-Sztainer D, Patterson J, Mellin A, et al. Weight control practices and disordered eating behaviors among adolescent females and males with type 1 diabetes. Diabetes Care. 2002;25(8):1289–1296.
- Goebel-Fabbri AE. Disturbed eating behaviours and eating disorders in type 1 diabetes: clinical significance and treatment recommendations. Curr Diab Rep. 2009;9(2):133–139.
- Watanabe M, Tuccinardi D, Ernesti I, et al. Scientific evidence underlying contraindications to the ketogenic diet: an update. Obesity Rev. 2020;21(10):10.
- Gray A. Nutritional recommendations for individuals with diabetes. Endotext Online (2019). Available at: https://www.ncbi.nlm.nih.gov/books/NBK279012. Available to 2019 May 27.
- Gang Jee K, Tortorici Y, Kalantar AR. Dietary protein intake, and chronic kidney disease. Clin Nutr Metab Care. 2017;20(1):77–85.
- Kalra S, Singla R, Rosha R, et al. Ketogenic diet: situational analysis of current nutritional guidelines. J Pak Med Assoc. 2018;68:No17.
- Scott A, o’Cathain A, Goyder E. Socioeconomic disparities in access to intensive insulin regimens for adults with type 1 diabetes: a qualitative study of patient and health care professional perspectives. Int J Equality Health. 2019;18(1):150.
- Morgan J, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. Br Med J. 1999;319(7223):1467–1468.
- Gurney VW, Halmi KA. Eating disorder curriculum for primary care providers. New York: Weill Medical College of Cornell University Westchester Division, White Plains; 2000.
- Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276(11):875–881.
- Food and agricultural organisation of the united nations. food-based dietary guidelines. Available at: https://www.fao.org/nutrition/education/food-dietary-guidelines/regions/countries/united-kingdom/en/. Available to May 15, 2022.
- Hart M, Pursey K, Smart C. Low carbohydrate diets in eating disorders and type 1 diabetes. Clin Child Psychol Psychiatry. 2021;26(3):643–655.
- Johnstone M. Reflective topical autobiography: an underutilized interpretive research method in nursing. In: Department of nursing and public health, faculty of biomedical and health sciences and nursing. University: Bundara West; 1999 doi:https://doi.org/10.1016/s1322-7696(08)60312-1