ABSTRACT
Introduction
As a key regulator of body water, sodium homeostasis forms an essential component of human physiology. Type 2 Diabetes Mellitus (T2D)-associated sodium overload stems from chronic renal retention of sodium, contributing toward the development of adverse cardiovascular sequelae.
Areas covered
Our traditional model of sodium regulation invokes two compartments: extracellular fluid (ECF [plasma and interstitial fluid]) and intracellular fluid (ICF). Data from the Mars program reveal inconsistencies with this two-space model, including mismatches between net body sodium and water. Recent data utilizing 23Na magnetic resonance imaging (MRI) show a preponderance of bound sodium within human dermis, consistent with a third space repository and providing compelling evidence to support a three-space model in which dermal sodium binding facilitates sodium homeostasis within the ECF and ICF. This buffer is impaired in T2D, with diminishment of dermal bound sodium that may promote deleterious sequelae of sodium overload within the ECF and ICF.
Expert opinion
Future studies should focus on novel therapeutic opportunities for sodium regulation in T2D and other conditions of sodium dysregulation. The ratio of free:bound dermal sodium (reflecting sodium storage capacity) could be utilized as a clinical biomarker for salt and water balance, to improve diagnostic accuracy and facilitate clinical decision-making.
1. Introduction
As a key regulator of body water content (including plasma volume and blood pressure), and osmolality within both the Extracellular Fluid (ECF) and Intracellular Fluid (ICF), sodium regulation forms an essential and vital component of human physiology. Perhaps not surprisingly, we have evolved complex and elaborate endocrine and neuro-humoral pathways implicating the Renin–Angiotensin–Aldosterone System, Brain Natriuretic Factor, and Atrial Natriuretic Factor to optimize sodium homeostasis, regulated on a minute-by-minute basis [Citation1,Citation2].
Clinically, aberrations of sodium homeostasis underlie and associate with much human disease. Broadly, these include sodium wasting (deficiency of total body sodium stemming from excessive loss of sodium [diarrhea, hemorrhage, or urinary losses] and/or diminished dietary sodium intake), and sodium overload (excessive total body sodium associated with chronic conditions like congestive cardiac failure and renal failure). However, despite the importance of such aberrations of sodium regulation and implications for pathophysiology in a broad range of human diseases, accurate clinical assessment of sodium balance (including in the acute-care and emergency settings) remains suboptimal. Furthermore, the clinical management of sodium aberrations is frequently challenging, especially in the context of sodium and fluid overload and hyponatremia with multiple causative factors.
In this concise review, we discuss the relevance of the ‘two-space’ model for sodium regulation (implicating the ECF and ICF compartments) in the context of recently published magnetic-resonance-based imaging studies, with identification and quantification of bound sodium within the dermis. Such data have led to a novel hypothesis for a ‘three-space’ model of sodium regulation, with the ECF compartment effectively split into two sub-compartments containing sodium in its bound and unbound (ionic) forms. We explore the role of glycosaminoglycans (GAGs) as a central component of the dermal ‘third space repository’ (TSR) for sodium. We then provide a description of Type 2 Diabetes Mellitus (T2D) as a sodium-overloaded condition, and the clinical implications for sodium handling in T2D from such renewed insights of a dermal TSR for sodium and a three-space model for sodium homeostasis. Finally, we consider future perspectives of how our renewed understanding of sodium regulation may translate into improvements in both the clinical assessment of body sodium status and the effective management of sodium aberrations.
2. Traditional ‘Two-Space’ Model of Sodium Regulation
Insights into the human physiological regulation of sodium have changed little over the last 40 years [Citation3], and this prevailing view has dominated our approach to medical education over this time. In essence, our traditional model of human sodium homeostasis invokes just two compartments or ‘spaces’: the ECF (including both plasma and non-plasma [interstitial fluid] constituents, the latter occurring primarily within the dermal and subcutaneous space), and ICF (the fluid compartment that exists within cells) [Citation3]. Importantly, this ‘two-space’ model of sodium regulation does not allow for sodium to exist in any compartment (or sub-compartment) other than the ECF and ICF. Furthermore, this model only allows for sodium to exist in its conventional and traditional physiological state as unbound ‘ionic’ form, with its attendant electrophysiological and osmotic effects. Accordingly, the two-space model of sodium homeostasis predicts that dietary sodium intake matches sodium output (including urinary sodium excretion and sodium loss in sweat), thereby maintaining sodium balance and equilibrium. Given the osmotic effects of sodium in its ionic form, another important prediction from the ‘two-space’ model of sodium homeostasis is that overall sodium balance within the body (i.e. net and cumulative sodium content within the ECF and ICF compartments) will always directly (and commensurately) correlate with total body water content. Furthermore, any change in the osmolality and sodium content of the ECF will have a direct impact on that of the ICF and vice versa.
Unfortunately, our whole notion of ‘two-space’ sodium regulation as outlined here is based on inherently flawed methodologies. The accurate measurement of dietary sodium intake is notoriously difficult. Existing methodologies that include single- and multiple-day food records and 24-hour dietary recall are inherently imprecise as a scientific measurement of dietary sodium intake [Citation4,Citation5], and are simply inadequate as a basis for any robust physiological model of sodium homeostasis. A further problem is that much of the literature on human sodium homeostasis focuses on 24-hour urinary collections for estimations of sodium excretion. This approach to the estimation of renal sodium excretion has even been considered as ‘gold-standard’ [Citation6]. However, when compared with alternate approaches to the measurement of urinary sodium excretion (such as spot, timed, or overnight urine collections), there is uncertainty regarding the utility of each approach [Citation6]. Furthermore, in studies of human participants in free-living environments that rely on each participant to collect an entire 24-hour collection of urine, there are a multitude of factors that may stymie such an approach, such as, for example, the need to use public toilets when away from home, or simply forgetting to collect urine on every void. Such mitigating factors may not always be acknowledged by researchers if there is reliance on self-recall. However, even if the 24-hour urinary collection was entirely accurate and precise for measuring renal sodium excretion, this methodology is designed on the premise that human sodium homeostasis plays out on a relatively short-lived timescale (with renal sodium excretion matching dietary sodium intake). Such an approach precludes the exploration of potentially longer-term fluctuations of sodium balance, that may ultimately lead us to question the validity and clinical utility of our traditional two-space model of sodium homeostasis.
3. From ‘Two-Space’ Model to Mars and beyond
Our traditional approach to measuring sodium intake and excretion, with all its inherent flaws and inaccuracies as outlined in the last section, ensured that the ‘two-space’ model of sodium homeostasis persisted for so long. However, this has all changed in recent years following the publication of data that emerged from the Mars program [Citation7]. To explore the feasibility of prolonged flights to Mars and potentially humans living on Mars for long periods of time, it was necessary for NASA to ascertain the effects of such an unusual scenario on many aspects of human physiology and psychology. To simulate prolonged space flight, the Mars program was established for cosmonauts to live in an Earth-based capsule for many weeks, during which rigorous and continuous observations and measurements would be made [Citation7]. Fortuitously, such measurements also included those on sodium intake and excretion. It is difficult to overstate the sea-change in our approach to measuring human sodium balance that the Mars program enabled, including both accurate and reliable measurements of urinary sodium excretion for periods much longer than a mere 24-hours, indeed, for weeks and even months. Furthermore, the highly controlled environment of the Mars program enabled very accurate and reliable measures of dietary sodium intake, without the reliance on, and limitations of, self-recall. In short, the Mars program [Citation7] offered a unique opportunity to explore human sodium regulation accurately, reliably, and scientifically, and to use these insights to confirm or refute our traditional ‘two-space’ model of sodium homeostasis.
The Mars program [Citation7] has revealed some fascinating data that could only have been achieved through such a controlled and prolonged approach to observation. Data that are particularly relevant to our discussion include frequent mismatches between net body sodium load (based on measurements of oral dietary intake and urinary excretion of sodium over long time periods) and body weight (from osmotically retained water) [Citation7]. This included relative excessive dietary sodium intake without the expected water retention [Citation7]. As outlined in the last section, the ‘two-space’ model of sodium regulation predicts that net body sodium load and body water content should correlate directly and commensurately. Therefore, these novel observations from the Mars program [Citation7] are entirely inconsistent with, and indeed refute our traditional ‘two-space’ model of sodium homeostasis [Citation3]. The implication is that not all sodium within the body exists within its unbound ionic form, with attendant osmotic effects. This allows for the possibility of an additional compartment or ‘space’ in which sodium may exist in its alternate bound form, isolated from any attendant electrostatic or osmotic effects. Further insight from the Mars program [Citation7] included the first demonstration of ultra-long weekly (infradian) and even monthly cycles of urinary excretion of sodium [Citation7,Citation8], which is also difficult to reconcile with the ‘two-space’ model of sodium regulation.
4. Magnetic-Resonance-Based Imaging and Quantification of Dermal Sodium
The ubiquity of sodium within the body makes it inherently difficult to study. Sodium literally occurs everywhere, within cells, within plasma and within the extracellular space surrounding cells. It is often said that life cannot exist without water. Whilst true, it is hard to envision how life as we know it could exist without sodium, as a key regulator of osmolarity, exerting effects in tandem with water. Therefore, far from searching for something hidden and elusive, it may seem like an odd venture to search for something as ubiquitous within the body as sodium. Recently, however, just such a search has occurred. A major impetus for this search was the data on sodium handling from the Mars program outlined in the last section [Citation7], the inconsistencies with the 'two-space' model of sodium regulation, and the possibility of a third space for sodium to exist in its bound form.
Although usually based on the spin of hydrogen atoms, magnetic resonance imaging (MRI) can also generate data on the spin of sodium ions, enabling 23Na MRI to search for a third space repository (TSR) for sodium [Citation9,Citation10]. Initial studies utilized whole-body 23Na MRI at 3 Tesla (3 T) magnetic field to demonstrate a preponderance of sodium signals originating from human skin [Citation10–12]. Furthermore, in chronic kidney disease, skin sodium content was observed to be closely associated with left ventricular mass and systolic blood pressure [Citation12,Citation13]. Interestingly, the 23Na MRI technique has also shown muscle sodium content to positively correlate with hypertrophic vascular remodeling in T2D [Citation14].
Unfortunately, an important limitation of whole body 3 T 23Na MRI is that the field strength of the magnet and the gradients provide insufficient resolution of images to discern between sodium in its different forms, or to provide accurate and reliable quantification of sodium content. A preponderance of dermal sodium has previously been demonstrated [Citation10–12]. However, these sodium signals originated from ionic sodium within the ECF, and bound sodium levels were not probed by 23Na MRI methods reported in these works. Moreover, assessment of bound sodium levels is crucial to provide key insights into sodium regulation, specifically regarding a possible TSR for sodium within the human dermis. To address these concerns, our own group published data from ex-vivo human skin samples utilizing a microimaging system with a three-fold stronger magnetic field of 9.4 T for 23Na MRI, which enabled the first clear discernment of unbound from bound sodium. Using this technique, the bound sodium was quantified accurately, as it has a slow diffusive movement on the timescale of the imaging used [Citation9,Citation15].
To summarize our journey so far, we have outlined the ‘two-space’ model of human sodium regulation that has prevailed for nearly half a century and the important limitations to our traditional methodologies for exploring sodium balance in humans. We then explored the unique data from the Mars program [Citation7], providing epiphanic novel insights into human sodium regulation that seriously call into question our traditional notion of the ‘two-space’ model, and which allows for an additional space, or TSR for sodium. Finally, we have outlined recently reported imaging studies based on 23Na MRI, showing a preponderance of sodium within the human dermis, and further evidence published by our own group to demonstrate this dermal sodium to exist in a bound form (in which sodium ions are weakly bound to macromolecules), entirely consistent with a dermal TSR for sodium [Citation9]. The stage is now set for a discussion of an alternate ‘three-space’ model of sodium regulation.
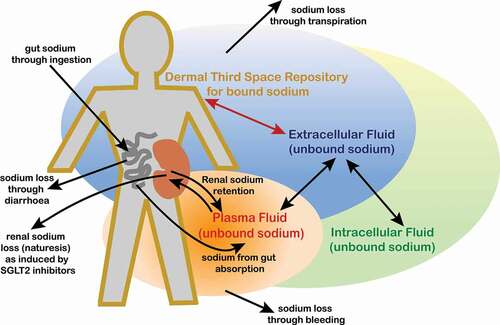
5. ‘Three-Space’ Model of Sodium Regulation
The imaging data outlined above are highly suggestive of a dermal TSR for sodium. Importantly, and as outlined in the next section [Citation9], sodium within its TSR exists bound to dermal GAGs. As such, contrary to what the ‘two-space’ model of sodium regulation predicts, there is no requirement for total body sodium to always match total body water, and certainly not over such a short timeframe.
The osmotic pressure associated with GAGs has been well studied due to its role in articular cartilage and correlation with ensuing osteoarthritis [Citation16]. Detailed experimental and computational studies have shown that the osmotic pressure increases with polysaccharide concentration, chain length, negative fixed charge density (number of sulfation groups), and sodium ion concentration [Citation17,Citation18]. This non-linear osmotic pressure increase from sodium ions is believed to be due to the molecular weight increase of the GAGs. For our purpose, the dermal GAGs can act as an effective sodium store that is separate from the ICF and ECF (the latter incorporating both plasma and interstitial fluid).
Regarding how a TSR for sodium would function physiologically and what its purpose would be, we envision an effective sodium store within the ECF, with the dermis ideally located due to its large volume, surface area, and accessibility (covering the entire body and incorporating a large proportion of interstitial fluid). Such a location would provide maximal ease of movement of sodium ions between the TSR and the contiguous ECF. Regarding its function, we hypothesize that a TSR for sodium would act like a storage buffer or depot, to release additional (potentially lifesaving) sodium to the ECF when body sodium is depleted (such as acute hemorrhage or diarrheal illness), providing an evolutionary drive for such a depot. Conversely, the deleterious effects of excessive amounts of ionic sodium in the body (including hypertension, peripheral edema, left ventricular strain and other hemodynamic effects), would be buffered by a dermal TSR for sodium. In this latter scenario of sodium excess, the dermal TSR would bind and store excessive sodium from the ECF, thereby protecting the ECF and ICF from any associated deleterious effects. In short, a dermal TSR for sodium would help to keep ECF (and therefore ICF) ionic sodium properly regulated, through acting as either a donor or buffer for ECF sodium when body sodium is depleted or excessive, respectively. The potential mechanisms by which such sodium migrations between the ECF and TSR are regulated are not yet understood and should form a focus for future research. It remains possible, though, that such a process could operate entirely physically (without the need for any endocrine or paracrine control), and based on the relative concentrations of sodium within the ECF and the binding capacity status of the dermal TSR for sodium.
It is worth reviewing the current evidence and observations that promote a three-space model for human sodium regulation:
The three-space model would explain perfectly (and even predict) the mismatches between total body sodium and body water from the Mars program [Citation7] as outlined earlier. Conversely, such observations entirely contradict a two-space model of sodium homeostasis.
Infradian cycles of urinary sodium excretion observed from the Mars program [Citation7] are difficult to explain through a two-space model. Whilst not entirely explainable through a three-space model either, the presence of a TSR for sodium would at least provide the potential for such infradian cycles to exist, through cyclical changes in the binding capacity of the TSR for sodium. Whilst such a mechanism is entirely speculative and requires more focused research to explore the details, the point here is that a three-space model for sodium would at least allow for infradian cycles of urinary sodium. Conversely, such infradian cycles would be entirely precluded from a two-space model.
The three-space model provides a perfect explanation for a conundrum that has long puzzled healthcare professionals who manage patients with Diabetes Mellitus (DM), regarding how the large quantities of renally retained exchangeable sodium are accommodated and in what locations [Citation19]. The absence of peripheral edema in patients with DM (outside of the context of co-existing conditions like heart failure), which is hard to explain through a two-space model, is readily explainable through a three-space model, in which a proportion of the sodium overload is stored bound (and osmotically inactive) within a dermal TSR for sodium.
The imaging studies outlined earlier provide evidence for a preponderance of bound sodium within the human dermis. Other than a TSR for sodium, it is difficult to provide a physiological explanation for such an observation, which is of course entirely consistent with a three-space model.
To date, evidence for a ‘three-space’ model of human sodium regulation as outlined here (and shown schematically in ) remains largely circumstantial, although as mounting evidence and consistent observations accrue, the case becomes ever-more compelling. Indeed, such a hypothesis has gained a broader acceptance among the scientific fraternity in recent times [Citation20]. Perhaps, the main element of contentiousness regarding a three-space model for sodium regulation stems more from the requirement for a paradigm shift to replace our longstanding two-space model (which most of us would have accepted as the truth when at medical school), with its associated dogma and the inherent and inevitable resistance to modifying such a long-held view. It is worth remembering that examples of such a mind-set, with ostensible resistance to change despite clear guiding evidence, plague the history of scientific breakthroughs.
6. The Role of Glycosaminoglycans in the Dermal TSR for Sodium
For the three-space model of sodium regulation and the dermal TSR for sodium to be physiologically viable, it is important to consider its structural location. We provided compelling evidence for the co-location of bound sodium with the dermal glycosaminoglycan (GAG) scaffold, through comparison of immunohistochemical staining for dermal GAGs with the 9.4 T 23Na MRI images [Citation9]. Other reported imaging studies are consistent with this hypothesis [Citation11,Citation12]. The dermal GAGs form an extensive hydrated-gel scaffold, consisting of polymers with a negative fixed charge density, known to bind strongly to both water and ions. This feature is important for the physiological function of GAGs in the skin, to the extent that synthetic scaffolds (for tissue engineering applications) frequently include GAGs for optimal effect [Citation21]. This gel creates turgor and a physical structure or scaffold (a dermal extracellular matrix), around which resides the ECF [Citation22]. Dermal GAGs include the non-sulfated hyaluronan (HA) and a family of highly sulfated complex linear polysaccharides, covalently attached to protein cores, forming proteoglycans [Citation23], and providing numerous functions that include the facilitation of cell migration, diffusion of nutrients, coordination of signaling molecules within the ECF and immune-regulation [Citation24]. The sodium-binding capacity of GAGs may play an important role in determining the concentration of dermal bound sodium. Whilst currently speculative, intuitively for a TSR for sodium, the location of the dermal GAGs within the non-plasma component of the ECF [Citation25] would enable direct migration of sodium ions between the dermal GAGs and the dermal ECF.
7. T2D as a Sodium-Overloaded Condition
T2D suffers as a clinical entity in the sense that its diagnosis (and until recently, much of its management options) is entirely based upon glycemic control. This unidimensional descriptor of T2D has an important impact on the way we envision this condition, and our clinical priorities, with ‘glucocentricity’ taking center stage. The reality, though, is that T2D has a complex pathogenesis that remains incompletely understood, and that dysglycemia is just one (albeit important) manifestation that arises from such complexity. From this milieu, there are other perspectives with which to view T2D, shifting our attention from purely glycemic control. Although not part of its diagnostic makeup, nor usually a priority for its management strategies, sodium overload is, arguably, as much a clinical and biochemical feature of T2D as is dysglycemia. Had sodium overload been integrated into the diagnosis of T2D, this would likely have placed a greater emphasis on optimizing sodium regulation in this condition, and improved awareness among healthcare professionals regarding sodium overloading in T2D.
Our understanding of T2D as a sodium-overloaded condition goes back many decades. Indeed, 30 years ago, it was shown that compared with euglycemic controls, people with T2D have an 8–10% increase in the amount of exchangeable sodium within the ECF (from 24Na-based radioisotope and volume expansion studies), even in the context of good glycemic control [Citation19,Citation26–28]. Such studies also focused on T2D in the context of nephropathy and/or congestive heart failure [Citation26]. The question is then how such sodium overload translates into meaningful clinical outcomes and sequelae of T2D. Our current understanding is that ‘T2D-associated sodium overload’ (TASO) plays an important role in the development of hypertension, left ventricular overload, and renal dysfunction in T2D, with strong epidemiological support for such a hypothesis [Citation19,Citation29]. More recently, our understanding of the pathophysiological links between T2D and hypertension has extended beyond mere sodium retention, to a scenario that also incorporates the effects of T2D-associated insulin resistance and heightened sympathetic drive [Citation30]. Perhaps not surprisingly, the increased mortality and morbidity attributed to T2D [Citation31] stems at least in part from TASO, with its attendant augmented risk of myocardial infarction and heart failure [Citation14]. Furthermore, the longer-term micro- and macrovascular glycemic complications of T2D are augmented by the co-occurrence of TASO-related hypertension [Citation29].
Given the unequivocal existence of TASO, it is important to explore its underlying mechanisms and to address how the realms of glucose and sodium dysregulation may influence one another. From one perspective, the renal retention of sodium in the context of hyperglycemia can be viewed as a protective phenomenon. Through the osmotic effects of glycosuria, those with T2D (particularly in the context of poor glycemic control) would be at risk of dehydration and cardiovascular collapse. From this perspective, the renal retention of sodium in T2D helps to mitigate this inherent risk of excessive renal water loss.
Renal sodium retention in T2D may stem from the sodium-retaining effects of hyperinsulinemia secondary to insulin resistance. However, this hypothesis remains contentious [Citation32]. Although early data from human-based euglycemic hyperinsulinemic clamp studies [Citation33] suggested a sodium-retaining effect of insulin [Citation32,Citation33], these studies were limited by the pharmacological doses of insulin used. Furthermore, the data were not reproducible at lower physiological doses of insulin [Citation34]. The renal reabsorptive sodium-retaining effects of insulin per se are further refuted somewhat by the apparent lack of sodium retention or hypertension in patients with insulinoma [Citation32]. Based on the observations outlined here, it seems unlikely that hyperinsulinemia (at least in the physiological range), is the sole cause of TASO.
Given the lack of data to implicate hyperinsulinemia per se in the development of TASO, it is important to extend our approach, to consider the effects of glucose itself. Glycosuria is known to stimulate the expression within the proximal nephron of a key renal transporter protein for sodium and glucose retention: ‘sodium glucose-like transporter 2’ (SGLT2) [Citation32,Citation35–37]. With worsening glycemic control in T2D, increased glycosuria results in commensurate upregulation of renal SGLT2 expression and enhanced renal sodium reuptake. Additionally, Serum and Glucocorticoid inducible Kinase 1 (SGK1) regulates glucose transport in the proximal nephron and is itself activated by glucose and insulin [Citation32]. Epithelial sodium channel (ENaC), located in the distal nephron, is stimulated via SGK1 [Citation32] and promotes further renal sodium reuptake in the context of glycosuria. Therefore, based on these observations, the development of TASO appears to stem from the co-occurrence of hyperglycemia, glycosuria, and hyperinsulinemia (features that typify T2D), through the stimulated renal expression of SGLT2, SGK1, and ENaC within the nephron [Citation32].
8. Clinical Implications of a Three-Space Model for Sodium Handling in T2D
As outlined, TASO likely contributes toward the development of hypertension and left ventricular overload in T2D [Citation19,Citation29]. Given our renewed understanding of sodium handling outlined earlier, it is important to explore how this may translate to T2D, both regarding its pathophysiology and therapeutic opportunities. Through comparison of skin biopsy samples from adult participants with T2D and euglycaemic controls, we demonstrated the first evidence of a substantial reduction in bound dermal sodium, presumably reflecting a diminished dermal sodium-binding capacity in T2D [Citation9]. We speculate that in T2D, diminishment of bound sodium within the dermal TSR would limit the buffering capacity for TASO, and that inevitably this would result in excessive ionic sodium within the ECF and adverse hemodynamic and cardiovascular sequelae. In effect, we hypothesize that TASO would stem from two complementary pathways: one involving renal retention of sodium, the other a limitation of sodium buffering via probable impaired binding capacity of the dermal GAGs. Assuming validation of such a hypothesis, it would follow that a logical therapeutic target for T2D would be to re-establish the sodium-binding capacity of the (dermal) GAGs, to limit the deleterious effects of TASO on excessive sodium content within the ECF and ICF and implications for cardiovascular functioning.
In recent times, there has been much interest in the SGLT2 Inhibitor class of drug therapies for T2D. SGLT2 inhibitors promote renal excretion of two sodium for every one glucose molecule from the renal tubules [Citation35], thereby inducing natriuresis and reduced plasma volume by 4% [Citation35,Citation38]. SGLT2 inhibitors associate with cardiovascular and renal benefits in T2D, including improved systolic blood pressure and reduced hospitalized heart failure [Citation35–37]. Numerous hypotheses have been proposed [Citation39], but supporting evidence remains inconclusive. Recent data suggest the effects of the SGLT2 Inhibitor class on endothelial function as a possible mediator of its cardiovascular and renal benefits [Citation40]. Accordingly, there has been a scientific re-awakening of the important role of sodium handling in T2D-related adverse cardiovascular effects. The favorable effects of the SGLT2 Inhibitor class of drug therapies on cardio-renal outcomes have fueled this renaissance. The extent to which the natriuretic effects of this class are implicated in these outcomes remains open to question, and one which is the focus of much ongoing research. However, in addition to its renal effects, it will be important to consider whether this class of therapies may also influence the sodium-binding capacity of the dermal GAGs, and whether this process may help to explain the underlying mechanisms of some of its favorable cardio-renal effects. Intriguingly, through the use of a 3 T 23Na MRI technique, it was shown that treatment with Dapagliflozin (an SGLT2 inhibitor therapy used for the management of T2D) reduces total dermal sodium content in patients with T2D [Citation10], although this may simply reflect an overall reduction in sodium within the ECF rather than specifically the dermal TSR for sodium.
In addition to the possible effects of SGLT2 Inhibitor therapies on the dermal TSR for sodium with implications for sodium handling within the ECF, this class of therapies may also influence sodium content within the ICF compartment. Indeed, SGLT2 Inhibitor-induced reduction of intracellular sodium likely underlies at least some of the cardio-protective effects of this class (including improved clinical outcomes in patients with heart failure), through the prevention of oxidative stress and cardiomyocyte death [Citation41]. Recent data reveal a possible effect of SGLT2 Inhibitor therapies on the Na/H exchanger (NHE), a group of membrane proteins implicated in the regulation of ICF sodium concentration, cell volume, and pH. In a study on rodent cardiomyoblasts, it was demonstrated that Empagliflozin (an SGLT2 Inhibitor) reduced angiotensin II–induced hypertrophy of cardiomyoblasts through inhibition of the expression of both SGLT1 and NHE1 [Citation42]. Further studies on ventricular myocytes from rabbits and rats reveal Empagliflozin-induced inhibition of NHE flux, with a reduction in cytosolic sodium and calcium concentrations, and a concomitant increase in mitochondrial calcium concentration [Citation43]. Moreover, the SGLT2-Inhibitor induced inhibition of cardiac NHE flux (and reduction of cardiomyocyte sodium concentration) appears to be a class-effect, with similar observations between the SGLT2-Inhibitor therapies, Empagliflozin, Dapagliflozin, and Canagliflozin, on rodent cardiomyocytes [Citation44].
The data outlined above provide intriguing insights into possible mechanisms by which the SGLT2 Inhibitor class of therapies exert their cardiovascular benefits. It should be noted that these insights stem primarily from rodent-based studies performed ex vivo. Furthermore, it is not clear how SGLT2 Inhibitor therapies may exert direct effects on cardiomyocytes given the lack of SGLT2 receptors within the heart [Citation45]. However, despite these caveats, NHE inhibition appears to be a potential therapeutic target in heart failure to optimize cardiomyocyte function and attenuate myocardial remodeling [Citation46]. Unfortunately, the clinical studies on NHE inhibition to date have revealed inconsistent results, with poor efficacy and serious side-effects [Citation46].
The cardioprotective effects of SGLT2 Inhibitor therapies remain an active field of research. One intriguing hypothesis relates to the fasting-like paradigm induced by this class, triggering the promotion of cellular homeostasis from the activation of nutrient deprivation pathways [Citation45]. These include enhanced gluconeogenesis and ketogenesis, activated by the molecular stimulus sirtuin-1 (SIRT1) and downstream mediators, proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and fibroblast growth factor 21 (FGF21) [Citation45]. These nutrient deprivation sensors can promote autophagy and improve oxidative stress, thereby promoting cardio-protection [Citation45]. Although this metabolic-induced indirect mechanism of cardio-protection from SGLT2 Inhibitor therapies does not implicate sodium regulation directly, the rodent data outlined above reveal possible effects of these therapies on cardio-protection stemming from the regulation of ICF sodium homeostasis. Future studies should explore this intriguing mechanism further, and how possible SGLT2 Inhibitor-induced changes (either direct or indirect) to the TSR for sodium may also impact on sodium concentrations in both the ECF and ICF compartments, and consequent cardiovascular function.
9. Expert Opinion
Our conceptualization of T2D has been strongly influenced by an emphasis on glucocentricity, with important implications for its diagnosis and management strategies. An alternate perspective is that T2D associates with renal sodium retention and TASO. Our notion of human sodium handling has remained static over the last 40 years and has followed the traditional two-space model. Recent data from the Mars program [Citation7] show inconsistencies with the two-space model. Furthermore, recently reported 23Na MRI-based imaging studies show a preponderance of bound sodium within the dermal GAGs that strongly supports a TSR for sodium [Citation9]. An alternate three-space model for sodium regulation would help to reconcile both the reported data from the Mars program [Citation7] and these recent imaging studies. Finally, T2D associates with diminished bound sodium within the dermal GAGs, which may open a novel therapeutic opportunity.
With the establishment of a three-space model for sodium regulation, an important question relates to its clinical implications, both diagnostically and therapeutically. The accurate and reliable clinical assessment of salt and water balance is notoriously difficult. Despite this, much clinical decision-making (including within the acute and emergency-care settings) relies upon such information. Common examples include the use of intravenous fluids for dehydration (and the correct choice of fluid depending on salt and water balance), or the use of diuretic agents for salt and water overload. Whilst extreme variants of such clinical scenarios are usually obvious clinically, in a large proportion of acutely unwell patients there may be multiple factors that influence salt and water balance contemporaneously, and the decision regarding its correct management can be challenging. Traditionally, biochemical assessments of serum and urine sodium concentrations and osmolality provide useful information, although there is usually a time-delay for such data. It remains possible that the ratio of free:bound dermal sodium (reflecting sodium storage capacity) could be utilized clinically as an accurate biomarker for salt and water balance, given that it likely correlates with overall ionic sodium within the ECF. This could prove invaluable in clinical decision-making regarding initial management of salt and water imbalance, but also guide ongoing management. Although not currently available, it is possible that future transportable mini-MRI detectors (to image dermal bound and unbound sodium) could enable improved diagnosis and clinical decision-making regarding salt and water balance, particularly within acute and emergency-care clinical settings.
Regarding T2D, a measure of bound sodium within the dermal TSR could provide an important criterion on which to assess overall salt and water balance. Such data could indicate a level of vulnerability of the ECF to TASO and be useful as a biomarker of response to therapies that induce natriuresis (such as SGLT2 Inhibitor therapies). Future studies should explore the extent to which the dermal GAGs form a TSR for sodium, and whether and how other dermal molecules may also be implicated. Furthermore, we should explore the underlying mechanisms whereby the dermal GAGs become depleted of bound sodium, including possible changes in GAG chain length, conformation, or sulfur content. Such insights, building on prior rodent-based studies [Citation47], would provide further therapeutic opportunities to intervene, with the aim of re-establishing the full buffering capacity of the dermal TSR for sodium.
Finally, viewing sodium regulation as a three-space model has the potential to transform our physiological understanding of sodium handling. Any model is only useful if it proves superior to its predecessor in predicting outcomes. In this case, sodium binding was previously thought to have insignificant effects, according to the two-space model. By accepting an updated three-space model that fits our current understanding, we can experimentally test its predictions. We should be open-minded to future modifications based on novel data, which may include, for example, the observation of bound sodium in locations other than the dermis, thereby extending the reach of the TSR for sodium. Based on our current data and understanding, the three-space model for sodium homeostasis represents our best model according to outcome predictions. For now, we should context our understanding by the three-space model and align our clinical and research efforts accordingly.
Article highlights
As a key regulator of body water content, sodium homeostasis forms an essential and vital component of human physiology.
Our traditional model of human sodium regulation invokes two compartments, including extracellular fluid (plasma and the interstitial fluid) and intracellular fluid. Within each, sodium exists in its ionic form, with associated electrostatic and osmotic effects.
Recently reported studies using a 23Na MRI have revealed a preponderance of sodium within the human dermis, our own group showing the first evidence for bound sodium within this location, co-locating to the glycosaminoglycan (GAG) scaffold.
A novel ‘three-space’ model for sodium regulation implicates an additional ‘third space repository’ (TSR) for sodium within the human dermis. Through non-covalent (ionic) binding with GAGs, sodium would not exert any electrostatic or osmotic effects whilst in its TSR, consistent with the mismatches between net sodium balance and body water content reported from the Mars program.
Type 2 Diabetes Mellitus (T2D) associates with sodium overload (from the renal retention of sodium), which in turn likely drives the development of hypertension, left ventricular overload and adverse cardiovascular sequelae.
In T2D, there is a significant reduction in bound dermal sodium. This may limit the buffering capacity for sodium overloading, and result in excessive ionic sodium within the ECF and ICF compartments, with hemodynamic and cardiovascular sequelae.
The ratio of free:bound dermal sodium (reflecting sodium storage capacity) could be utilized as a clinical biomarker for salt and water balance, to improve diagnostic accuracy and facilitate clinical decision-making.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank all the participants and healthcare professionals involved in the studies cited in this review. We thank the Arden Tissue Bank at UHCW for their support with skin samples. We thank AstraZeneca (funding awarded to TMB) and the Medical Research Council (MC_PC_15074 to GEP) for their generous funding support contributing toward the data cited in this review.
Additional information
Funding
References
- Patel S, Rauf A, Khan H, et al. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–325.
- Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: hormones secreted from the heart. Peptides. 2019;111:18–25.
- Manning RD Jr., Guyton AC. Dynamics of fluid distribution between the blood and interstitium during overhydration. Am J Physiol. 1980;238(5):H645–51.
- Bentley B. A review of methods to measure dietary sodium intake. J Cardiovasc Nurs. 2006;21(1):63–67.
- McLean RM, Farmer VL, Nettleton A, et al. Assessment of dietary sodium intake using a food frequency questionnaire and 24-hour urinary sodium excretion: a systematic literature review. J Clin Hypertens (Greenwich). 2017;19(12):1214–1230.
- Ji C, Sykes L, Paul C, et al. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev Panam Salud Publica. 2012;32(4):307–315.
- Birukov A, Rakova N, Lerchl K, et al. Ultra-long-term human salt balance studies reveal interrelations between sodium, potassium, and chloride intake and excretion. Am J Clin Nutr. 2016;104(1):49–57.
- Jantsch J, Schatz V, Friedrich D, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 2015;21(3):493–501.
- Hanson P, Philp CJ, Randeva HS, et al. Sodium in the dermis colocates to glycosaminoglycan scaffold, with diminishment in type 2 diabetes mellitus. JCI Insight. 2021; 6(12). DOI: https://doi.org/10.1172/jci.insight.145470
- Karg MV, Bosch A, Kannenkeril D, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17(1):5.
- Wiig H, Luft FC, Titze JM. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiol. 2018;222(3):e13006.
- Kopp C, Linz P, Maier C, et al. Elevated tissue sodium deposition in patients with type 2 diabetes on hemodialysis detected by (23)Na magnetic resonance imaging. Kidney Int. 2018;93(5):1191–1197.
- Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85(5):520–526.
- Kannenkeril D, Jung S, Harazny J, et al. Tissue sodium content correlates with hypertrophic vascular remodeling in type 2 diabetes. J Diabetes Complications. 2021;35(12):108055.
- Schepkin VD, Neubauer A, Nagel AM, et al. Comparison of potassium and sodium binding in vivo and in agarose samples using TQTPPI pulse sequence. J Magn Reson. 2017;277:162–168.
- Maroudas A, Bannon C, Silberberg A. Measurement of swelling pressure in cartilage and comparison with the osmotic pressure of constituent proteoglycans. Biorheology. 1981;18(3–6):619–632.
- Bathe M, Rutledge GC, Grodzinsky AJ, et al. A coarse-grained molecular model for glycosaminoglycans: application to chondroitin, chondroitin sulfate, and hyaluronic acid. Biophys J. 2005;88(6):3870–3887.
- Chahine NO, Chen FH, Hung CT, et al. Direct measurement of osmotic pressure of glycosaminoglycan solutions by membrane osmometry at room temperature. Biophys J. 2005;89(3):1191–1197.
- O’Hare JP, Corrall RJ. De natrio diabeticorum. increased exchangeable sodium in diabetes. Diabet Med. 1988;5(1):22–26.
- Ellison DH, Welling P, Ingelfinger JR. Insights into salt handling and blood pressure. N Engl J Med. 2021;385(21):1981–1993.
- Piccirillo G, Feuerer N, Carvajal Berrio DA, et al. Hyaluronic acid-functionalized hybrid gelatin-Poly-L-Lactide scaffolds with tunable hydrophilicity. Tissue Eng Part C Methods. 2021;27(11):589–604.
- Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339(1):237–246.
- Merry CLR, Lindahl U, Couchman J, et al. Proteoglycans and sulfated glycosaminoglycans. In: th VA, Cummings RD, Esko JD, et al., editors. Essentials of glycobiology. Long Island, New York: Cold Spring Harbor Laboratory Press; 2022. p. 217–232.
- Zhang L. Glycosaminoglycans in development, health and disease. Preface Prog Mol Biol Transl Sci. 2010;93:xvii–xviii.
- Oien AH, Wiig H. Electrostatic, elastic and hydration-dependent interactions in dermis influencing volume exclusion and macromolecular transport. J Theor Biol. 2016;400:80–91.
- O’Hare JA, Ferriss JB, Brady D, et al. Exchangeable sodium and renin in hypertensive diabetic patients with and without nephropathy. Hypertension. 1985;7(6 Pt 2):II43–8.
- de Chatel R, Weidmann P, Flammer J, et al. Sodium, renin, aldosterone, catecholamines, and blood pressure in diabetes mellitus. Kidney Int. 1977;12(6):412–421.
- De Chatel R, Toth M, Barna I, et al. Body sodium, atrial natriuretic peptide and blood pressure in diabetes mellitus. Acta Biomed Ateneo Parmense. 1992;63(1–2):153–161.
- Pavlou DI, Paschou SA, Anagnostis P, et al. Hypertension in patients with type 2 diabetes mellitus: targets and management. Maturitas. 2018;112:71–77.
- Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380(9841):601–610.
- Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17(1):83.
- Brands MW, Manhiani MM. Sodium-retaining effect of insulin in diabetes. Am J Physiol Regul Integr Comp Physiol. 2012;303(11):R1101–9.
- DeFronzo RA. The effect of insulin on renal sodium metabolism. A review with clinical implications. Diabetologia. 1981;21(3):165–171.
- Brimble A, Corrall R, Mattocks J, et al. Insulin and the renal response to volume expansion in man (A). J Physiol (London) 1985; 66
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357.
- Dekkers CCJ, Gansevoort RT, Heerspink HJL. New diabetes therapies and diabetic kidney disease progression: the role of SGLT-2 inhibitors. Curr Diab Rep. 2018;18(5):27.
- Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–1658.
- Salvatore T, Caturano A, Galiero R, et al. Cardiovascular benefits from gliflozins: effects on endothelial function. Biomedicines. 2021;9(10):1356.
- Palmiero G, Cesaro A, Vetrano E, et al. Impact of SGLT2 inhibitors on heart failure: from pathophysiology to clinical effects. Int J Mol Sci. 2021;22(11):5863.
- Abdulrahman N, Ibrahim M, Joseph JM, et al. Empagliflozin inhibits angiotensin II-induced hypertrophy in H9c2 cardiomyoblasts through inhibition of NHE1 expression. Mol Cell Biochem. 2022;477(6):1865–1872.
- Baartscheer A, Schumacher CA, Wust RC, et al. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia. 2017;60(3):568–573.
- Uthman L, Baartscheer A, Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61(3):722–726.
- Packer M. Cardioprotective effects of sirtuin-1 and its downstream effectors: potential role in mediating the heart failure benefits of SGLT2 (sodium-glucose cotransporter 2) inhibitors. Circ Heart Fail. 2020;13(9):e007197.
- Karmazyn M. NHE-1: still a viable therapeutic target. J Mol Cell Cardiol. 2013;61:77–82.
- Cechowska-Pasko M, Palka J, Bankowski E. Decrease in the glycosaminoglycan content in the skin of diabetic rats. The role of IGF-I, IGF-binding proteins and proteolytic activity. Mol Cell Biochem. 1996;154(1):1–8.