1. Introduction
GO is an inflammatory process associated with autoimmune thyroid diseases and characterized by increased adipogenesis and enlargement of the extraocular muscles resulting in the expansion of the retro-orbital content.
The optimal management of GO relies on the assessment of disease activity and severity, as anti-inflammatory/immunosuppressive treatments are effective on active GO, whereas long-term consequences of inactive GO require rehabilitative surgery, including orbital decompression, squint, and lid surgery [Citation1,Citation2].
GO activity is assessed with the Clinical Activity Score (CAS) () [Citation3], whereas severity is graded, based on the EUGOGO score, as mild, moderate-to-severe, and sight-threatening [Citation4]. This classification mirrors the treatment needs (), as milder cases are managed conservatively while moderate-to-severe GO requires immunosuppressive treatments, aimed at inactivating GO, in an attempt to avoid rehabilitative surgery.
In Europe, intra-venous methylprednisolone (ivMP) ± sodium mycophenolate for three months, in weekly infusions is the recommended first-line treatment for active GO [Citation5]. To maximize benefits and minimize adverse effects, the ivMP route is modulated based on GO severity: the highest doses (7.5 g) are prescribed to patients with severe forms [Citation5] while intermediate doses (4.5 g) to the milder cases. Sight-threatening GO requires medical decompression, followed by urgent bony orbital decompression, if unresponsive in a few weeks. Medical decompression consists of three ivMP pulses (500–1000 g) given on consecutive or alternate days [Citation6], repeated after one week and followed by weekly infusions not exceeding 8 g.
Over the last two decades, the therapeutic toolkit for active GO has been enriched with new biological disease-modifying drugs including rituximab, tocilizumab, and teprotumumab. Currently, these molecules are recommended as second-line treatments alternatively to orbital radiotherapy (OR) or to a second course of ivMP. Nonetheless, randomized controlled trials (RCT), meta-analysis, reviews, and statistical analyses on pooled data suggest that these drugs are effective and will probably enter into clinical practice more and more frequently in the near future. Indeed, in the US, following FDA approval, teprotumumab is now used by several physicians as first-line treatment for GO. Nevertheless, the proportion of relapses after teprotumumab is similar to that reported after ivMP (15–30%) and some safety concerns have been raised following the observation of increased risk of hearing loss with teprotumumab [Citation7–10].
Response to treatment can be objectively measured by scores such as the composite index, which assess changes of lid retraction, inflammatory signs, exophthalmos, and eye muscle ductions () [Citation11].
Unfortunately, available therapies are still inadequate since a successful treatment measured as the change of biological parameters, does not imply per se an improvement of patients’ quality of life (Qol). Indeed, GO patients exhibit long-term visual, appearance, and psychosocial disability and suffer from emotional stress and occupational impairment even many years after being treated with immunosuppressants and surgery [Citation12,Citation13].
2. Incidence of ‘relapsing’ GO
The natural history of GO is self-limiting according to the Rundle curve, characterized by an initial ‘active phase’ in which the severity increasingly worsens, followed by an ‘inactive phase’ of improvement of inflammation and subsequent tissue remodeling and fibrosis.
Rarely, the course of the disease diverges from this monophasic curve because of relapses during the follow-up as outlined in .
Figure 1. The biphasic course of GO according to the model of Rundle (Panel A). The shaded area represents the severity of GO graded according with the EUGOGO score (right axes). The black continuous line represents GO activity measured by the clinical activity score (CAS) (left axes). Dotted arrows and text in regular font outline the recommended therapies for each stage (active/inactive) and grade (mild, moderate to severe and sight-threatening). In italics fonts the treatments that might be useful in the future, but for which data are insufficient to support a recommendation. The gray arrow is the 6- weeks-follow up assessment and thereafter not-responders undergo a second line treatment. Patients developing sight-threatening GO (DON, corneal breakdown and sublussation of the eye globe) require urgent medical/surgical decompression.
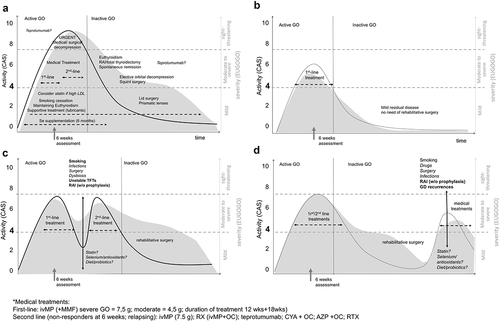
GO relapses following the first diagnosis can be defined as the recurrence of inflammatory signs and symptoms after a period of remission or improvement of variable length.
To simplify this issue, we can grossly divide these patients into two groups: early and late relapsing.
Early recurrences occur after few months (e.g. <6 months) of inactive disease. This depends on a prolonged active phase due to an asynchronous/asymmetric involvement of the orbits and to a variable response to first-line therapy with a 15–30% of the patients not responding/relapsing after ivMP [Citation14].
Late recurrences may occur in a minority of patients who flare after a period of quiescent disease lasting several months/years. The reported frequency is variable, ranging from 5 to 16% according to the diagnostic criteria used [Citation15,Citation16]. In a retrospective study, Patel et al. reported a recurrence rate as high as 16%, in most of the cases occurring from 2 to 5 years after first diagnosis [Citation17]. This percentage is likely overestimated as late relapses are observed very rarely in a real-life clinical context. Late recurrences must be differentiated from chronic congestive GO, in which the impaired orbital vascular flow causes edema, eyelid/conjunctival erythema, and chemosis mimicking active inflammation with high CAS. In this condition, only surgical treatment is expected to improve GO.
3. Hypothetical risk factor/triggering events associated with GO relapses
High TRAb levels, older age, male gender, longer duration of GD/GO symptoms, smoking habit, oxidative stress, poor control of the underlying thyroid dysfunctions and radioiodine treatment are known risk factors for GO worsening and poor response to treatments. The same factors are also associated with an increased risk of GO in newly diagnosed GD patients [Citation2,Citation17], and hypothetically could influence GO relapse after treatment.
Orbital rehabilitative surgery but also cataract surgery have been associated with late GO recurrences, being smokers more at risk. In the study of Patel, periocular surgery was the most frequent (12%) trigger event associated with GO recurrences, followed by thyroid dysfunctions (5%). Nonetheless, the overall risk related with surgery is quite low as Baldeschi and coworkers reported GO recurrences after bony decompression in about 1% (3/239) of patients [Citation18].
Recently, additional risk factors have been identified opening new perspectives in personalized risk-assessment and targeted prevention.
GD patients with high LDL levels have an increased risk to develop GO, and the administration of atorvastatin to hypercholesterolemic GO patients undergoing ivMP improved the clinical outcomes. Nevertheless, it is unclear whether the enhanced response is due to the anti-inflammatory or lipid lowering properties of atorvastatin [Citation19].
Gut microbiota composition seems to be different in GO/GD patients compared to controls with increasing levels of Prevotella, Lactobacillus, and Veillonella, although it is still debated if this is a cause or a consequence of the disease. In animal models of GO, differences in gut microbiota influenced the heterogeneity of thyroid and ocular disease being Firmicutes associated with orbital-adipogenesis. The transplant of fecal material from GO donor to these mice increased the severity of GD/GO. Unpublished data of the study named ‘Investigation of Novel biomarkers and Definition of the role of the microbiome In Graves’ Orbitopathy (INDIGO)’ (https://cordis.europa.eu/project/id/612116/reporting/it) suggested that the administration of a probiotic (LAB4, Cultech Ltd.) to GD patients reduced the risk of hyperthyroidism relapse, without influencing GO severity/progression.
4. Can we avoid GO flares?
The majority of the studies focused on preventing progression of GO from mild to severe forms and on the treatment moderate-to-severe active GO, while scarce data are available on how to avoid GO relapses after first-line treatment. Although hypothetical, it seems reasonable that the same supportive therapies and lifestyle changes useful to prevent GO progression may also reduce the relapse rate after first-line therapy.
Cautious use of radioiodine (RAI) therapy, smoking cessation, proper treatment of thyroid dysfunction, and selenium supplementation, have a proven effectiveness and have been extensively reviewed elsewhere [Citation20].
The most obvious modifiable risk factor is cigarette smoking, which is associated with a poorer outcome after medical treatments and orbital radiotherapy, higher risk of GD relapse, increased likelihood of GO worsening/occurrence after radioiodine and increased risk of early and late GO recurrences [Citation21]. Both active and passive smokings are associated with tissue hypoxia and increased generation of reactive oxygen species and free radicals, enhanced production of inflammatory cytokines, imbalance of Th1/Th2/Th17 responses, alteration of intestinal microbiome [Citation19]. These effects are thought to be dose-dependent and reversible upon cessation. Noteworthy, more than a half of quit attempts fails within few weeks when patients are left without any assistance. Thus, specialized behavioral support and pharmacotherapy to relieve the symptoms of nicotine withdrawal should be offered to GO patients [Citation22].
Stable restoration of euthyroidism, avoiding phases of uncontrolled hypo and hyperthyroidism, is a crucial factor to improve response to treatment and prevent early and late recurrences. In these patients, whether the treatment of hyperthyroidism would be conservative (anti-thyroid drugs) or definitive (radioiodine, thyroidectomy, or both) is still matter of debate. RAI treatment can trigger GO in about 15–20% of cases being this event prevented by a short-term course of low-dose oral/iv glucocorticoids prophylaxis [Citation2,Citation23,Citation24]. RAI is contraindicated in patients with active GO but can be considered in those with burn-out GO after immunosuppressive treatment. One advantage of thyroidectomy is the faster and permanent remission of hyperthyroidism, and this would be preferable in patients with severe residual GO in order to speed up the rehabilitative surgery.
Selenium has been reported to decrease the ocular involvement and to reduce GO progression of mild Graves’ orbitopathy even after the treatment is stopped [Citation25]. Nevertheless, in this study selenium levels were not measured, and it is thus unknown whether these beneficial effects were due to its antioxidant effects or to the correction of an associated selenium deficiency. Currently, selenium supplementation is not recommended in moderate-to-severe GO, except in cases of evident dietary deficiency.
The effectiveness of lipid-lowering therapies on relapse rates is currently unknown, however we believe it is appropriate recommending statin at least in all GO patients meeting the criteria for primary and secondary cardiovascular prevention.
Observations concerning gut microbiota open a number of interesting scenarios as diet, physical exercise, antibiotics, probiotics, nutritional n-3 polyunsaturated fatty acids, and antioxidants that may modulate its composition. More research and carefully designed clinical trials are needed before suggesting probiotics or changes in diet, even though these practices have gained popularity over the last few years among patients, such as self-prescribed dietary restrictions (e.g. vegan or gluten-free diet).
Finally, the appropriate management of first-line treatments has an impact on the likelihood of subsequent GO flares [].
Table 2. Efficacy of available treatments for GO. The response and relapse rate and efficacy in preventing dysthyroid optic neuropathy (DON) are reported.
Time is increasingly recognized as a key factor in treatment planning, and the choice of treatment may depend on disease duration. For instance, the efficacy of ivMP and rituximab is greater in highly active and recent GO, whereas of teprotumumab it is effective also in patients with a longer disease duration [Citation22]. Teprotumumab is associated with a greater improvement in proptosis as compared with ivMP, however data from the OPTIC and OPTIC-x trials showed a relapse rate of nearly 30% after a first course of teprotumumab with one patient developing dysthyroid optic neuropathy (DON) [Citation7]. The whole of these data suggests that ivMP is still appropriate in the majority of patients affected with GO and it seems unlikely that steroids will be rapidly supplanted by this or other novel treatment [Citation8,Citation9].
Since GO recurrences, including DON, may occur at ivMP withdrawal, patients should continue ivMP treatment for several weeks. To reduce the frequency of GO flares, some clinicians administer oral prednisone between iv pulses and during the tapering period after pulses discontinuation, even though this practice seems not to increase the overall response rate [Citation26]. Some retrospective studies suggested that OR may be employed in preventing GO flares after steroids titration [Citation27], but prospective trials are not available to fully support its routine use.
As the majority of patients with active GO responsive to ivMP improve as early as 6 weeks, it has been suggested that non-responders can be switched to other treatments alone or in combination with steroids without excessive delay [Citation5,Citation28]. Nevertheless, a shared decision-making discussion with the patient should be encouraged, since some of these cases may become responders at 12 weeks of treatment [Citation29].
In RCTs or open studies, both rituximab and tocilizumab improved GO activity and severity in patients who were non-responsive or flared after steroids. Albeit with conflicting evidence [Citation30,Citation31], the monoclonal anti CD20 antibody rituximab seems associated with a lower recurrence rate compared to steroids and teprotumumab, although at present, it is still recommended as second-line therapy [].
5. Conclusions
The course of GO can be influenced by preventive measures that might reduce the progression rate of GO to more severe forms. Several risk factors for GO occurrence and poor response to treatments have been unequivocally acknowledged (smoking, uncontrolled thyroid dysfunction, administration of radioiodine without a proper prophylaxis, not timely treatment of the active phase), while others remain unclear (dyslipidaemia, intestinal dysbiosis). Although very reasonable, it is unclear if the correction of these risk factors may also be helpful in preventing recurrences of GO after immunosuppressive treatments.
Given the large heterogeneity of GO manifestations, a more tailored approach is desirable. Further studies are warranted to identify the characteristics of subgroups patients with the highest chances to respond to a certain treatment.
Until more effective and specific drugs are discovered in the future, long-term surveillance and counseling are recommended for all GO patients.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Campi I, Vannucchi G, Salvi M. THERAPY OF ENDOCRINE DISEASE: endocrine dilemma: management of Graves’ orbitopathy. Eur J Endocrinol. 2016;175:R117–R133.
- Bartalena L. Graves’ orbitopathy: imperfect treatments for a rare disease. Eur Thyroid J. 2013;2:259–269.
- Mourits MP, Prummel MF, Wiersinga WM, et al. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clinical Endocrinol. 1997;47:9–14.
- Bartalena L, Baldeschi L, Dickinson A, et al. Consensus statement of the european group on graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158:273–275.
- Bartalena L, Kahaly GJ, and Baldeschi L, et al. The 2021 European group on graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185:G43–G67.
- Currò N, Covelli D, Vannucchi G, et al. Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid. 2014;24:897–905.
- Douglas RS, Kahaly GJ, and Ugradar S, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X study. Ophthalmology. 2022;129:438–449.
- Tanda ML, Gallo D, Ippolito S, et al. Treatment of moderate-to-severe and active Graves’ orbitopathy: a step forward from the OPTIC study. J Endocrinol Invest. 2020;43:1523–1525.
- Kahaly GJ, Douglas RS, Holt RJ, et al. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021;9:360–372.
- Bartalena L, Marinò M, Marcocci C, et al. Teprotumumab for Graves’ orbitopathy and ototoxicity: moving problems from eyes to ears? J Endocrinol Invest. 2022;45:1455–1457.
- Bartalena L, Wiersinga WM. Proposal for standardization of primary and secondary outcomes in patients with active, moderate-to-severe Graves’ orbitopathy. Eur J Endocrinol. 2020;9:3–16.
- Terwee C, Wakelkamp I, Tan S, et al. Long-term effects of Graves’ ophthalmopathy on health-related quality of life. Eur J Endocrinol. 2002;146:751–757.
- Ponto KA, Pitz S, Pfeiffer N, et al. Quality of life and occupational disability in endocrine orbitopathy. Dtsch Arztebl Int. 2009;106:283–289.
- Salvi M, Campi I. Medical treatment of Graves’ orbitopathy. Horm Metab Res. 2015;47:779–788.
- Selva D, Chen C, King G. Late reactivation of thyroid orbitopathy. Clin Exp Ophthalmol. 2004;32:46–50.
- Patel P, Khandji J, Kazim M. Recurrent Thyroid Eye Disease. Ophthalmic Plast Reconstr Surg. 2015;31(6):445–448.
- Wiersinga W, Žarković M, Bartalena L, et al. Predictive score for the development or progression of Graves’ orbitopathy in patients with newly diagnosed Graves’ hyperthyroidism. Eur J Endocrinol. 2018;178:635–643.
- Baldeschi L, Lupetti A, Vu P, et al. Reactivation of Graves’ orbitopathy after rehabilitative orbital decompression. Ophthalmology. 2007;114:1395–1402.
- Lanzolla G, Sabini E, and Leo M, et al. Statins for Graves’ orbitopathy (STAGO): a phase 2, open-label, adaptive, single centre, randomised clinical trial. Lancet Diabetes Endocrinol. 2021;9:733–742.
- Bartalena L, Piantanida E, and Gallo D, et al. Epidemiology, natural history, risk factors, and prevention of Graves’ orbitopathy. Front Endocrinol. 2020;11:615993.
- Hegediüs L, Brix TH, Vestergaard P. Relationship between cigarette smoking and Graves’ ophthalmopathy. J Endocrinol Invest. 2004;27:265–271.
- Rigotti NA, Kruse GR, Livingstone-Banks J, et al. Treatment of tobacco smoking: a review. JAMA. 2022;327:566–577.
- Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73–78.
- Vannucchi G, Covelli D, Campi I, et al. Prevention of orbitopathy by oral or intravenous steroid prophylaxis in short duration Graves’ disease patients undergoing radioiodine ablation: a prospective randomized control trial study. Thyroid. 2019;29:1828–1833.
- Lanzolla G, Marinò M, Marcocci C. Selenium in the treatment of Graves’ hyperthyroidism and eye disease. Front Endocrinol. 2021;11:608428.
- Zang S, Ponto KA, Kahaly GJ. Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96:320–332.
- Sobel RK, Aakalu VK, Vagefi MR, et al. Orbital radiation for thyroid eye disease: a report by the American Academy of Ophthalmology. Ophthalmology. 2022;129:450–455.
- Vannucchi G, Covelli D, and Campi I, et al. The therapeutic outcome to intravenous steroid therapy for active Graves’ orbitopathy is influenced by the time of response but not polymorphisms of the glucocorticoid receptor. Eur J Endocrinol. 2013;170:55–61.
- Bartalena L, Veronesi G, Krassas GE, et al. Does early response to intravenous glucocorticoids predict the final outcome in patients with moderate-to-severe and active Graves’ orbitopathy? J Endocrinol Invest. 2017;40:547–553.
- Salvi M, Vannucchi G, Currò N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100:422–431.
- Stan MN, Salvi M. MANAGEMENT OF ENDOCRINE DISEASE: rituximab therapy for Graves’ orbitopathy - lessons from randomized control trials. Eur J Endocrinol. 2017;176:R101–R109.
