1. Introduction
Glucocorticoids (GCs) are the most prescribed drugs in the management of inflammatory musculoskeletal diseases, such as rheumatoid arthritis (RA). However, a very recent review accurately retraced the 70-year history of the “Nobel Prize” GCs for the treatment of several rheumatic conditions [Citation1,Citation2].
The reasons for this “survival”, apparently at present without a deadline, as well as the motivations for the persistent and significant efficacy as anti-inflammatory/immune modulatory drugs of GCs, even on the first line in RA, are the matter of the present short review.
RA is mainly characterized by bilateral inflammatory involvement of joints with synovial hyperplasia, bone erosion, and progressive loss of function. Patients complain of pain, morning stiffness, and limitations of the range of joint movement. From the beginning of their use, GCs showed to quickly induce relief of RA symptoms with proven effectiveness, as components of the stress response system (), and over the years it was discovered that GCs act at multiple and complex levels in the pathogenesis of the disease.
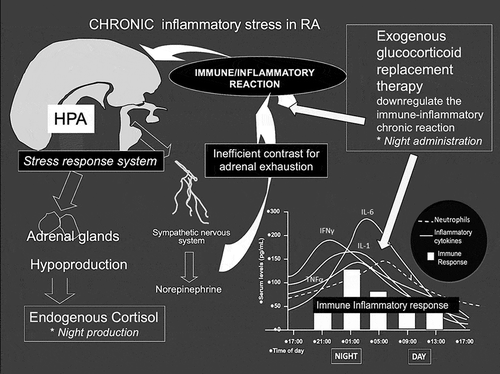
Figure 1. Mechanisms of reduced production of endogenous cortisol in chronic active rheumatoid arthritis, and replacement hormonal function for exogenous low-dose glucocorticoids with optimized anti-inflammatory effects when available at the night (respect of the circadian rhythms). TNFα: Tumor Necrosis Factor α; IL-1: Interleukin-1; IFNγ: Interferon γ; IL-6: Interleukin-6
2. Endogenous cortisol and exogenous glucocorticoids in rheumatoid arthritis
The chronic inflammatory stimulus exerted by RA causes a persistent activation of the hypothalamic-pituitary-adrenal (HPA) axis, with a progressive insufficient production of endogenous corticotropin-releasing hormone (CRH), adrenocorticotropic hormone (ACTH), and cortisol. A cascade of events is the final consequence of the effects of pro-inflammatory cytokines (e.g. TNFα, IL-1, IL-6) that in addition up-regulate hepatic 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1). In fact, this enzyme converts peripheral inactive cortisone into cortisol, therefore exerting a negative feedback on hypothalamic ACTH release and consequently on adrenal cortisol production. The mechanism is called systemic “cortisone-to-cortisol” shuttle [Citation3].
Interestingly, as demonstrated in both murine models and human RA, 11β-HSD1 is also represented in the inflammatory joints, but its potential beneficial action is counteracted by 11β-HSD2. 11β-HSD2 is highly expressed by macrophages and activated T-lymphocytes and has the function to enhance cortisol degradation, with the final result of down-regulating its concentration in the inflamed sites [Citation4,Citation5].
In this way, the exogenous supplementation of GCs should act as a “replacement therapy” in order to restore up to sufficient serum levels of cortisol ().
It is also possible to rebalance the HPA axis at hypothalamic level. In fact, the Food and Drug administration has recently approved repository corticotropin injection (RCI) as adjunctive short-term therapy for RA flares as already used in the past already from 1959, but with some difficulties in efficient handling of such approach over the decades [Citation6,Citation7]. However, ACTH doesn’t only induce adrenal cortisol production as previously assumed, but it also exerts anti-inflammatory effects by directly targeting melanocortin receptors present in immune cells and devoid of steroidogenic actions [Citation6].
3. Circadian rhythms of cortisol in rheumatoid arthritis
One of the most recent significant achievement for the optimization and “survival” of long-term low-dose treatment of RA with GCs seems originating from the validation of their nightly administration, respecting their physiological circadian production as anticipated in 2003 by Cutolo et al. [Citation8].
As matter of fact, serum cortisol follows a circadian oscillation, with maximum levels in the morning and lowest levels at midnight. This also seems to be a normal consequence of the cytokines rise (e.g. TNF and IL-6) during the night, that stimulate the progressive raising production of cortisol around 3 am. Therefore, cortisol reaches its serum peak around 5 am and then slowly decreases during the day; however, its night production in chronic inflammatory conditions like RA it is low/insufficient, as discussed, and as consequence the natural night anti-inflammatory GCs function is dramatically reduced with the later appearance of morning symptoms.
For these reasons, in the presence of increased night cytokine production like observed in RA, it is much more optimized to make available the exogenous replacement GCs doses around 3–4 am, then at breakfast as usually done around 7–9 am ().
The respect of circadian rhythms of the rise of the inflammatory reaction in the night and the administration of low doses of GCs at the same nighttime, according to a most physiological anti-inflammatory actions, are from a decade a possible option due to the availability of a modified-release prednisone formulation ().
The circadian GCs formulation is assumed at 11 pm and the prednisone is released then at 3 am in the stomach/gut, mimicking in such a manner the physiological endogenous GCs action against pro-inflammatory cytokines () [Citation9].
Two randomized clinical trials demonstrated the efficacy of this formulation in the management of signs and symptoms of RA, in particular, on morning stiffness, with the presence of superior biological effects versus morning immediate release prednisone [Citation10,Citation11]. Modified-release prednisone was found also effective in the management of polymyalgia rheumatica and seems a promising formulation to better control the inflammatory process in newly diagnosed patients with giant cell arteritis [Citation12,Citation13].
Of note, the 2017 Nobel Prize in Physiology or Medicine was jointly awarded to three scientists for their recent discoveries of molecular mechanisms controlling the cell circadian rhythms [Citation14].
4. Targets of action of glucocorticoids in rheumatoid arthritis
GCs show pleiotropic functions, with a wide range of genomic and non-genomic effects, whose in-depth discussion go beyond our aim. For summary purposes, high amounts of GCs exert fast and non-genomic effects, while low doses of GCs exert genomic effects a bit slower, by regulating simultaneously the transcription of thousands of genes.
The latter is the case of RA, where the immunomodulatory and immunosuppressive effects of GCs are required to down-regulate the complex activation of pro-inflammatory cells. Indeed, GCs decrease activation of monocytes-macrophages, T cells, with a less pronounced perturbation of B cells, as well as eosinophils, basophils, circulating adhesion molecules, and prostaglandins [Citation15].
Interestingly, during the course of RA, GCs seem able to polarize active proinflammatory macrophages (M1) toward an alternatively activated phenotype (M2), with anti-inflammatory effects [Citation16,Citation17]. The end result is an effective and lasting downregulation of the pathophysiological processes in RA.
5. Oral low dose and long-term glucocorticoids in rheumatoid arthritis
Various routes of administrations of GCs are available (intrarticular, intramuscular, intravenous, oral and others). Intramuscular and intravenous formulations allow rheumatologists to administer high dosage of GCs, but this is rarely useful in course of RA. In fact as discussed above, in chronic therapy of RA, physicians research mainly genomic effects of GCs together with a safer management, and this is obtained as mentioned with oral low-dose therapy [Citation15].
In fact, the dosage of 5 mg/day of prednisolone was found in a multicenter, double-blind, placebo-controlled trial to retard progression of radiographic RA joint damage after two-years of treatment: on the contrary, high dosages, showed a significant risk of five-year radiographic damage progression in another large investigation [Citation18,Citation19].
Today, there is clear evidence that RA treatment regimens including low-dose GCs given early in RA slow radiographic progression, meeting the definition of a real disease-modifying antirheumatic drug (DMARD). In addition, the evidence suggests that the combination of GCs with other DMARDs, favorably alter the disease course even after their discontinuation [Citation20].
Moreover, a European League Against Rheumatism (EULAR) group of experts provided reassurance/evidence on the low-risk profile for side effects with a long-term dosage ≤5 mg prednisone equivalent per day, advising physicians to take also into account and to try to improve the whole lifestyle of the RA patients even in order to prevent the complications GCs-related [Citation21].
In fact, despite the well-proven efficacy of GCs, both rheumatologists and patients are still concerned about the side effects of these drugs (mainly insulin-resistance, arterial hypertension, infections, cataract, frailty fractures, skin atrophy, behavior disorders) [Citation22]. However, the reported high incidence of complications is often extrapolated from inhomogeneous cohorts of patients, treated with variable high dosages of GCs during the course of the disease and without trying to optimize the administration with the respect of the correct timing.
To avoid all these biases of evaluation the Glucocorticoid Low-dose Outcome in RA project (GLORIA), that is a randomized, double-blind, clinical trial supported by the EU Agency (HORIZON 2020), is ongoing to accurately evaluate the safety and the effectiveness of low dose GCs (5 mg per day of prednisolone versus placebo) in elderly RA patients over 65 years. The results of this study are expected in the coming months [Citation23].
6. Glucocorticoids are more than a bridge
Current American and European guidelines for the treatment of RA still indicate only a temporary utility for GCs. The 2015 American College of Rheumatology (ACR) Guideline for the treatment of RA considers using of low-dose GCs (however identified in the text with a medium-dose of ≤10 mg/day of prednisone or equivalent) in patients that start conventional synthetic DMARDs (csDMARDs) or in patients with a disease flare. The shortest use time is also recommended.
Similarly, the 2019 updated EULAR recommendations for the management of RA confirm the already long-time suggested use of low-dose GCs as first line drug, but only for a short-term period (about 3 months), tailoring them as a ‘bridging therapy’ until concomitant csDMARDs show their clinical effects.
As previously discussed, these recommendations reflect the concerns deriving from an incorrect use of GCs (e.g. high dosages for long term, incorrect timing of administration, too potent GCs) that risks penalizing RA patients by depriving them of an effective therapy. Moreover, a recent double-blind, randomized controlled trial on RA patients receiving a biological DMARD together with prednisone 5 mg per day for 24 weeks has confirmed that a too quick tapering of GCs is associated with a worse control of the disease and appearance of more flares [Citation24].
7. Conclusions and future perspectives
New first-in-class drugs might be discovered/synthesized in the next future to treat the immune/inflammatory reaction in RA, but the repurpose and/or further optimization of old drug(s) also offers promise of safety and efficacy.
In fact, for all the aforementioned reasons, GCs, especially low-doses (under 5 mg prednisone/day) plays an essential role in the management of RA and their quick abandonment is inadvisable and counterproductive.
Low-dose long-term and possibly night release GCs, could delay/reduce the use of biological DMARDs in RA.
Data results of GLORIA trial will report the real side effects of exogenous GCs low-dose over long time in a large population of elderly RA patients. However, an international survey within the GLORIA study, already showed that RA patients with self-reported exposure to GCs express high levels of satisfaction with low-dose GCs efficacy, as do rheumatologists [Citation22].
A fascinating new field of research could be the impact of chronic GCs therapy also on gut microbiota of RA patients, that is recognized as one of the main drivers in the immune-inflammatory response. Dedicated studies are desirable in the coming years [Citation25].
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership, or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
M. Cutolo, S. Paolino, and E. Gotelli are members of the EULAR Study Group on Neuroendocrine Immunology of Rheumatic Diseases (NEIRD).
Additional information
Funding
References
- Hench PS, Kendall EC, Slocumb CH, et al. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch Intern Med (Chic). 1950;85:545–666.
- Palmowski Y, Buttgereit T, Buttgereit F. The 70th anniversary of glucocorticoids in rheumatic diseases: the second youth of an old friend. Rheumatology (Oxford). 2019;58:580–587.
- Straub RH, Cutolo M. Glucocorticoids and chronic inflammation. Rheumatology (Oxford). 2016;55:ii6–ii14.
- Schmidt M, Weidler C, Naumann H, et al. Reduced capacity for the reactivation of glucocorticoids in rheumatoid arthritis synovial cells: possible role of the sympathetic nervous system? Arthritis Rheum. 2005;52(6):1711–1720.
- Fenton C, Martin C, Jones R, et al. Local steroid activation is a critical mediator of the anti-inflammatory actions of therapeutic glucocorticoids. Ann Rheum Dis. 2021;80:250–260.
- Hayes K, Panaccio MP, Goel N, et al. Patient characteristics and indicators of treatment initiation with repository corticotropin injection in patients with rheumatoid arthritis: a claims database analysis. Rheumatol Ther. 2021 Jan 5. Epub ahead of print. DOI:10.1007/s40744-020-00272-x.
- Savage O, Davis PS, Chapman L, et al. Corticotrophin (ACTH) in rheumatoid arthritis. Ann Rheum Dis. 1959;18:100–110.
- Cutolo M, Seriolo B, Craviotto C, et al. Circadian rhythms in RA. Ann Rheum Dis. 2003;62:593–596.
- Cutolo M. Circadian rhythms and rheumatoid arthritis. Joint Bone Spine. 2019 May;86:327–333.
- Buttgereit F, Doering G, Schaeffler A, et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet. 2008;371:205–214.
- Buttgereit F, Mehta D, Kirwan J, et al. Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2). Ann Rheum Dis. 2013;72:204–210.
- Cutolo M, Hopp M, Liebscher S, et al. Modified-release prednisone for polymyalgia rheumatica: a multicentre, randomised, active-controlled, double-blind, parallel-group study. RMD Open. 2017;3:e000426.
- Miler E, Stapleton PP, Mapplebeck S, et al. Circulating interleukin-6 as a biomarker in a randomized controlled trial of modified-release prednisone vs immediate-release prednisolone, in newly diagnosed patients with giant cell arteritis. Int J Rheum Dis. 2019;22:1900–1904.
- Lorenz LJ, Hall JC, Rosbash M. Expression of a drosophila mRNA is under circadian clock control during pupation. Development. 1989;107:869–880.
- Berardicurti O, Ruscitti P, Pavlych V, et al. Glucocorticoids in rheumatoid arthritis: the silent companion in the therapeutic strategy. Expert Rev Clin Pharmacol. 2020;13:593–604.
- Koenen M, Culemann S, Vettorazzi S, et al. Glucocorticoid receptor in stromal cells is essential for glucocorticoid-mediated suppression of inflammation in arthritis. Ann Rheum Dis. 2018;77:1610–1618.
- Tardito S, Martinelli G, Soldano S, et al. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev. 2019;18:102397.
- Wassenberg S, Rau R, Steinfeld P, et al. Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:3371–3380.
- Louveau B, De Rycke Y, Lafourcade A, et al. Effect of cumulative exposure to corticosteroid and DMARD on radiographic progression in rheumatoid arthritis: results from the ESPOIR cohort. Rheumatology (Oxford). 2018;57:1563–1573.
- Bijlsma JW. Disease control with glucocorticoid therapy in rheumatoid arthritis. Rheumatology (Oxford). 2012;51(Suppl 4):iv9–13.
- Strehl C, Bijlsma JW, De Wit M, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis. 2016;75:952–957.
- Santiago T, Voshaar M, De Wit M, et al. Patients’ and rheumatologists’ perspectives on the efficacy and safety of low-dose glucocorticoids in rheumatoid arthritis-an international survey within the GLORIA study. Rheumatology (Oxford). 2021;keaa785. Epub ahead of print. doi:10.1093/rheumatology/keaa785
- Hartman L, Rasch LA, Klausch T, et al. Harm, benefit and costs associated with low-dose glucocorticoids added to the treatment strategies for rheumatoid arthritis in elderly patients (GLORIA trial): study protocol for a randomised controlled trial. Trials. 2018;19:67.
- Burmester GR, Buttgereit F, Bernasconi C, et al. Continuing versus tapering glucocorticoids after achievement of low disease activity or remission in rheumatoid arthritis (SEMIRA): a double-blind, multicentre, randomised controlled trial. Lancet. 2020;396:267–276.
- Paolino S, Pacini G, Patanè M, et al. Interactions between microbiota, diet/nutrients and immune/inflammatory response in rheumatic diseases: focus on rheumatoid arthritis. Reumatologia. 2019;57:151–157.
