ABSTRACT
Background: In this report we describe, for the first time, the activation of the peripheral immune compartment in a patient with a CRB1 linked retinal degenerative disease, masquerading as intermediate uveitis.
Methods: To monitor the immune system during systemic immunosuppressive treatment, given for the initial diagnosis of intermediate uveitis, blood samples were taken before and during therapy, for analysis of peripheral blood mononuclear cell-subsets and circulating immune mediators.
Results: The levels of various pro-inflammatory immune mediators (including MIF, TSLP, CCL2/MCP-1, CXCL9, CXCL10, IFN-β, IL-6, IL-17, IL-21, IL-22, and IL-23) were elevated in serum at the first time point, and decreased under immunosuppressive treatment. In parallel, the frequency of activated (CD86+) CD1c+ myeloid dendritic cells in blood was proportional to the central foveal thickness measured by optical coherence tomography.
Conclusions: These observations challenge the current view on the distinct pathophysiology of retinal degenerative and retinal inflammatory conditions in this patient.
1. Introduction
Retinal dystrophies are a heterogeneous group of severe inherited retinal diseases characterized by progressive deteriorating of photoreceptors that are visually debilitating and potentially blinding. One of the more than 200 genes linked to retinal dystrophies is the retinal gene Crumbs homologue 1(CRB1) that encodes a protein critical to canonical retinal development [Citation1]. Over 150 genetic mutations in CRB1 have been reported in patients with retinal dystrophies. The clinical manifestation of retinal dystrophies linked to mutations in sequence of the CRB1 gene may vary considerably and includes conditions such as retinitis pigmentosa, Leber congenital amaurosis, and cone–rod dystrophy [Citation2]. Although the CRB1-related disease spectrum hallmarks fundus-specific patterns and retinal dysfunction typical to ‘degenerative’ conditions, recently Hettinga et al. [Citation3] described several patients with among others CRB1-related retinal dystrophy masquerading as intraocular inflammation. Here, we performed longitudinal immunophenotyping in the blood of one of the patients from this series and report on hitherto unknown activation of various pro-inflammatory pathways and immune cells in blood.
1.1. Case description
A 14-year-old girl, without medical history, was referred to our tertiary hospital with visual complaints including blurred vision and floaters. At presentation, the best corrected visual acuity (BCVA) was 20/32 and 20/25 (Snellen equivalent) for the right and left eye, respectively. Ophthalmological examination revealed inflammation of the vitreous, cystoid macular edema (CME), and multifocal choroiditis-like lesions, indicative for intraocular inflammation (). Screening by a pediatric rheumatologist and clinical immunologist did not detect any underlying systemic disease. Diagnostic screening included extensive laboratory testing (including full blood count, C-reactive protein, blood sedimentation rate, angiotensin converting enzyme, antinuclear antibodies, human leukocyte antigen typing, antibody titers for various infectious agents, and liver and kidney function tests), urine screening for proteins, tuberculin skin test for tuberculosis, X-ray and computed tomography imaging of the lungs, and magnetic resonance imaging of the brain. The patient was initially diagnosed with idiopathic intermediate uveitis, which was supported by a second opinion at another tertiary referral center. Consequently, she was treated with several local and systemic anti-inflammatory and immunomodulatory agents, including systemic corticosteroids, methotrexate, mycofenolate mofetil, tumor necrosis factor-α inhibitors, and eventually tocilizumab. All therapies were equally ineffective in controlling the disease. During tocilizumab therapy (the period described in this study), BCVA of both eyes increased slightly from 20/50 to 20/40 (Snellen equivalent) for the right eye and from 20/66 to 20/33 for the left eye. However, though improvements in visual acuity were seen, the overall clinical picture did not show any permanent improvement, and visual acuity continued to decrease. Subsequent genetic testing revealed a heterozygous mutation in the CRB1 gene, and the diagnosis of retinal dystrophy was established.
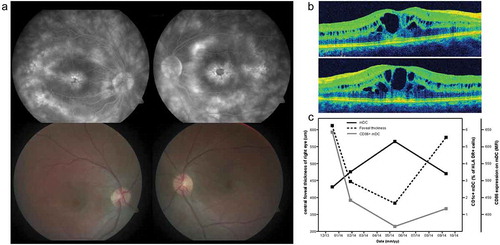
Figure 1. Cystoid macular edema at several time points during immune-monitoring. (a) Upper left and right: fluorescein angiography of the right and left eye of an patient with retinal dystrophy at presentation revealing extensive fluorescein leakage in the macular area. Lower left en right: fundoscopy color images of the right and left eye of the same patient at presentation. (b) Optical Coherence Tomography images of the macula of the right (upper image) and left (lower image) eye at presentation revealing CME. (c) Dynamics of foveal thickness (measured by OCT), the frequency of CD1c+ mDC cells in blood and their activation status (measured by expression of activation marker CD86) through time.
CME: Cystoid Macular Edema; mDC: myeloid dendritic cell; MFI: mean fluorescence intensity; OCT: Optical Coherence Tomography.
2. Methods
During this period, we monitored the circulating immune compartment of this patient. After informed consent, blood was drawn before the start of tocilizumab therapy (time point I) and after 2, 5, and 6 months (time points II–IV, respectively). Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque density gradient centrifugation and analyzed by multicolor flow cytometry (Fortessa, BD Biosciences, San Jose, CA, USA). PBMCs were directly stained with fluorochrome-conjugated antibodies to analyze myeloid dendritic cells (anti-CD3-PerCP-CY5.5, anti-CD19-PerCP-CY5.5, anti-CD56-PerCP-CY5.5, anti-HLA-DR-PB, anti-CD16-BV500, anti-CD14-APC-H7, anti-CD141-PE, anti-CD303-FITC, anti-CD86-PE-Cy7, and anti-CD1c-APC; BD Biosciences). Acquired data were analyzed using FlowJo software (FlowJo, LLC, OR, USA). Forty-six immune mediators were simultaneously measured in undiluted serum using our in-house developed and validated (ISO9001 certified) multiplex immunoassay based on Luminex technology [Citation4].
3. Results
The levels of various pro-inflammatory immune mediators (including macrophage migration inhibitory factor, thymic stromal lymphopoietin, chemokine CC ligand-2 [also known as monocyte chemoattractant protein-1], CXCL9 (chemokine CXC ligand) and CXCL10, interferon-beta, and interleukin [IL]-6, IL-17, IL-21, IL-22, and IL-23) were elevated in serum at the first time point and decreased in subsequent time points (see also ). In parallel, the frequency of activated (as indicated by CD86+) CD1c+ myeloid dendritic cells in blood was proportional to the central foveal thickness measured by optical coherence tomography ().
Table 1. Cytokine analysis in serum of a patient with retinal dystrophya.
4. Discussion
Retinal dystrophies comprise a group of progressive retinal disorders frequently caused by mutations in the CRB1 gene [Citation2]. Studies on the CRB1 gene revealed its critical role in retinal development and homeostasis, and an extensive line of evidence demonstrates that genetic disruption of the function of this retinal gene results in the death of photoreceptors and progressive deterioration of retinal function with associated vision loss [Citation1]. Consequently, efforts for better understanding the CRB1-linked degenerative disease are mostly focused on studying the molecular implications of genetic mutations on retinal dysfunction. However, endogenous or environmental factors outside the retina may also play a significant role in modulating the phenotype in patients, complicating its diagnosis, and potentially alter its clinical evolution.
Indeed, recent studies have revealed circumstantial evidence that suggests a contributing role of inflammatory pathways in retinal dystrophies: Patients with retinal degeneration may manifest with inflammation of the vitreous and show elevated levels of pro-inflammatory cytokines and chemokines in ocular fluids. Curiously, as described for ocular inflammatory diseases, patients with retinal dystrophies may show peripheral immune responses toward retinal antigens – indicating co-occurring and possible harmful autoimmune mechanisms directed against the retina [Citation5–Citation7].
In this study, we further substantiate this concept and describe additional hitherto unknown inflammatory changes that can be detected in the blood of such a patient. Most strikingly, cytokines such as IL-17 and IL-23, which are hallmark cytokines for T-helper 17 cells, a subset of T cells that are considered to play a central role in autoimmune uveitis [Citation8], were elevated in blood and decreased after immunosuppressive/modulatory treatment that included tocilizumab. In addition, activated CD1c+ myeloid dendritic cell frequency was proportional to the degree of foveal thickness, which makes it tempting to suggest a role for this cell subset in the development of CME in this patient. Interestingly, this immune cell was recently linked to disease activity in noninfectious uveitis commonly associated with macular edema [Citation9,Citation10]. These findings support a role of several inflammatory factors in the pathogenesis of retinal dystrophies. The inflammatory signatures may perhaps be the result of a (secondary) immune response directed against the damaged ocular tissues. The exact mechanism remains poorly understood; however, pro-inflammatory cytokines and chemokines produced by activated retina resident microglia [Citation11–Citation14], Müller cells, and the retinal pigment epithelium have been implicated [Citation15]. Whether the activation of microglia is induced by photoreceptor cell death or actually causes this photoreceptor cell death remains to be determined [Citation12]; however, accumulating evidence supports the concept that activated microglia play an active role in photoreceptor cell death through phagocytosis and the production of pro-inflammatory mediators [Citation13,Citation14].
Although the influence of inflammation on the natural course of disease remains to be elucidated, the here described case reveals that various inflammatory signatures are well observed at the clinical and molecular levels, which is a thought-provoking observation that makes it tempting to speculate on its implications for the care of this subgroup of patients. Considering it is a primary ocular genetic defect, anti-inflammatory therapy is currently not indicated for retinal dystrophy because it will not resolve the cause of disease. However, the emerging role of inflammation in retinal dystrophies may provide opportunities for influencing the natural course and secondary complications of this group of diseases. Although we are at the dawn of gene therapy to correct the genetic defect in retinal dystrophies, currently, gene therapy is not yet widely available. In contrast, various potent immunomodulatory agents are available that could potentially be used to influence the inflammatory responses to prevent secondary complications (macular edema) to delay vision loss and improve quality of life. Research in this area, which is currently lacking, is likely to receive more attention in the near future [Citation16,Citation17]. In the past, treatment with intravitreal corticosteroid has been applied for treatment of secondary macular edema in retinitis pigmentosa patients with mixed results [Citation18,Citation19]. It is important to note that the temporary improvements in visual function in the case described here may also be the reflection of the natural disease course, and further studies are necessary to further elucidate the contribution of inflammation on the clinical course of CRB1-linked retinal dystrophies.
In summary, these observations substantiate that in our patient with CRB1-linked retinal dystrophy masquerading as intraocular inflammation, the disease is accompanied by molecular activation of inflammatory cytokine pathways and immune cells in the blood. As such, these results challenge the current view on the distinct pathophysiology of retinal degenerative and retinal inflammatory conditions in this patient.
Key issues
This report describes laboratory evidence of systemic activation of the immune system in a patient with a CRB1 linked retinal degenerative disease.
The here reported case substantiates a role for the inflammation in the pathogenesis of retinal dystrophy.
The frequency of CD1c+ myeloid dendritic cells in the peripheral blood, and their activation status, was proportional to central foveal thickness, an important clinical parameter with serious potential consequences for visual acuity
Contributorship statement
F.H. Verhagen: data collection, analysis and interpretation of data, literature search, writing the article, final approval of the version to be published.
J.J.W. Kuiper: concept and design, acquisition of data, analysis and interpretation of data, writing the article, final approval of the version to be published.
S. Nierkens: acquisition of data, analysis and interpretation of data, drafting the article, final approval of the version to be published.
Saskia M. Imhof: acquisition of data, revising the article critically for intellectual content, final approval of the version to be published.
T.R.D. Radstake: acquisition of data, revising the article critically for intellectual content, final approval of the version to be published.
J.H. de Boer: concept and design of the study, acquisition of data, revising the article critically for intellectual content, final approval of the version to be published.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Alves CH, Pellissier LP, Wijnholds J. The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog Retin Eye Res. 2014;40:35–52.
- Ehrenberg M, Pierce E, Cox GF, et al. CRB1: one gene, many phenotypes. Semin Ophthalmol. 2013;28(5–6):397–405.
- Hettinga YM, Van Genderen MM, Wieringa W, et al. Retinal dystrophy in 6 young patients who presented with intermediate uveitis. Ophthalmology. 2016;123(9):2043–2046.
- De Jager W, Velthuis H, Prakken BJ, et al. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10(1):133–139.
- Tamm S, Whitcup SM, Gery I, et al. Immune response to retinal antigens in patients with gyrate atrophy and other hereditary retinal dystrophies. Ocul Immunol Inflamm. 2001;9(2):75–84.
- Yoshida N, Ikeda Y, Notomi S, et al. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013;120(1):100–105.
- Stunkel M, Bhattarai S, Kemerley A, et al. Vitritis in pediatric genetic retinal disorders. Ophthalmology. 2015;122(1):192–199.
- Prete M, Dammacco R, Fatone MC, et al. Autoimmune uveitis: clinical, pathogenetic, and therapeutic features. Clin Exp Med. 2016;16(2):125–136.
- Chen P, Tucker W, Hannes S, et al. Levels of blood CD1c+ mDC1 and CD1chi mDC1 subpopulation reflect disease activity in noninfectious uveitis. Investig Ophthalmol Vis Sci. 2015;56(1):346–352.
- Chen P, Urzua C, Knickelbein JE, et al. Elevated CD1c + myeloid dendritic cell proportions associate with clinical activity and predict disease reactivation in noninfectious uveitis. Investig Opthalmology Vis Sci. 2016;57(4):1765–1772.
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003 Apr;76(4):463–471.
- Hughes EH, Schlichtenbrede FC, Murphy CC, et al. Generation of activated sialoadhesin-positive microglia during retinal degeneration. Investig Opthalmology Vis Sci. 2003;44(5):2229–2234.
- Yoshida N, Ikeda Y, Notomi S, et al. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013;120(1):e5–e12.
- Zhao L, Zabel MK, Wang X, et al. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med. 2015;7(9):1179–1197.
- Rutar M, Natoli R, Chia RX, et al. Chemokine-mediated inflammation in the degenerating retina is coordinated by Müller cells, activated microglia, and retinal pigment epithelium. J Neuroinflammation. 2015;12:8.
- Viringipurampeer I, Bashar AE, Gregory-Evans CY, et al. Targeting inflammation in emerging therapies for genetic retinal disease. Int J Inflam. 2013;2013:1–7.
- Guadagni V, Novelli E, Piano I, et al. Pharmacological approaches to retinitis pigmentosa: a laboratory perspective. Prog Retin Eye Res. 2015;48(1):62–81.
- Ozdemir H, Karacorlu M, Karacorlu S. Intravitreal triamcinolone acetonide for treatment of cystoid macular oedema in patients with retinitis pigmentosa. Acta Ophthalmol Scan. 2005;83(2):248–251.
- Srour M, Querques G, Leveziel N, et al. Intravitreal dexamethasone implant (Ozurdex) for macular edema secondary to retinitis pigmentosa. Graefe’s Arch Clin Exp Ophthalmol. 2013;251(6):1501–1506.
