1. Introduction
Coronavirus disease-19 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has a wide clinical spectrum with variable severity [Citation1–3]. Whilst most symptomatic patients experience mild manifestations, hospitalised individuals often develop a pronounced hyperinflammatory state, leading to acute respiratory distress syndrome with severe hypoxemia, multi-organ dysfunction, and death [Citation2,Citation3].
Repurposing approved drugs has been an effective therapeutic approach in the management of COVID-19 and treatment regimens continue to evolve [Citation2,Citation3]. Early in the disease course, treatments target viral replication and include antivirals (remdesivir) and neutralising monoclonal antibodies (sotrovimab, or casirivimab with imedevimab) [Citation4]. Immunomodulatory drugs target the later, hyperinflammatory phase of infection and include dexamethasone and interleukin (IL)-6 inhibitors (tocilizumab or sarilimab) [Citation4]. Whilst immunomodulatory medications are effective in suppressing pulmonary inflammation, they carry a risk for delayed pathogen clearance and an increased risk of secondary opportunistic infections [Citation1].
Baricitinib is an oral targeted synthetic Janus-kinase (JAK)1/JAK2 inhibitor, approved for the treatment of active rheumatoid arthritis (RA) [Citation2,Citation3]. Compared with other JAK inhibitors (JAKi), baricitinib may exert direct anti-viral effects [Citation2,Citation3,Citation5,Citation6]. This was suggested in February 2020 using artificial intelligence (AI) algorithms (BenevolentAI) that examined a large repository of structured medical and drug data [Citation7]. Baricitinib therefore gained interest as a potential therapeutic option for COVID-19, based on its known anti-cytokine effects and possible anti-viral mechanisms [Citation8].
2. Mechanism of action of baricitinib in COVID-19
The SARS-CoV-2 pro-inflammatory cytokine storm is understood to involve signal transduction pathways mediated by JAK and signal transducer and activator of transcription (STAT) [Citation3]. Cytokine-mediated signalling is initiated upon binding their extracellular cognate receptors, inducing a conformational change resulting in activation (by phosphorylation) and recruitment of their associated JAKs [Citation3]. There are four JAK isoforms (JAK1, JAK2, JAK3, and tyrosine kinase 2) [Citation3]. JAK activates and phosphorylates intracellular tyrosine residues on the cytokine receptors, which act as binding sites for STATs [Citation3]. Activated JAKs phosphorylate STATs, which thereon dissociate from their receptors and dimerise to form phosphorylated STAT-STAT [Citation3]. This dimer translocates to the cell nucleus to bind specific DNA regions as a transcription factor, thereby modulating gene expression [Citation3]. JAKi target these pathways and are approved for inflammatory-mediated pathologies [Citation3]. Baricitinib selectively (and reversibly) inhibits JAK1/JAK2, interrupting IL-2, IL-6, IL-10, interferon (IFN)-gamma and granulocyte-macrophage colony-stimulating factor, which are elevated in COVID-19 inflammatory states [Citation3].
Baricitinib is thought to interfere with viral cellular entry. In COVID-19, viral endocytosis is initiated when SARS-CoV-2 binds its spike protein to angiotensin-converting-enzyme 2 (ACE2) receptors expressed on type two pneumocytes (AT2 cells), which are particularly vulnerable to SARS-CoV-2 infection [Citation7]. AI algorithms suggest that baricitinib inhibits adaptor-related protein-2-associated protein kinase-1 (AAK1) and, to a lesser extent, cyclin-G-associated kinase enzymes (GAK) in AT2 cells [Citation5,Citation7,Citation9]. Both of these kinases are members of the numb-associated kinases groups and are known regulators of clathrin-mediated viral endocytosis [Citation5,Citation7,Citation9]. Based on this mechanism, baricitinib is thought to interfere with the intracellular entry and assembly of SARS-CoV-2 () [Citation6,Citation7]. This is supported by in vitro evidence where baricitinib has been shown to inhibit the entry of SARS-CoV-2 into spheroid liver organelles [Citation9].
Figure 1. A summary of the proposed anti-inflammatory and anti-viral actions of baricitinib in the treatment of COVID-19.
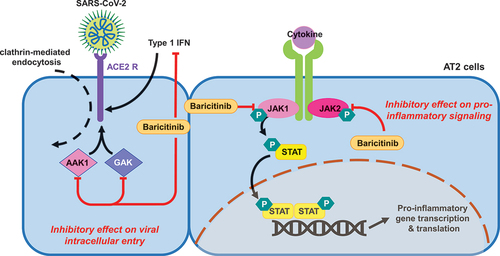
It is also thought that baricitinib offers an inhibitory effect on IFN signalling [Citation6]. This has been demonstrated experimentally using in vitro models [Citation9,Citation10]. As type one (and two) IFNs upregulate ACE2 expression in respiratory epithelia, suppressing the IFN response may reduce ACE2 expression thereby impeding ACE2-mediated SARS-CoV-2 endocytosis [Citation11]. This is supported by work in organotypic liver spheroids where baricitinib has been shown to inhibit IFN-mediated ACE2 induction [Citation9]. Contrastingly, this finding was not replicated in models of lung tissue suggesting that the effects of baricitinib may depend on the organ system [Citation9]. The effect of ACE2 suppression is further complicated by the protective effects of ACE2 against renin-angiotensin-aldosterone-system related organ injury in acute illness [Citation12]. Following internalisation, SARS-CoV-2 downregulates ACE2, therefore precluding ACE2 protective effects [Citation12]. Baricitinib’s type one IFN suppression may exacerbate ACE2 downregulation, further impeding its protective effects. In summary, the effect of baricitinib on net IFN suppression and SARS-CoV-2 cellular entry is multifactorial and therefore, the overall effect is likely to depend on patients’ immune status and phase of infection [Citation11].
3. Clinical evidence for the use of baricitinib in COVID-19
The efficacy and safety of JAK1/JAK2 inhibition in the management of COVID-19 has been investigated in both observational and randomised control trials (RCTs). The three largest RCTs to date include the ACTT-2 trial, the COV-BARRIER study and the RECOVERY trial [Citation8,Citation13–15]. All RCTs assessing baricitinib for the treatment of COVID-19 to-date are summarized in .
Table 1. A summary of the RCTs investigating the efficacy of baricitinib in the management of adult inpatients with COVID-19. The table outlines differences in size, recruitment period, standard of care (SOC) therapies, primary outcomes, and AEs between trials. Note that RCTs investigating other JAK inhibitors were excluded from this summary
The ACTT-2 trial (NCT04401579) investigated the efficacy of baricitinib therapy alongside remdesivir [Citation13]. It followed the ACTT-1 study where remdesivir was shown to shorten recovery time in hospitalised adults with COVID-19 [Citation16]. In the ACTT-2 trial, 1033 hospitalized patients treated with remdesivir were randomized to receive baricitinib or placebo. In the treatment arm, participants received 2 or 4 mg baricitinib daily with the dose dependent on renal function. Baricitinib was administered for 14 days or until hospital discharge. Participants randomised to receive baricitinib recovered more quickly than those receiving placebo (median 7 days versus 8 days, p = 0.03). Baricitinib was found to be most efficacious in reducing the time to recovery in patients with severe disease and in patients who required noninvasive ventilation or high flow oxygen, the median time to recovery was 10 days versus 18 days in the placebo group (confidence interval (CI) 1.10 to 2.08). Furthermore, participants in the baricitinib arm were more likely to show a clinical improvement at day 15 (odds ratio for improvement 1.3; 95% CI 1.0–1.6). A significant benefit of baricitinib therapy was demonstrated on the incidence of new oxygen requirement (−17.4%; 95%CI −31.6 to −2.1) and invasive ventilation or extracorporeal membrane oxygenation (ECMO) (−5.2%; 95%CI −9.5 to −0.9). Baricitinib-treated participants experienced significantly fewer adverse events (AEs) compared to controls (16% versus 21%; p = 0.03). Importantly, the baricitinib group experienced fewer infection-related AEs than controls and a similar number of thromboembolic events (1% difference, 95% CI −1.3 to 3.3) [Citation13].
The COV-BARRIER trial (NCT04421027) evaluated the efficacy and safety of baricitinib in combination therapy with standard treatment for hospitalised adults with COVID-19 [Citation8]. It randomized 764 participants to the baricitinib group and 761 to the placebo group [Citation8]. During the course of the trial, the standard of care for COVID-19 management changed significantly following the results from the landmark RECOVERY trial published in February 2021 [Citation17]. This study demonstrated an 11% relative reduction in the mortality of hospitalized COVID-19 patients with corticosteroid therapy and dexamethasone was thereon incorporated into the routine inpatient management of COVID-19. All participants enrolled into the COV-BARRIER trial received standard of care therapy including antivirals (18.9%) and systemic corticosteroids in the majority (79.3%). Although the study did not meet its primary endpoint and there was no significant difference in disease progression overall, participants treated with baricitinib demonstrated a significantly reduced 28-day mortality 10% (n = 79) versus 15% (n = 116) (hazard ratio 0 · 62 [95% CI 0 · 47–0 · 83]; p = 0 · 0050).
Most recently, the RECOVERY group published the largest RCT of baricitinib in the management of COVID-19 to-date (NCT04381936) [Citation14]. In this trial, 8156 patients were randomized to receive baricitinib or placebo. Baricitinib was administered at a 4 mg daily oral dose for 10 days or until hospital discharge. All participants received standard of care therapy; 95% of participants received corticosteroids and 23% received the IL-6 inhibitor tocilizumab. The study reported a 13% proportional reduction in 28-day mortality in participants receiving baricitinib (RR 0.87 (0.77–0.98), p = 0.026) [Citation14]. Furthermore, participants on baricitinib were less likely to progress to invasive mechanical ventilation (RR 0.87 (0.74–1.01), p = 0.06) and were more likely to be discharged at day 28 (RR 1.10 (1.04–1.15), p < 0.001). The benefits were similar in all subgroups, including those receiving corticosteroids or IL-6 inhibitors, demonstrating that baricitinib can be safely given alongside other immunomodulatory therapies [Citation14].
The RECOVERY group published a meta-analysis of all RCT’s investigating the use of JAK-inhibitors in hospitalised patients with COVID-19 [Citation14]. Eight previous trials involving a total of 3732 participants were included in the analysis. In these 8 trials, randomisation to treatment with a JAK inhibitor was associated with a 43% reduction in mortality rate (RR 0.57; 95% CI 0.45–0.72). This was significantly greater than the survival benefit reported by the RECOVERY trial and when this RCT was included in the meta-analysis, only a 20% proportional reduction in mortality was reported (RR 0.8; 95% CI 0.71–0.89; p < 0.001) [Citation14].
4. Limitations of RCT data
Although significant evidence now supports the use of baricitinib in the management of severe COVID-19, data must be interpreted with caution and there remain areas of uncertainties. Firstly, the magnitude of mortality risk reduction differs markedly between different RCTs [Citation14]. This is partly due to the large degree of between-study heterogeneity and differences in study population, treatment protocols, and standard of care therapies as summarised in . Given these limitations, it remains unclear as to which patient subgroup would benefit most from baricitinib therapy and which patients should be treated with multiple immunomodulatory therapies.
5. Adverse effects of baricitinib
The AEs of baricitinib have been well described in patients with RA. In these cohorts, the most significant reported AEs include infection, venous thromboembolism (VTE), major adverse cardiovascular events (MACE) and malignancy [Citation18]. Infection is the most commonly reported treatment emergent AE and baricitinib is associated with the reactivation of latent infections including herpes simplex, tuberculosis, hepatitis B, Epstein-Barr virus and herpes-zoster virus [Citation19,Citation20]. The most commonly reported serious infections include pneumonia, herpes zoster reactivation, urinary tract infection, and cellulitis [Citation18]. Long term safety data in RA patients has shown that baricitinib is associated with a small but significant risk for VTE with an estimated 5 cases of thrombosis per 1000 patient years [Citation19]. This was a particular concern in COVID-19 patients who are at increased risk for VTE due to a hypercoagulable disease state. Other side effects include hypersensitivity reactions, liver toxicity, gastrointestinal side effect and cardiovascular effects [Citation19]. Despite initial concerns regarding AEs in COVID-19 cohorts, there has been no association between baricitinib therapy and serious AEs in clinical data-to-date [Citation8,Citation13,Citation14].
6. Summary
In conclusion, baricinitib is an immunomodulatory medication that has been repurposed for the treatment of COVID-19. There have been four RCTs supporting the efficacy and safety of baricitinib in the management of hospitalised adult COVID-19 patients. On the basis of this evidence, both the World Health Organization and the FDA published guidance in early 2022 recommending the use of baricitinib for patients with severe COVID-19 [Citation21,Citation22]. In March 2022, the results from the RECOVERY trial confirmed a significant mortality benefit of baricitinib in those with severe COVID-19. Significantly, this study showed that baricitinib could be safely given alongside alternative immunomodulatory therapies including IL-6 inhibitors [Citation14]. Overall, this evidence suggests that baricitinib is a safe and effective treatment for severe COVID-19.
Although the evidence for baricitinib in COVID-19 is encouraging, there remains unanswered research questions. In children, the role for JAK-inhibition in COVID-19 is not defined. Whilst pediatric cases are generally mild, a small subset develop Pediatric Inflammatory Multisystem Syndrome temporally associated with COVID-19 (PIMS-TS). PIMS-TS is a life-threatening condition that is characterized by significant hyperinflammation and cytokine storm [Citation23]. Treatment of PIMS-TS involves immunomodulation including corticosteroids, intravenous immunoglobulin (IVIg) and in some cases anti-cytokine agents [Citation24]. The RECOVERY trial protocol is investigating the use of different immunomodulatory treatments for the management of PIMS-TS and further work is needed to ascertain whether JAK-inhibition plays a role in this condition [Citation25].
Uncertainties in the management of adults with COVID-19 remain. For instance, it is not known which immunomodulatory therapy is most efficacious and there have been no head-to-head studies comparing baricitinib to IL-6 inhibitors or cheaper alternatives such as IVIg. Furthermore, it is unclear which group of patients is most likely to benefit from baricitinib therapy. In the context of increasing therapeutic options for COVID-19, future work is needed to characterise the optimal management regimes for individual patients.
Declaration of interest
N Sofat was an investigator for the COV-BARRIER trial sponsored by Eli Lilly. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, et al. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatol (United Kingdom). 2021. DOI:https://doi.org/10.1093/rheumatology/keaa587.
- Jorgensen SCJ, Tse CLY, Burry L, et al. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020. DOI:https://doi.org/10.1002/phar.2438
- Akbarzadeh-Khiavi M, Torabi M, Rahbarnia L, et al. Baricitinib combination therapy: a narrative review of repurposed Janus kinase inhibitor against severe SARS-CoV-2 infection. Infection. 2021;1–14. DOI:https://doi.org/10.1007/s15010-021-01730-6
- NICE. Rapid policy statement interim clinical commissioning policy: neutralising monoclonal antibodies and intravenous antivirals in the treatment of COVID-19 in hospitalised patients commissioning position. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2021/09/C1529-interim-clinical-comm-policy-neutralising-monoclonal-antibodies-and-intravenous-antivirals.pdf (2021).
- Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402.
- Spinelli FR, Conti F, Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci Immunol. 2020;5(47). DOI:https://doi.org/10.1126/sciimmunol.abc5367
- Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31.
- Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407–1418.
- Stebbing J, Sánchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7(1). DOI:https://doi.org/10.1126/sciadv.abe4724
- Petrone L, Petruccioli E, Alonzi T, et al. In-vitro evaluation of the immunomodulatory effects of Baricitinib: implication for COVID-19 therapy. J Infect. 2021;82(4):58–66.
- Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e19.
- Vaduganathan M, Vardeny O, Michel T, et al. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659.
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807.
- Horby PW, Emberson JR, Mafham M, et al. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analy-sis. medRxiv. [PREPRINT]. 2022. DOI:https://doi.org/10.1101/2022.03.02.22271623.
- Ely EW, Ramanan AV, de Bono S, et al. Baricitinib plus standard of care for hospitalised adults with COVID-19 on invasive mechanical ventilation or Extracorporeal membrane oxygenation: results of a randomised, placebo-controlled trial. medRxiv. 2021;2021(10.11.21263897). DOI:https://doi.org/10.1101/2021.10.11.21263897
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383(19):1813–1826.
- RECOVERY collaborative group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021. DOI:https://doi.org/10.1056/nejmoa2021436.
- Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis. 2022;81(3):335–343.
- Peng L, Xiao K, Ottaviani S, et al. A real-world disproportionality analysis of FDA adverse event reporting system (FAERS) events for baricitinib. Expert Opin Drug Saf. 2020;19(11):1505–1511.
- Smolen JS, Genovese MC, Takeuchi T, et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol. 2019;46(1):7–18.
- Kmietowicz Z. Covid-19: WHO recommends baricitinib and sotrovimab to treat patients. BMJ. 2022;376:376:o97.
- FDA. FDA emergency use authorisation for Baricitinib in the treatment of COVID-19.
- Buonsenso D, Riitano F, Valentini P. Pediatric inflammatory multisystem syndrome temporally related with SARS-CoV-2: immunological similarities with acute rheumatic fever and toxic shock syndrome. Front Pediatr. 2020;8. DOI:https://doi.org/10.3389/fped.2020.00574.
- Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory Syndrome in children — initial therapy and outcomes. N Engl J Med. 2021;385(1):23–34.
- Fleming PF, Gale C, Molloy EJ, et al. Paediatric research in the times of COVID-19. Pediatr Res. 2021;90(2):267–271.
