ABSTRACT
Introduction
We summarize evidence for the role of therapeutic plasma exchange (TPE) in the treatment of anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV). TPE rapidly removes ANCA IgG, complement and coagulation factors important in the pathogenesis of AAV. TPE has been used in patients with rapidly deteriorating renal function to achieve early disease control, allowing time for immunosuppressive agents to prevent resynthesis of ANCA. The PEXIVAS trial challenged the utility of TPE in AAV, as it did not show benefit of adjunctive TPE on a combined end point of end stage kidney disease (ESKD) and death.
Areas covered
We analyze data from PEXIVAS and other trials of TPE in AAV, an up-to-date meta-analysis, and recently published large cohort studies.
Expert opinion
There remains a role for the use of TPE in AAV in certain groups of patients, in particular those with severe renal involvement (Cr >500 μmol/L or dialysis-dependent). It should be considered in patients with Cr >300 μmol/L and rapidly deteriorating function, or with life-threatening pulmonary hemorrhage. A separate indication is patients double positive for anti-GBM antibodies and ANCA. TPE may have the greatest benefit as part of steroid-sparing immunosuppressive treatment strategies.
1. Introduction to ANCA-associated vasculitis
Anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV) is an autoimmune condition characterized by pauci-immune inflammation of small blood vessels, with endothelial injury and tissue damage [Citation1]. It is thought that ANCA are directly pathogenic, and they are directed against one of two major targets: proteinase 3 (PR3) and myeloperoxidase (MPO), which are both enzymes present in neutrophil granules [Citation2]. Historically, AAV is classified according to clinical phenotypes of granulomatosis with polyangiitis (GPA, predominantly associated with PR3-ANCA), microscopic polyangiitis (MPA, predominantly associated with MPO-ANCA), and eosinophilic granulomatosis with polyangiitis (EGPA) [Citation3]. However, the clinical presentation and pattern of vessel involvement exhibit considerable overlap between these syndromes and they are often grouped together as ANCA-associated vasculitis [Citation4].
1.1. Epidemiology of AAV
ANCA-associated vasculitis is a rare disease with an incidence estimated to be between 13 and 20 per million per year in Europe, and a prevalence of 300 patients per million worldwide [Citation5,Citation6]. Of these patients, between 70% and 90% exhibit kidney involvement [Citation7]. Severity of kidney disease varies widely between individual cases, and ranges from asymptomatic abnormalities on urinalysis to end stage kidney disease (ESKD). Early detection of urinary abnormalities, despite normal blood tests, may lead to diagnosis of significant renal involvement via kidney biopsy, allowing prompt treatment and improved prognosis [Citation8].
Despite the rarity of AAV, it represents one of the most frequent causes of rapid progressive glomerulonephritis (RPGN). RPGN is a clinical description of the rapid loss of renal function over a short period (days to weeks), corresponding to the development of crescentic glomerulonephritis. Patients who present with advanced kidney injury induced by AAV have a poor prognosis, with only 50% of patients surviving to 1 year with independent kidney function in historic cohorts [Citation9–11]. There have been improvements in patient survival in more modern cohorts, attributable to advances in both treatment regimens and supportive care, as reported by Hilhorst et al. [Citation12]. This 30-year longitudinal study showed that even in patients with a Cr >500 μmol/L, the 1-year patient survival improved from 61.5% in patients treated between 1979 and 1989 to 92.3% in those treated between 2001 and 2009. Even in contemporary cohorts, the overall mortality remains high, with a standardized mortality ratio (SMR) of 2.3 [Citation13]. Long-term renal outcomes remain poor in patients presenting with life-threatening manifestations of AAV, with Gulati et al. reporting ESKD-free survival at 36 months improving from 52.5% in those treated between 2006 and 2009 to 65.5% in those treated with a different immunosuppressive regimen between 2010 and 2020 [Citation14]. This highlights the ongoing need for improvements in our management strategies to reduce the burden of disease in these patients.
Clinically, AAV may present with nonspecific symptoms such as weight loss, fatigue, arthralgia, and myalgia. This may delay diagnosis and patients have often presented to multiple clinicians before being identified [Citation15]. The key manifestations of AAV relate to the development of small vessel vasculitis lesions, which may affect any organ, with a high degree of inter-individual variability [Citation16]. The focus of this article is on those patients presenting with the most life- and organ – threatening manifestations. These include pulmonary hemorrhage and severe acute kidney injury due to underlying crescentic glomerulonephritis.
1.2. Pathogenesis of AAV
The key pathway in the development of AAV is thought to be the loss of immunological T cell and B cell tolerance, leading to development of autoantibodies against neutrophils in genetically susceptible individuals [Citation1]. These antibodies activate neutrophils that have been primed by infection or inflammation, leading to endothelial and tissue injury via neutrophil degranulation and neutrophil extracellular trap (NET) formation [Citation1Citation17–24]. This is potentiated by complement activation largely via the alternative pathway [Citation25,Citation26]. In addition, auto-antigens in the NETs may be presented to auto-reactive effector T-cells by local professional antigen presenting cells, leading to a self-reinforcing cycle of autoimmunity, inflammation, endothelial damage, and eventual tissue fibrosis [Citation22,Citation27–29].
Multiple clinical studies support the idea that ANCA are the critical pathogenic mediators of AAV, and have shown that ANCA titers often correlate with disease activity, especially in those with kidney involvement [Citation30]. There have also been case reports of maternal fetal transfer of anti-MPO antibodies, leading to vasculitis with renal-pulmonary syndrome in the neonate [Citation31]. In addition, there is evidence that ANCA are predictive of relapse in patients with renal involvement, and a rising titer has been shown to be a sensitive marker of relapse [Citation32,Citation33]. However, the relationship between ANCA and active vasculitis is complex, and ANCA are not necessarily always pathogenic [Citation31,Citation34]. For example, ANCA can persist despite clinical remission, recur without evidence of clinical relapse, especially in patients with non-renal disease, and have been identified at low levels in healthy individuals [Citation35–37]. This latter finding may be due to differences in avidity, titer, and IgG subclass of pathogenic ANCA compared to those found in the normal population. Olson et al. compared longitudinal serum samples from 27 patients prior to their AAV diagnosis to 27 matched controls, and found that the majority of patients with AAV had elevated PR3-ANCA titers prior to diagnosis (63%), and even prior to subclinical inflammation as indicated by a rise in CRP [Citation38]. They also found that AAV patients had higher levels of PR3-ANCA even within the normal range compared to controls, and that these levels increased over time, while control PR3-ANCA titers remained static. This likely indicates that further triggers are required following the development of autoantibodies to induce inflammation. Thus, the pathogenicity of ANCA is widely accepted and underpins the use of TPE to lower the levels [Citation26,Citation39–46]. However, it seems clear that although ANCA are likely to be pathogenic, a second insult is required to trigger clinical disease [Citation38–40,Citation47].
The underlying etiology of AAV is poorly understood; however, both genetic and environmental factors have been implicated. Genome-wide association studies have linked the presence of circulating PR3-ANCA with polymorphisms in HLA-DP, PRTN3 (which encodes proteinase 3) and SERPINA1 (which encodes α1-antitrypsin, a circulating inhibitor of PR3); similarly, the presence of MPO-ANCA has been associated with variations in HLA-DQ [Citation48,Citation49]. With respect to environmental precipitants, infection has been reported to trigger induction of AAV, and precipitate relapse [Citation6,Citation50,Citation51]. Infectious agents may promote loss of tolerance by inducing autoantigen exposure through formation of NETs, or inducing autoimmunity through molecular mimicry. In addition, they can directly prime neutrophils for ANCA-induced activation [Citation52,Citation53]. Certain drugs, such as propylthiouracil, and exposure to silica have also been implicated in development of AAV [Citation54–58].
2. Treatment strategies in AAV
Currently, patients with severe AAV are treated with a combination of high-dose glucocorticoids, cytotoxic agents, and targeted anti-B-cell therapies to induce remission, followed by sustained low-dose immunosuppressive treatment to prevent relapse. These strategies have dramatically improved outcomes for patients with AAV; however, excess morbidity and mortality remain high [Citation59–62]. This is likely due to both disease factors and treatment-related toxicities, the latter of which contributes to the significant risk of serious infections in this patient population [Citation63,Citation64]. There is evidence that glucocorticoids cause significant harm, with increasing cumulative dosing leading to worsening outcomes [Citation65–67]. As such, a major focus of the vasculitis community has been to minimize glucocorticoid use to reduce associated toxicities [Citation67–70]. In patients with severe AAV, therapeutic plasma exchange (TPE) has been used in conjunction with glucocorticoids, cyclophosphamide (CYC) and/or rituximab (RTX) to gain more rapid disease control. The main aim is to reduce the risk of death and irreversible organ damage, for example ESKD, but a further aim is to reduce the doses of immunosuppressive drugs and hence their toxicities.
3. Introduction to therapeutic plasma exchange
Therapeutic plasma exchange (TPE) or plasmapheresis is an extracorporeal blood purification treatment, in which a patient’s plasma is removed from whole blood and replaced with either donor plasma or human albumin solution [Citation71]. TPE removes pathogenic substances, including autoantibodies, immune complexes, and lipoproteins [Citation71]. It was first described in 1914 in acute kidney failure in dogs, where whole blood was manually removed and replaced with red blood cells suspended in colloid solution [Citation72]. TPE was first utilized in humans in the 1950s for the treatment of hematological conditions such as Waldenström’s macroglobulinaemia, as TPE was found to be effective in removing paraproteins; however, the rapid re-accumulation of these abnormal proteins initially prevented its widespread use [Citation73]. Chronic, intermittent TPE was trialed and found to be effective in treating the associated hyperviscosity syndromes. Since then, TPE has become an essential component of treating many antibody-mediated conditions, including hematological, neurological, and renal diseases [Citation74].
In nephrology, it was first utilized in 1975 to treat a patient with pulmonary-renal syndrome due to anti-glomerular basement (anti-GBM) disease [Citation75]. Lockwood et al. went on to publish a case series, which showed great efficacy in early and rapid improvement in renal function and pulmonary hemorrhage [Citation76]. All of the patients had reduction or complete suppression of autoantibody levels; however, it was clear that there was a requirement for ongoing immunosuppression to prevent resynthesis of the pathogenic autoantibodies [Citation76]. This led Lockwood et al. to hypothesis that TPE would also be effective in treating patients presenting with RPGN and vasculitis in the absence of anti-GBM antibodies [Citation77]. The success of this approach will be discussed later. At the time, it was postulated that there was an as yet unidentified immune complex driving the classical histological changes of crescentic glomerulonephritis. In retrospect, these patients probably had AAV with renal involvement.
Studies have since shown that TPE rapidly removes ANCA [Citation78,Citation79]. Therefore, it has been used to try and reduce early organ damage from active disease while waiting for other immunosuppressive agents to suppress inflammation and prevent further production of ANCA. Another mechanism by which TPE may have benefit in AAV is through removal of complement and coagulation factors, which have been implicated in pathogenesis [Citation76,Citation80–84].
3.1. Practical aspects of TPE
Plasma exchange can be performed using two main automated systems: centrifugal and membrane filtration [Citation71]. In the centrifugal system, whole blood is extracted via venous access, and the plasma separated via centrifugation from cellular components [Citation85,Citation86]. The plasma supernatant is removed, with the remaining blood returned to the patient after being mixed with appropriate replacement fluid. The membrane filtration system pumps the patient’s blood through a hollow fiber filter, the membrane of which allows only the passage of plasma, which is subsequently removed, but not cells. The main difference between the two methods is that in membrane-based TPE, only molecules that are less than three million Daltons can be removed, whereas the centrifugal method does not have an upper limit. This implies that membrane-based TPE leads to less effective clearance of larger molecules such as IgM, cryoglobulins, and immune complexes [Citation85,Citation86].
There are specific adverse effects associated with both systems. The membrane system has been implicated in leucocyte and complement activation due to interaction with the artificial membrane itself [Citation87–90]. However, this may be limited to specific types of filters, and it has been suggested that this effect can be reduced if a different filter is utilized [Citation89]. In addition, hemolysis may occur if the transmembrane pressures are too high during plasma filtration [Citation91]. In comparison, the use of centrifugal devices can lead to increased risk of platelet loss. There have been a few trials directly comparing the two widely available systems, and the majority favor the centrifugal system due to a lower risk of clotting events and increased efficiency of plasma removal [Citation92,Citation93]. However, one advantage of the membrane-based automated system, particularly relevant to patients with AAV, is that this system can also be used with dialysis filters to perform TPE together with continuous renal replacement therapy.
Originally, arteriovenous shunts were used for access with associated high infection risk; but these have largely been replaced by strategies utilizing central venous access with a resultant reduction in infection-related adverse events. The centrifugal technique can operate at a lower flow rate, so peripheral intravenous cannulae can be used, which may help to further reduce access-related infection and thrombosis. The most frequently used replacement fluid in TPE employing either technique is 5% human albumin solution (HAS). Fresh frozen plasma (FFP) may be used when the patient has an underlying coagulopathy, active bleeding (e.g. pulmonary hemorrhage in AAV), or within 24 hours peri-procedure (e.g. renal biopsy). Heparin or citrate anti-coagulation may be used as required by the system concerned. TPE has been shown to effectively remove ANCA, with exchanges of 1 to 1.5 plasma volumes being effective at removing up to 70% of IgG in the intravascular compartment [Citation85]. However, redistribution of IgG occurs, meaning that repeated treatment (usually 5 or more) is required for a sustained effect.
3.2. Adverse effects associated with TPE
TPE is an invasive and expensive treatment, requiring special equipment and highly trained staff to ensure safe delivery. However, as most reactions are mild and easily resolved, it is overall felt to be a safe treatment. The most common adverse event is reported to be citrate toxicity from anti-coagulation leading to hypocalcemia, especially in cases where FFP is used as the replacement fluid. There is also an increased bleeding risk due to the removal of normal plasma constituents, including clotting factors, potentially predisposing to coagulopathy. Other adverse effects include symptomatic hypovolaemia, anaphylactoid reactions to FFP, and infection. In the two randomized controlled trials of TPE that reported adverse events, there was no significant difference in adverse events, not including infections, in patients that did or did not receive plasma exchange [Citation94]. The average risk of complications is estimated at <10% [Citation95] with only 0.4% of procedures having severe adverse effects [Citation96]. Reactions are most commonly associated with the use of FFP as the replacement fluid.
In some studies, serious infections are amongst the most common adverse effects of TPE [Citation14,Citation68,Citation97,Citation98]. This has been recently confirmed in a meta-analysis, which showed that TPE increased the risk of serious infection at 12 months with a relative risk of 1.27 (1.08 to 1.49) [Citation94]. However, AAV patients have up to seven times higher risk of serious infection than the general population, and this is persistent even after 8-years of follow-up [Citation98]. Consistent risk factors include increased age and parenchymal lung disease [Citation14]. Furthermore, the patients that have more often been treated with TPE are generally the critically unwell patients, requiring dialysis access and/or endotracheal intubation, which may be compounded by the functional immunoparesis of advanced kidney disease. In addition, patients treated with concomitant TPE are also often treated with high-dose intravenous glucocorticoid therapy, which is associated with more frequent infection, particularly in the elderly and those with organ-threatening disease [Citation66,Citation99].
To address this increased infection risk in a vulnerable patient group, it is important to tailor the concomitant immunosuppression with a reduction in glucocorticoid exposure where possible, and with the routine use of prophylactic antibiotics. A recent post-hoc analysis from the RAVE trial demonstrated that the use of trimethoprim-sulfamethoxazole as prophylaxis against Pneumocystis jirovecii is associated with a reduction in severe infection in patients with AAV, independent of the remission induction regimen used [Citation100]. We also advocate monitoring immunoglobulin levels, with the prophylactic use of intravenous immunoglobulin to address any TPE or immunosuppression-associated hypogammaglobulinemia.
There are other practical considerations for patients being treated with TPE. It is important to ensure that samples for diagnostic testing are taken prior to starting TPE, as the accuracy of serology-based diagnostic tests will be impacted by removal of circulating endogenous IgG. In addition, drugs that are protein-bound are removed by TPE, which may have implications for drug dosing and timing. Specifically, in the context of AAV, the monoclonal IgG antibody rituximab, which is commonly used for induction therapy, is efficiently removed by TPE [Citation101]. As such, RTX should be prescribed at the end of a course of TPE. Despite the detection of lower peak and trough levels of RTX in those undergoing TPE, there is no evidence this impacts the degree of peripheral B cell depletion.
4. Summary of evidence of utility of TPE in patients with AAV
4.1. Early studies of TPE in AAV
Lockwood et al. reported the first cohort study showing a possible benefit of TPE in a series of nine patients with fulminating pauci-immune crescentic nephritis treated with intensive plasma-exchange, corticosteroids, and cytotoxic drugs [Citation77]. Although this report pre-dated discovery of ANCA, the patients had a clinical diagnosis of vasculitis and likely had AAV. This case series showed that five patients rapidly recovered renal function, three had either prevention of further deterioration or a delayed improvement, and only one patient did not recover renal function.
Important to note is that these nine patients were perhaps mistakenly described as having immune-complex glomerulonephritis, due to the presence of circulating ‘complexes’ on C1q-deviation test. However, there was no evidence of anti-GBM antibodies, no positive serology for lupus, no cryoglobulins identified, and there was normal serum complement in all cases. The biopsies otherwise showed pauci-immune glomerulonephritis with no dominant IgA staining, and this was accompanied with typical clinical features of active vasculitis.
There are several other small case series and non-randomized trials of the utility of TPE in patients with RPGN, without evidence of anti-GBM antibodies, but these have shown varying results [Citation29,Citation102]. There are also two small retrospective cohort studies that utilize TPE as a rescue therapy in patients who continue to progress or have persistent, active disease, at 2 to 4 weeks after initial presentation [Citation103,Citation104]. One of the studies showed that five of six patients that were dialysis-dependent at presentation became dialysis-independent by 3 months and that these patients had comparable outcomes to control subjects [Citation104]. However, due to the retrospective reporting of small numbers of patients, it is difficult to interpret whether TPE used in this way was beneficial, and this approach has not been widely adopted. The 2021 KDIGO Glomerular Diseases Guidelines also suggest that TPE can be considered as a treatment strategy for patients with refractory disease [Citation105].
4.2. Randomized controlled trials of TPE in AAV
In 1988, Glockner et al. published a small, randomized study of 26 patients receiving standard immunosuppressive therapy with or without the addition of TPE for patients with crescentic glomerulonephritis without known autoantibodies [Citation106]. These patients had marked improvement in renal function; however, there was no significant advantage of adjunctive TPE compared to immunosuppression alone. In 1992, the Canadian Apheresis group published a randomized controlled trial of immunosuppressive therapy alone vs immunosuppressive therapy with supplementary TPE in 63 patients with a histopathological diagnosis of crescentic glomerulonephritis [Citation107]. This study also found no benefit from the addition of TPE. However, these studies are difficult to interpret, as although some of the cases were probably due to AAV, a proportion could easily be due to other systemic diseases including systemic lupus erythematosus, IgA vasculitis, and cryoglobulinemia.
The first moderately sized randomized controlled trial was reported in 1991 by Pusey et al. [Citation108]. Recruitment into this trial started in 1978, before ANCA were first identified in 1985, but patients all had vasculitis with biopsy proven crescentic glomerulonephritis, without anti-GBM antibodies. Patients were stratified into three groups according to renal function: those that were dialysis-dependent, those with independent kidney function but with Cr >500 µmol/L, and those with Cr <500 µmol/L. This study found a significant benefit from TPE in patients who were initially dialysis dependent (19/49 cases). Of the dialysis-dependent group, 10 of 11 patients that received TPE vs 3 of 8 patients in the control group recovered renal function at 1-month post presentation. Crucially, this benefit was sustained in longer-term follow-up. In another randomized trial reported by Szpirt et al. in 2011, 32 patients with ANCA positive GPA were randomized to treatment with or without TPE in addition to standard immunosuppression [Citation109]. Renal survival after 12 months and five years was better in those receiving TPE, but there was no difference in mortality. Multivariate analysis showed that TPE improved renal survival in those with initial creatinine greater than 250 µmol/L. Plasma exchange consequently became widely adopted for use as remission-induction therapy in patients with renal involvement in AAV, as it was acknowledged that dialysis-free survival, even transiently (months or years) was a meaningful outcome for patients.
Despite limited or no evidence for the therapeutic benefit of high doses of steroids, which have substantial dose-dependent adverse effects, they have remained a cornerstone of management in patients with AAV [Citation65]. It was unclear whether intravenous methylprednisolone (MP) or TPE was more effective in gaining the rapid control of disease required for patients with severe renal involvement. This question was addressed in the ‘Randomised Trial of Plasma Exchange or High-Dose Methylprednisolone as Adjunctive Therapy for Severe Renal Vasculitis’ (MEPEX) study, a collaborative trial conducted by the European Vasculitis Society (EUVAS) [Citation11]. This trial recruited 137 vasculitis patients with severe kidney involvement between 1995 and 2002. This was an important trial as this specific patient cohort had previously been excluded or under-represented in trials. Severe kidney involvement was defined as patients presenting with rapidly progressive glomerulonephritis with a Cr >500 µmol/L or requiring dialysis for less than 2 weeks at the time of enrollment. Patients received either 7 exchanges or 3 grams of IV MP in addition to standard treatment. The results showed significant benefit from TPE compared to high dose IV MP, with 69% of patients in the TPE arm remaining alive and dialysis-free at 3 months, compared to 49% of patients in the MP arm. This benefit continued to 12 months, where there remained a 50% relative risk reduction in the need for ongoing renal replacement therapy (RRT). However, there was no mortality benefit at 12 months, with similar serious adverse event rates between the two arms. Infections were the major cause of death in both groups, with only TPE associated with thrombocytopaenia and thrombosis risk. The MEPEX study classified all enrolled patients according to their biopsy results, and there was no difference between treatment arms in severity or frequency of any histopathological findings. The degree of tubulointerstitial fibrosis was generally predictive of poor kidney outcomes.
A further report from MEPEX analyzing longer-term follow-up did not show a sustained benefit from TPE on patient mortality [Citation110]. However, fewer patients that were treated with adjunctive TPE (23 patients, 33%) required long-term RRT, compared to patients treated with high-dose IV MP (33 patients, 49%). There was concern that if there was a reduction in ESKD due to treatment with TPE, then there should be evidence of improved mortality, given the known association between the need for RRT and reduced life expectancy [Citation110]. The authors suggested that as patients have rapid onset of ESKD in severe, active AAV, then they may not have accumulated the same chronic morbidity prior to initiating dialysis as seen in other etiologies of ESKD [Citation110,Citation111]. There was a significant mortality rate in the entire study cohort, with >50% of patients having died by 5 years post presentation. This could be related to non-vasculitis morbidities causing death in both groups due to the advanced age of the patients, or the treatment regimens may have increased mortality via mechanisms independent of ESKD (e.g. infection).
The uncertainty of the value of TPE in AAV led the vasculitis community to develop a large international trial ‘Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis’ (PEXIVAS) [Citation68]. The PEXIVAS study recruited 704 patients between 2010 and 2016, with severe, active AAV. It had a 2-by-2 factorial design, to address both the benefit of plasma exchange in addition to standard therapy, and to assess the non-inferiority of lower glucocorticoid dosing to standard dosing, with a composite endpoint of death or ESKD. Patients were enrolled if they had active urinary sediment and/or a kidney biopsy showing focal necrotizing glomerulonephritis and an eGFR <50 mL/min. Patients with diffuse alveolar hemorrhage (DAH) were included if they had radiographic changes with no alternative diagnosis, plus one of: (i) broncho-alveolar lavage confirming the diagnosis, (ii) frank hemoptysis, (iii) elevated diffusion capacity of carbon monoxide, or (iv) drop in hemoglobin 3 2 g/dL without other causes. Patients were randomized so that there was equal representation of patients with the most severe kidney disease (requiring dialysis or a Cr >500 µmol/L). More patients received CYC (85%) than RTX induction therapy. In this study, all patients received IV MP, with two different options of steroid taper thereafter, so that one arm had significantly less overall glucocorticoid exposure. Patients treated with TPE received 7 exchanges over 14 days, which is a slower rate than in many other studies where daily plasma exchanges are often delivered. The median creatinine at enrollment was 330 µmol/L. Severe renal manifestations were present in 205 patients (29% of the study cohort) with either a Cr >500 µmol/L and/or a requirement for RRT. DAH was evident in 27% of the cohort.
The main finding of the trial was that there was no benefit of TPE on the primary composite outcome of death or ESKD (hazard ratio 0.86, 95% CI: 0.65–1.13) after a median follow-up of 2.9 years [Citation68]. There were also no differences in secondary outcomes including serious adverse events. The reduced-dose steroid regimen was shown to be non-inferior to the standard dose (ARR 2.3%; 95% CI −4.5–9.1) with significantly fewer serious infections in the first year in the reduced-dose group (incidence rate ratio 0.69, 95% CI 0.52–0.93). This trial was a remarkable achievement for the vasculitis community in that it enrolled a large number of patients with a rare disease, and brought together a collaborative network of investigators from 95 centers in 16 countries. Numbers lost to follow-up were small, and treatment adherence was good despite the open label design. One of the criticisms of the PEXIVAS trial was not to stipulate the requirement for a kidney biopsy at entry [Citation112]. This has meant that it has not been possible to evaluate the influence of kidney histology on the outcomes of the trial. It should be noted that patients that have a more relapsing-remitting course may have significant tubulointerstitial and glomerular scarring prior to diagnosis, which could affect response to treatment.
Although both the MEPEX and PEXIVAS trials aimed to evaluate the role of adjunctive TPE in patients with AAV, there were significant differences in trial design and settings that may partly explain why the outcomes were so different. Firstly, a greater proportion of patients enrolled in the MEPEX trial had more severe renal involvement than in the PEXIVAS cohort. The average creatinine in patients in MEPEX was higher (735 vs 330 μmol/L), as was Birmingham Vasculitis Activity Score (BVAS) (21 vs 9) and C-Reactive Protein (CRP) (93 vs 45 mg/dL). The number of patients that were RRT-dependent was also much higher in MEPEX (70%) compared to PEXIVAS (20%). Analysis of the subgroup of patients that had a creatinine of >500 μmol/L or required RRT at presentation in PEXIVAS (n = 205) showed a trend to benefit of TPE on the primary outcome, but this was non-significant (HR 0.77, 95% CI 0.53–1.11).
Secondly, the induction regimens used in the studies were different. All patients enrolled in PEXIVAS were treated with IV MP, whereas in MEPEX, patients in the TPE arm of the study received no MP. It may be that the benefit of TPE is greatest in patients when used as a replacement for high-dose intravenous glucocorticoids, thus avoiding steroid side effects. Furthermore, MEPEX used daily oral CYC at 2.5 mg/kg per day, which was reduced for patients >60 years old [Citation11]. The CYCLOPS trial, which directly compared daily oral versus intravenous CYC, showed no differences in survival but a lower risk of relapse in the daily oral limb (HR 0.5, 95% CI 0.26 to 0.93; p = 0.029) [Citation113]. However, this did not translate to any differences in renal function or adverse events at the end of the study. The trial investigators of PEXIVAS felt there were no significant advantages to either route and opted to leave the choice of intravenous or oral CYC or RTX to the local investigator, to allow centers to operate within their own experience [Citation68]. The impact of this on trial results remains unclear.
Thirdly, the trials were different in the dates they were conducted, and the number of different centers involved. There have been advances in supportive care over the 15-year interval between recruitment windows of the two trials, and in the experience of clinicians treating patients with cytotoxic medications. It has been well established that there is a temporal improvement in overall outcomes in AAV [Citation12,Citation114,Citation115]. This is reflected in the lower-than-predicted event rates in PEXIVAS, which required an increase in the number of patients from 500 to 700 in order to meet the pre-specified event rate. The heterogeneity of how patients were treated over a long-term follow-up period, in many different centers, may also have reduced the capacity of PEXIVAS to detect a benefit of TPE. Since the publication of PEXIVAS, some centers have changed their practice to stop using TPE for all patients with AAV. However, there should be caution in how the trial is interpreted, as TPE could have a role in selected patients.
4.3. Recent cohort studies of TPE in AAV
A large retrospective cohort study from the Mayo Clinic group reported outcomes of 251 patients with significant renal involvement between 1996 and 2015, as defined by an eGFR <30 mL/min [Citation116]. Of these, 54 patients (21.5%) required dialysis at presentation, of which 12 (22.2%) discontinued dialysis. Patients received a variety of treatment combinations, decided on a case-by-case basis by the treating physician. Of the cohort, 51 patients received TPE, with patients that had more severe renal disease being more likely to receive TPE. Notably, most patients that received TPE also received IV MP (90.2%). Overall, the study found no significant benefit for TPE in this cohort, even after attempts at reducing bias via propensity score matching analyses. However, only a minority of patients were dialysis dependent, and a lower percentage of them recovered renal function than in other studies, suggesting a difference in clinical characteristics.
Pepper et al. showed, in a cohort of 42 patients with AAV from Hammersmith Hospital, that TPE could be used in conjunction with IV CYC as an effective alternative to oral CYC (as used in MEPEX) to treat patients with AAV who were dialysis-dependent at presentation [Citation117]. In comparison to patients enrolled in the TPE arm of the MEPEX trial, this cohort of patients had favorable renal outcomes with 65% of patients being alive with independent kidney function vs 51% in the MEPEX cohort at 1 year. Gulati et al., in a more recent cohort of 64 patients, also from the Hammersmith Hospital, showed that a combination of TPE in addition to low-dose IV CYC and RTX, with no routine use of IV MP and a low-dose oral steroid regimen, could be effective in patients with severe manifestations of AAV [Citation14]. This included patients with DAH or severe renal impairment, as defined by a Cr >500 µmol/L and/or a requirement for RRT. Of the patients with a Cr >500 µmol/L without a requirement for dialysis at presentation, 86% survived with independent kidney function at 1 year. Of the patients that required RRT at presentation, 67% recovered independent kidney function, and 90% of these patients survived with independent kidney function at one year. Approximately half of this cohort (n = 32) received at least one dose of IV MP prior to transfer to the center. The addition of pulsed IV MP did not improve early patient or kidney survival and was associated with greater risk of adverse events [Citation118,Citation119]. This was similar to the findings from Chanouzas et al., who found that IV glucocorticoids did not impact patient or kidney survival and was associated with a greater risk of infection and diabetes in patients with severe AAV using a conventional CYC-based remission-induction regimen and TPE [Citation66]. The compounding effect of using IV MP and TPE may explain the increased rates of infection seen in the TPE arm of PEXIVAS.
Morel et al. recently published a multi-center retrospective study between 2005 and 2017 which enrolled 153 patients [Citation120]. This study aimed to address whether CYC or RTX-based regimens were more effective in AAV, and if there was any benefit from the addition of TPE. This cohort included patients with moderately severe kidney involvement, with a Cr >350 µmol/L or an eGFR <15. Patients had to have a positive ANCA, active urinary sediment and/or pauci-immune crescentic GN on kidney biopsy. Patients were excluded if they were treated with a combination of RTX and CYC. Similar to the Mayo series, there was a temporal shift toward the use of RTX, but the majority of patients were treated with CYC (134 patients, 88%) rather than RTX (19 patients, 12%) [Citation116,Citation120]. Any patient that received at least one plasma exchange was included in the TPE group of 81 patients. Almost all patients also received high doses of IV MP with at least 1.5 grams, and up to 3 grams. Oral glucocorticoid dosing was comparable to the standard dosing arm of the PEXIVAS trial [Citation68,Citation120]. By 3 months post treatment, 95% of patients were in clinical remission. The study was only able to compare the differences between patients who received TPE in the CYC cohort, as only 5 patients were treated with RTX and TPE. Patients with more severe disease were more likely to receive TPE, introducing selection bias into the comparison. Of the patients receiving TPE, the median Cr was 578 µmol/L and 49 (64%) of them required RRT; in comparison, those not receiving TPE had a median Cr of 418 µmol/L and 15 (26%) of them required RRT. Propensity score weighting was used to adjust for confounders, and results showed that dialysis-free survival was higher in patients who received TPE and CYC than CYC alone at 6 months (72% vs 62%) and 12 months (74% vs 60%). Adverse events were similar in both the TPE and CYC alone groups (0.9 vs 0.8 infections/patient year respectively). Of the 8 deaths in the study, 6 patients died due to a severe infection, of whom 3 had received TPE as well as CYC. This could be related to the high doses of glucocorticoids used in this study. The authors observe that the renal survival at 1 year was higher in their series than in the MEPEX trial, although this may be due to the high proportion of patients in the MEPEX trial that had renal fibrosis at diagnosis (26%) [Citation11,Citation120]. However, there appears to be a benefit of adjunctive TPE for patients with more severe renal involvement in this study.
4.4. The impact of kidney histology on utility of TPE in AAV
Two main risk scores have been developed that utilize histopathological data in patients with AAV: the Berden classification and the Brix Renal Risk Score. The Berden classification proposes four categories of lesion: focal, crescentic, mixed, and sclerotic, and this was shown to be useful in prediction of renal outcomes [Citation121]. The Brix Renal Risk Score is a clinicopathological classification incorporating the proportion of normal glomeruli, degree of tubular atrophy and interstitial fibrosis, and eGFR at time of biopsy, and assigns a weighted score [Citation122]. The patients are then divided into three categories: low, medium, and high risk. It has been shown to accurately predict ESKD at 36 months (0%, 26%, and 68%, respectively, across the three groups). Brix et al. and Hilhorst et al. both emphasize that the percentage of normal glomeruli is the strongest individual predictors of end stage kidney disease risk [Citation122,Citation123].
Nezam et al. recently published outcome data from a large retrospective cohort study, to try and understand if histopathological findings help identify patients more likely to benefit from TPE [Citation124]. They enrolled 425 patients diagnosed with AAV between 2004 and 2019, who had severe kidney involvement across multiple centers in France, and who had a kidney biopsy at diagnosis. In this cohort, 188 patients received TPE. They used their outcome data to develop a prediction model based on the average treatment effect of TPE, to estimate whether the use of TPE could reduce the probability of death or ESKD by one year post treatment initiation. They utilized statistical modeling to compensate for significant patient, disease, and treatment-related heterogeneity. For example, the TPE treated group had significantly more patients with crescentic class on their biopsy (39%) compared to those in the control group (19%). There were also differences in median creatinine at presentation (550 µmol/L in the TPE group vs 282 µmol/L in the control group). However, the authors found that using histopathological findings in conjunction with clinical characteristics it was possible to identify a subgroup of patients that would benefit from TPE. The average treatment effect of TPE for those recommended treatment was a 25% reduction in death or RRT. Parameters such as degree of kidney injury (Cr >600 µmol/L), crescentic class according to Berden classification, and a clinical syndrome consistent with a diagnosis of MPA led to significantly higher scores predictive of additional benefit of TPE in these patients.
In the study of TPE in severe AAV reported by Gulati et al., histopathological data were available for the majority of patients [Citation14]. Both Berden class and the Brix Renal Risk Scores helped stratify patients at risk of progression to ESKD. Patients who presented with dialysis-dependent kidney failure and an intermediate Brix score were more likely to recover independent kidney function than those with high Brix scores. This was largely due to a lower percentage of tubular atrophy, median of 10% vs 30% in those who recovered kidney function vs those who did not respectively (p = 0.02). Correspondingly, patients who recovered also had a higher proportion of normal glomeruli (median 14% vs 5%, respectively, p = 0.03). This suggests that adjunctive TPE is most beneficial in patients with active inflammatory disease, prior to substantial scarring, by achieving rapid disease control.
5. Utility of TPE in AAV with diffuse alveolar hemorrhage
Diffuse alveolar hemorrhage is a life-threatening feature of AAV and has been proposed as an indication for TPE since the earliest reports, because of the rapid removal of pathogenic molecules [Citation76]. The effectiveness of TPE in DAH was reported from a single center in the USA, in which there was resolution of DAH in all 20 patients treated with TPE, compared with a historical control group in which three of 11 patients died [Citation125]. In a multi-center study from France, 29 of 30 patients with severe DAH treated with TPE recovered and mechanical ventilation was discontinued in all cases [Citation126]. The benefit of TPE was also reported in a national database study from Japan including 254 patients with AAV and DAH. After propensity matching, it was shown that there was a significant reduction in in-hospital mortality with TPE compared to those who did not receive it (35.6% vs 54.2%) [Citation127].
However, two other retrospective cohort studies have not suggested benefit from TPE in DAH. Firstly, Cartin-Ceba et al. reported the outcomes of a cohort of 73 patients with confirmed DAH on bronchoscopy [Citation128]. Most patients (41/73, 56%) had severe lung involvement requiring ICU admission; however, 10/73 (14%) did not require medical intervention and were managed as an outpatient. Thirty-two patients (44%) were treated with TPE, with the more critically unwell patients receiving this treatment. Authors used propensity score analysis to try and overcome the bias of confounding due to indication, and found no benefit of TPE in either remission at 6 months or mortality. They also aggregated the results of 11 other studies and found that of 172 patients with clear outcome results, that there was no difference in mortality at time of hospital discharge; 69 (66%) patients treated with TPE were alive at hospital discharge compared to 51 (75%) patients who were not treated with TPE. However, this did not consider the severity of DAH, and other factors affecting patient mortality. Secondly, Quartuccio et al. published the outcomes of 106 patients with DAH [Citation129]. Of these, 46 patients (44.7%) were treated with TPE. Again, they did not show a reduction in mortality, but they acknowledged the significant bias that the most severe/refractory cases were treated with TPE. The authors concluded that for patients with life-threatening DAH, they would still recommend the use of TPE.
Although many randomized controlled trials, including MEPEX, excluded patients with DAH, they were included in PEXIVAS [Citation68,Citation130]. A total of 191 patients with DAH were recruited (27% of entire cohort) of whom 61 were classed as severe, with hypoxia. There was a non-significant benefit of TPE with HR 0.64 for non-severe and 0.67 for severe cases [Citation68]. These patients have not yet been reported separately, but it seems likely that the study was underpowered to detect a significant benefit of TPE. Based on the information available, it is unlikely that TPE is of benefit in patients with mild DAH. However, we suggest that it is considered in patients with severe DAH with significant hypoxia, and in those with concomitant organ threatening kidney involvement.
6. Utility of TPE in patients with AAV and anti-GBM disease (double positive cohort)
It is well recognized that some patients with AAV, between 10% and 15% in most series, also have anti-GBM antibodies. Kidney biopsy will usually reveal linear deposits of IgG along the GBM in the context of a crescentic glomerulonephritis. Two early case series showed that the initial renal outcome of double positive patients was similar to that of those with anti-GBM disease, leading to the suggestion that these patients were treated as for anti-GBM disease with TPE and immunosuppression [Citation131,Citation132]. In a more recent multicentre European study the outcome of 37 double positive, 41 single positive anti-GBM, and 568 AAV patients was compared. The double positive patients had disease features similar to anti-GBM disease at presentation, with 38% DAH and 57% dialysis-dependent kidney failure. There was a trend toward better recovery from dialysis in the double positive patients compared to single positive anti-GBM cases (33% versus 17%), although renal survival was not significantly better. On long-term follow-up, the double positive patients relapsed at a similar rate to those with AAV whereas none of the single positive anti-GBM cases relapsed [Citation133]. This suggests that double positive patients show a mixed clinical picture with features of both diseases, so we would suggest initial treatment as for anti-GBM disease, which includes TPE, and maintenance therapy as for AAV. Similar results were recently shown by Hu et al., therefore supporting the use of TPE [Citation134].
7. Meta-analysis of trials of TPE in AAV
The meta-analysis by Walsh et al. in 2011 and a Cochrane review in 2019 both examined the evidence for TPE in AAV [Citation135,Citation136]. The meta-analysis found that TPE may reduce the composite end point of death and ESKD at 1 year; however, this was driven by a decreased risk of ESKD and no reduction in the risk of death [Citation136]. The Cochrane review similarly found that there was evidence that TPE prevented ESKD at both 3 and 12 months. There was no effect found on mortality, duration of remission or total number of adverse events through the use of TPE [Citation135].
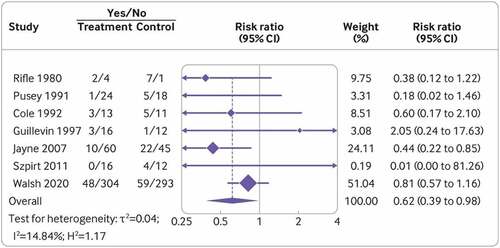
A more recent meta-analysis was published by Walsh et al. in 2022 to include data from nine clinical trials in AAV, including PEXIVAS [Citation11,Citation68,Citation94,Citation107–110,Citation137–140]. This incorporated data from 1060 patients, with the majority of cases extracted from the PEXIVAS trial. There was a median follow-up of 3 years. The study found no significant effect on all-cause mortality during follow-up. However, there was a reduction in risk of ESKD at 1 year with the use of adjunctive TPE (RR 0.62, CI 0.39–0.98), as summarized in . Patients who were identified to have the highest risk of progressing to ESKD at presentation benefited the most from TPE. This meta-analysis also showed an increased risk of serious infection from TPE at 1 year (RR 1.27, CI 1.08–1.49), with this effect persisting longer-term. However, there were no differences in any other serious adverse events. The interaction between TPE, high-dose glucocorticoids, other cytotoxic agents, and underlying patient- and disease-related factors are likely to impact this risk. A difficulty in interpreting these results, as noted when comparing MEPEX and PEXIVAS trials, was the clinical heterogeneity across the studies, with standard of care evolving over the course of enrollment into these trials.
8. Clinical guidelines for use of TPE as adjunctive therapy in AAV
Two major clinical guidelines (KDIGO and ACR) for management of patients with AAV differ in emphasis on the utility of TPE in current practice; however, both highlight that there remains a role for its use in individual cases [Citation105,Citation141]. The KDIGO (2021) guidance advises consideration of TPE in patients with life- or organ-threatening disease that presents with DAH or RPGN [Citation105]. The ACR 2021 guidance for the management of AAV has advised against TPE as routine addition to induction-remission therapies. However, it recommends consideration of TPE in patients deemed to be at higher risk of progression to ESKD, in those that are likely to be able to tolerate more serious infections, and those that are critically unwell as a salvage therapy [Citation141].
The latest meta-analysis by Walsh et al. has informed the independent BMJ Rapid Recommendations for AAV, suggesting that TPE should be considered for patients with moderate to high (Cr 300–500 µmol/L) or high (Cr >500 µmol/L or dialysis-dependent) risk for developing ESKD [Citation94,Citation142]. In these patients, the benefits were likely to outweigh the increased risk of infection or the risks of vascular-access-related complications, especially if access is required for administering dialysis anyway. In addition, Kaplan et al. reported that during the EULAR 2022 Congress in Copenhagen, the EULAR recommendation on AAV treatment concluded that ‘TPE may be considered as a part of therapy in GPA/MPA for those with a serum Cr >300 µmol/L due to active glomerulonephritis’ [Citation143].
9. Conclusions
The vasculitis research community has been inspirational in its development of collaborative, multi-center networks, which have allowed for large randomized controlled trials to be carried out in a rare disease. PEXIVAS has cemented the idea that TPE is not a suitable treatment for all patients with renal involvement in AAV [Citation68]. However, there must be caution in applying this to all patients without acknowledging limitations of the trial, including the absence of histopathological data for all patients, and incomplete data on the subgroups of patients with the most severe renal disease or hypoxic alveolar hemorrhage.
All patients enrolled in PEXIVAS also received high dose intravenous corticosteroids, which may have masked the benefits of TPE [Citation68]. Data from the MEPEX trial and the cohort study from Gulati et al. show that TPE may have further benefit when used as part of a steroid-sparing regimen [Citation14,Citation130]. This is in keeping with one of the major aims of vasculitis research, which is to allow a shift away from the use of high-dose steroids, and toward more targeted therapies with fewer associated toxicities. PEXIVAS did lead to consensus in the community that lower cumulative steroid dosing was as effective as the standard dose in AAV, with fewer associated adverse events [Citation68].
If we further inspect the data from PEXIVAS, in conjunction with more recent cohort studies and meta-analyses, there is substantial evidence that there remains a targeted role for the use of TPE as an adjunctive treatment in patients with the most severe renal disease [Citation14,Citation68,Citation94,Citation116,Citation120,Citation124,Citation142]. This has been reflected in many of the recent guidelines in AAV [Citation105,Citation141]. We suggest using TPE as an adjunct to a steroid sparing regimen in patients with a Cr >500 µmol/L or requiring dialysis at presentation, particularly in those who are more likely to be able to tolerate potential infectious complications. TPE could also be considered in those with a creatinine of >300 µmol/L and rising, or with severe DAH.
10. Patient opinion
It is becoming increasingly clear that we also need to strive not only to improve outcomes in renal and patient survival, but also to improve patient quality of life. The PEXIVAS trial showed no differences in quality of life after 12 months, depending on use of TPE [Citation68]. Recently, a large survey concerning the use of TPE was circulated to adults with AAV in Canada, the United Kingdom, and the United States [Citation144]. There were 470 respondents, with a mean age of 58.6 years. Patients were most likely to choose TPE if there was a substantial risk of kidney failure, although 145 patients (30.9%) consistently chose plasma exchange across all scenarios, regardless of increased infection risk. Conversely, 17% of patients would decline TPE regardless of the indication or risk. Interestingly, in the UK, patients were more likely to choose TPE if they had previously received dialysis or undergone TPE. Nonetheless, there was no consistent decision between all patients with AAV, again highlighting the requirement for individual risk assessment and shared decision-making.
11. Expert opinion
The use of TPE in AAV is underpinned by research into the pathogenicity of ANCA and the role of inflammatory mediators such as complement and coagulation factors. Benefit from TPE is likely to be due to the speed of removal of these pathogenic molecules from the circulation, pending the effects of immunosuppressive and anti-inflammatory drugs. Hence, TPE would be predicted to be most effective in severe acute disease, where rapid treatment is required to prevent organ damage. The clinical trials and meta-analysis discussed above confirm that TPE is of benefit in preventing ESKD in patients with active and advanced rapidly progressive glomerulonephritis [Citation11,Citation68,Citation94,Citation142]. TPE may prevent tissue damage from progressing beyond the point of no return in cases where drug treatment alone would not be sufficiently rapid. The prevention of the development of ESKD, with the requirement for renal replacement therapy, is of clinical, social, and economic benefit. It seems unlikely following PEXIVAS that further large trials of the use of TPE in AAV will be conducted. However, further work could be performed on identifying the characteristics of those patients who might respond to this treatment, as described in the histopathology study discussed above. A variety of immunological, genetic, and pathological biomarkers are being investigated in AAV. While there is strong evidence for the benefit of TPE in severe renal AAV, there is no such evidence supporting the use of intravenous MP; indeed, some uncontrolled studies suggest it is of no additional benefit. Hence, avoiding the use of IV MP (as in MEPEX), or of high doses of oral steroids (as in PEXIVAS), when using TPE could be a sensible approach [Citation68,Citation130]. Hence, TPE may be seen as a steroid sparing treatment. Supporting this approach, several recent studies in less severe AAV have demonstrated that the high steroid doses used historically are not more effective than lower doses and confer more adverse effects [Citation69,Citation145]. Future research will also need to examine the use of new therapeutic agents which could be used instead of, or together with, TPE. Avacopan, a C5a receptor blocker, has been shown to be non-inferior to steroids for induction treatment of AAV in the ADVOCATE trial [Citation70]. There appeared to be more rapid and greater improvement in kidney function in patients receiving avacopan, although the trial did not include those with an eGFR <15 ml/min. Further studies of avacopan, including patients with more severe kidney involvement, would be interesting. There is also a recent report of the use of imlifidase, an endopeptidase which breaks down IgG, in anti-GBM disease [Citation146,Citation147]. There was a rapid and complete fall in anti-GBM antibodies by about six hours, which is faster than can be achieved using TPE. More patients remained independent of dialysis at six months compared with historical controls, suggesting a clinical benefit. This approach could also be examined as an alternative to TPE in AAV, although it is specific for autoantibodies and will not affect inflammatory mediators.
So in our opinion there is sufficient evidence to recommend the use of TPE in patients with AAV and active glomerulonephritis with a creatinine >500 µmol/l. Based on the recent meta-analysis and the BMJ Rapid Recommendations [Citation142], it should also be considered in patients with a creatine of >300 µmol/l and rising. Although the evidence is less strong, TPE could be considered in patients with hypoxic diffuse alveolar hemorrhage. However, it seems clear that TPE is not indicated more widely in AAV. Decisions about treatment should always involve consideration of the clinical characteristics of each individual patient, for example their susceptibility to infection, and the patient’s wishes. The possibility of the avoidance of the need for long-term renal replacement therapy is a major incentive for patients and clinicians alike.
Article highlights
Anti-neutrophil cytoplasm antibodies (ANCA) are thought to be directly pathogenic in ANCA-associated vasculitis (AAV), by activating neutrophils and monocytes leading to endothelial damage.
TPE has been shown to rapidly and effectively remove ANCA and other pathogenic mediators of inflammation, including complement.
Early cohort studies and MEPEX, a randomized controlled trial, showed benefit of TPE in patients with organ-threatening manifestations of AAV.
The largest, and most recent randomized controlled trial of TPE in AAV, PEXIVAS, did not confirm significant benefit in all patients.
Recent meta-analysis has shown that TPE confers risk reduction of ESKD in patients with AAV and severe renal involvement.
BMJ Clinical Rapid Recommendations guidelines recommend the use of TPE in patients with Cr >500 μmol/L, and advise its consideration in those patients with Cr >300 μmol/L.
Declaration of interest
K Gulati is currently undertaking a clinical research training fellowship funded by Mr and Mrs Ken and Mary Minton (Imperial Health Charity). C Pusey has consulted for Vifor and Alentis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Kitching AR, Anders HJ, Basu N, et al. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6(1):71.
- RJ F, RS T, LA C, et al. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990;87:4115–4119.
- Xiao H, Hu P, Falk RJ, et al. Overview of the pathogenesis of ANCA-associated vasculitis. Kidney Dis (Basel). 2016;1:205–215.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised international Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1): 1- 11.
- Geetha D, JA J. ANCA-associated vasculitis: core curriculum 2020. Am J Kidney Diseases. 2020;75:124–137.
- Scott J, Hartnett J, Mockler D, et al. Environmental risk factors associated with ANCA associated vasculitis: a systematic mapping review. Autoimmun Rev. 2020;19:102660.
- Kronbichler A, Il SJ, Lee KH, et al. Clinical associations of renal involvement in ANCA-associated vasculitis. Autoimmun Rev. 2020;19:102495.
- McAdoo SP, Tanna A, Randone O, et al. Necrotizing and crescentic glomerulonephritis presenting with preserved renal function in patients with underlying multisystem autoimmune disease: a retrospective case series. Rheumatology (Oxford). 2015;54:1025–1032.
- Lee T, Gasim A, Derebail VK, et al. Predictors of treatment outcomes in ANCA-associated vasculitis with severe kidney failure. Clin J Am Soc Nephrol. 2014;9(5): 905- 913.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis. 2003;41:776–784.
- Jayne DRW, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18(7): 2180- 2188.
- Hilhorst M, Wilde B, van Paassen P, et al. Improved outcome in anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis: a 30-year follow-up study. Nephrol Dial Transplant. 2013;28:373–379.
- Wallace ZS, Fu X, Harkness T, et al. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology Internet. 2020;59:2308–2315.
- Gulati K, Edwards H, Prendecki M, et al. Combination treatment with rituximab, low-dose cyclophosphamide and plasma exchange for severe antineutrophil cytoplasmic antibody-associated vasculitis [Internet]. Kidney Int. 2021;100:1316–1324.
- Hunter RW, Welsh N, Farrah TE, et al. ANCA associated vasculitis. BMJ. 2020;369:m1070.
- Houben E, Bax WA, van Dam B, et al. Diagnosing ANCA-associated vasculitis in ANCA positive patients: a retrospective analysis on the role of clinical symptoms and the ANCA titre. Medicine (Baltimore). 2016;95:e5096.
- Tang S, Zhang Y, Yin S-W, et al. Neutrophil extracellular trap formation is associated with autophagy-related signalling in ANCA-associated vasculitis. Clin Exp Immunol. 2015;180:408–418.
- Yoshida M, Sasaki M, Sugisaki K, et al. Neutrophil extracellular trap components in fibrinoid necrosis of the kidney with myeloperoxidase-ANCA-associated vasculitis. Clin Kidney J. 2013;6:308–312.
- Sangaletti S, Tripodo C, Chiodoni C, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120:3007–3018.
- Kessenbrock K, Krumbholz M, Schönermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625.
- Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241.
- Wang H, Wang C, Zhao M-H, et al. Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol. 2015;181:518–527.
- Kraaij T, Kamerling SWA, van Dam LS, et al. Excessive neutrophil extracellular trap formation in ANCA-associated vasculitis is independent of ANCA. Kidney Int. 2018;94:139–149.
- Jennette JC, Falk RJ. Pathogenesis of the vascular and glomerular damage in ANCA-positive vasculitis. Nephrol Dial Transplant. 1998;13(Suppl 1):16–20.
- Xiao H, Dairaghi DJ, Powers JP, et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol. 2014;25:225–231.
- Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963.
- Xiao H, Schreiber A, Heeringa P, et al. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64.
- Huugen D, van Esch A, Xiao H, et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71:646–654.
- Prendecki M, Pusey CD. Recent advances in understanding of the pathogenesis of ANCA-associated vasculitis [version 1; peer review: 2 approved]. F1000Res.Internet. 2018;7. [cited 22 Mar 2022]. Available from: https://f1000research.com/articles/7-1113/v1
- Fussner LA, Hummel AM, Schroeder DR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol. 2016;68:1700–1710.
- Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol. 2004;93:398–401.
- Tervaert JWC, van der Woude FJ, Fauci AS, et al. Association between active Wegener’s Granulomatosis and anticytoplasmic antibodies. Arch Intern Med Internet. 1989;149:2461–2465.
- Kemna MJ, Damoiseaux J, Austen J, et al. ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol. 2015;26:537–542.
- Schlieben DJ, Korbet SM, Kimura RE, et al. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Am J Kidney Dis. 2005;45:758–761.
- Tomasson G, Grayson PC, Mahr AD, et al. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis–a meta-analysis. Rheumatology (Oxford). 2012;51:100–109.
- Jeffs LS, Peh CA, Nelson A, et al. IgM ANCA in healthy individuals and in patients with ANCA-associated vasculitis. Immunol Res. 2019;67:325–336.
- Girard T, Mahr A, Noël LH, et al. Are antineutrophil cytoplasmic antibodies a marker predictive of relapse in Wegener’s granulomatosis? A prospective study. Rheumatology (Oxford). 2001;40:147–151.
- Olson SW, Owshalimpur D, Yuan CM, et al. Relation between asymptomatic proteinase 3 antibodies and future granulomatosis with polyangiitis. Clin J Am Soc Nephrol. 2013;8:1312–1318.
- Kobayashi K, Shibata T, Sugisaki T. Aggravation of rat nephrotoxic serum nephritis by anti-myeloperoxidase antibodies. Kidney Int. 1995;47:454–463.
- Little MA, Al-Ani B, Ren S, et al. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS One. 2012;7:e28626.
- Pfister H, Ollert M, Fröhlich LF, et al. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood. 2004;104:1411–1418.
- Little MA, Smyth L, Salama AD, et al. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol. 2009;174:1212–1220.
- Schreiber A, Xiao H, Falk RJ, et al. Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J Am Soc Nephrol. 2006;17:3355–3364.
- Jennette JC, Xiao H, Falk R, et al. Experimental models of vasculitis and glomerulonephritis induced by antineutrophil cytoplasmic autoantibodies. Contrib Nephrol. 2011;169:211–220.
- Heeringa P, Brouwer E, Klok PA, et al. Autoantibodies to myeloperoxidase aggravate mild anti-glomerular-basement-membrane-mediated glomerular injury in the rat. Am J Pathol. 1996;149:1695–1706.
- Kinjoh K, Kyogoku M, Good RA. Genetic selection for crescent formation yields mouse strain with rapidly progressive glomerulonephritis and small vessel vasculitis. Proc Natl Acad Sci U S A. 1993;90:3413–3417.
- Brouwer E, Huitema MG, Klok PA, et al. Antimyeloperoxidase-associated proliferative glomerulonephritis: an animal model. J Exp Med. 1993;177:905–914.
- Merkel PA, Xie G, Monach PA, et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 2017;69:1054–1066.
- Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–223.
- Popa ER, Stegeman CA, Abdulahad WH, et al. Staphylococcal toxic-shock-syndrome-toxin-1 as a risk factor for disease relapse in Wegener’s granulomatosis. Rheumatology (Oxford). 2007;46:1029–1033.
- Rathmann J, Stamatis P, Jönsson G, et al. Infection is associated with increased risk of MPO- but not PR3-ANCA-associated vasculitis. Rheumatology (Oxford). 2022;61(12): 4817–4826.
- Kain R, Exner M, Brandes R, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088–1096.
- Pendergraft WF 3rd, GA P, RR S, et al. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10:72–79.
- Pendergraft WF 3rd, LC H, Thornley-Brown D, et al. Nephrotoxic effects of common and emerging drugs of abuse. Clin J Am Soc Nephrol. 2014;9:1996–2005.
- Yu F, Chen M, Gao Y, et al. Clinical and pathological features of renal involvement in propylthiouracil-associated ANCA-positive vasculitis. Am J Kidney Dis. 2007;49:607–614.
- de Lind van Wijngaarden RAF, van Rijn L, EC H, et al. Hypotheses on the etiology of antineutrophil cytoplasmic autoantibody associated vasculitis: the cause is hidden, but the result is known. Clin J Am Soc Nephrol. 2008;3:237–252.
- Gómez-Puerta JA, Gedmintas L, Costenbader KH. The association between silica exposure and development of ANCA-associated vasculitis: systematic review and meta-analysis. Autoimmun Rev. 2013;12:1129–1135.
- ten Holder SM, Joy MS, Falk RJ, et al. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130–147.
- Flossmann O, Berden A, de Groot K, et al. Long-term patient survival in ANCA-associated vasculitis. Ann Rheum Dis. 2011;70:488–494.
- Fauci AS, Haynes BF, Katz P, et al. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85.
- Houben E, Groenland SL, van der Heijden JW, et al. Relation between duration of the prodromal phase and renal damage in ANCA-associated vasculitis. BMC Nephrol. 2017;18:378.
- Basu N, Karabayas M, Pusey C. Prognosis and future developments in vasculitis. Best Pract Res Clin Rheumatol. 2018;32:148–165.
- Rathmann J, Jayne D, Segelmark M, et al. Incidence and predictors of severe infections in ANCA-associated vasculitis: a population-based cohort study. Rheumatology (Oxford). 2021;60:2745–2754.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–498.
- Floyd L, Morris A, Joshi M, et al. Glucocorticoid therapy in ANCA vasculitis: using the glucocorticoid toxicity index as an outcome measure. Kidney360. 2021;2:1002–1010.
- Chanouzas D, Jag M, Nightingale P, et al. Intravenous pulse methylprednisolone for induction of remission in severe ANCA associated Vasculitis: a multi-center retrospective cohort study. BMC Nephrol Internet. 2019;20:58.
- McGregor JG, Hogan SL, Hu Y, et al. Glucocorticoids and relapse and infection rates in anti-neutrophil cytoplasmic antibody disease. Clin J Am Soc Nephrol. 2012;7: 240LP – 247.
- Walsh M, Merkel PA, Peh C-A, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis [Internet]. N Engl J Med. 2020;382:622–631.
- Pepper RJ, McAdoo SP, Moran SM, et al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody–associated vasculitis [Internet]. Rheumatology. 2019;58:260–268.
- Jayne DRW, Merkel PA, Schall TJ, et al. Avacopan for the treatment of ANCA-associated vasculitis [Internet]. N Engl J Med. 2021;384:599–609.
- Clark WF, Huang SS. Introduction to therapeutic plasma exchange. Transfus Apheresis Sci. 2019;58:228–229.
- Jj ABEL, LG ROWNTREE, Bb TURNER. Plasma removal with return of corpuscles (plasmaphaeresis). J Pharmacol Exp Ther. 1914;5: 625LP – 641.
- Adams WS, Blahd WH, Bassett SH. A method of human plasmapheresis. Experimental Biology and Medicine. 1952;80(2):377–379.
- Fernández-Zarzoso M, Gómez-Seguí I. de la Rubia J. Therapeutic plasma exchange: review of current indications. Transfus Apher Sci. 2019;58:247–253.
- Lockwood CM, Boulton-Jones JM, Lowenthal RM, et al. Recovery from Goodpasture’s syndrome after immunosuppressive treatment and plasmapheresis. Br Med J. 1975;2:252–254.
- Lockwood CM, Rees AJ, Pearson TA, et al. Immunosuppression and plasma-exchange in the treatment of Goodpasture’s syndrome. Lancet. 1976;1:711–715.
- Lockwood CM, Pinching AJ, Sweny P, et al. Plasma-exchange and immunosuppression in the treatment of fulminating immune-complex crescentic nephritis. Lancet. 1977;309:63–67.
- Reeves HM, Winters JL. The mechanisms of action of plasma exchange. Br J Haematol. 2014;164:342–351.
- Keller AJ, Urbaniak SJ. Intensive plasma exchange on the cell separator: effects on serum immunoglobulins and complement components. Br J Haematol. 1978;38:531–540.
- Chirnside A, Urbaniak SJ, Prowse CV, et al. Coagulation abnormalities following intensive plasma exchange on the cell separator. II. Effects on factors I, II, V, VII, VIII, IX, X and antithrombin III. Br J Haematol. 1981;48:627–634.
- Xie X, Lv J, Shi S, et al. Plasma exchange as an adjunctive therapy for crescentic IgA nephropathy. Am J Nephrol. 2016;44:141–149.
- Gou S-J, Yuan J, Chen M, et al. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 2013;83:129–137.
- Gou S-J, Yuan J, Wang C, et al. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol. 2013;8:1884–1891.
- Derksen RH, Schuurman HJ, Meyling FH, et al. The efficacy of plasma exchange in the removal of plasma components. J Lab Clin Med. 1984;104:346–354.
- Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34:171–354.
- Winters JL. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. Hematology Am Soc Hematol Educ Program. 2012;2012:7–12.
- Plasmapheresis MF. Technical aspects and indications. Crit Care Clin. 2002;18:375–392.
- Pusey CD, Levy JB. Plasmapheresis in immunologic renal disease. Blood Purif. 2012;33:190–198.
- Burnouf T, Eber M, Kientz D, et al. Assessment of complement activation during membrane-based plasmapheresis procedures. J Clin Apher. 2004;19:142–147.
- Reimann PM, Mason PD. Plasmapheresis: technique and complications. Intensive Care Med. 1990;16:3–10.
- Marlu R, Naciri Bennani H, Seyve L, et al. Comparison of three modalities of plasmapheresis on coagulation: centrifugal, single-membrane filtration, and double-filtration plasmapheresis. J Clin Apher. 2021;36:408–419.
- Hafer C, Golla P, Gericke M, et al. Membrane versus centrifuge-based therapeutic plasma exchange: a randomized prospective crossover study. Int Urol Nephrol. 2016;48:133–138.
- Keklik M, Çelik S, Yıldızhan E. Comparison of centrifugal and membrane filtration modalities on therapeutic plasma exchange. J Clin Apher. 2022;37:217–222.
- Walsh M, Collister D, Zeng L, et al. The effects of plasma exchange in patients with ANCA-associated vasculitis: an updated systematic review and meta-analysis. BMJ. 2022;376:e064604.
- Kiprov D, Sanchez A, Pusey C. Therapeutic Apheresis. In: Daugirdas J, Blake P, IngT, editors. Handbook of dialysis. 5th ed. Philadephia: Wolters Kluwer ed. 2007.
- Mörtzell Henriksson M, Newman E, Witt V, et al. Adverse events in apheresis: an update of the WAA registry data. Transfus Apher Sci. 2016;54:2–15.
- Jones RB, Tervaert JWC, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–220.
- Sarica SH, Dhaun N, Sznajd J, et al. Characterizing infection in anti-neutrophil cytoplasmic antibody-associated vasculitis: results from a longitudinal, matched-cohort data linkage study. Rheumatology (Oxford). 2020;59:3014–3022.
- Waki D, Nishimura K, Tokumasu H, et al. Initial high-dose corticosteroids and renal impairment are risk factors for early severe infections in elderly patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: a retrospective observational study. Medicine (Baltimore). 2020;99:e19173.
- Odler B, Riedl R, Gauckler P, et al. Risk factors for serious infections in ANCA-associated vasculitis. Ann Rheum Dis. 2023;1- 7. [cited 26 Jan 2023]. Available from: http://ard.bmj.com/content/early/2023/01/26/ard-2022-223401.abstract
- Puisset F, White-Koning M, Kamar N, et al. Population pharmacokinetics of rituximab with or without plasmapheresis in kidney patients with antibody-mediated disease. Br J Clin Pharmacol. 2013;76:734–740.
- Smith PK, d‘Apice JF. Plasmapheresis in rapidly progressive glomerulonephritis. Am J Med.1978;65(4):564–566.
- Slot MC, Tervaert JWC, Franssen CFM, et al. Renal survival and prognostic factors in patients with PR3-ANCA associated vasculitis with renal involvement. Kidney Int. 2003;63:670–677.
- de Joode AAE, Sanders JSF, Smid WM, et al. Plasmapheresis rescue therapy in progressive systemic ANCA-associated vasculitis: single-center results of stepwise escalation of immunosuppression. J Clin Apher. 2014;29:266–272.
- KDIGO. Clinical practice guideline for the management of glomerular diseases. Kidney Int. United States. 2021;2021:S1–S276.
- Glöckner WM, Sieberth HG, Wichmann HE, et al. Plasma exchange and immunosuppression in rapidly progressive glomerulonephritis: a controlled, multi-center study. Clin Nephrol. 1988;29:1–8.
- Cole E, Cattran D, Magil A, et al. A prospective randomized trial of plasma exchange as additive therapy in idiopathic crescentic glomerulonephritis. the Canadian apheresis study group. Am J Kidney Dis. 1992;20:261–269.
- Pusey CD, Rees AJ, Evans DJ, et al. Plasma exchange in focal necrotizing glomerulonephritis without anti-GBM antibodies. Kidney Int. 1991;40:757–763.
- Szpirt WM, Heaf JG, Petersen J. Plasma exchange for induction and cyclosporine A for maintenance of remission in Wegener’s granulomatosis – a clinical randomized controlled trial. Nephrol Dial Transplant. 2011;26:206–213.
- Walsh M, Casian A, Flossmann O, et al. Long-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney Int. 2013;84:397–402.
- Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663.
- Kronbichler A, Il SJ, Wang C-S, et al. Plasma exchange in ANCA-associated vasculitis: the pro position. Nephrol Dialysis Transplantation. 2021;36:227–231.
- Harper L, Morgan MD, Walsh M, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up. Ann Rheum Dis. 2012;71:955–960.
- Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63:257–266.
- Rhee RL, Hogan SL, Poulton CJ, et al. Trends in long-term outcomes among patients with antineutrophil cytoplasmic antibody-associated vasculitis with renal disease. Arthritis Rheumatol. 2016;68:1711–1720.
- Casal Moura M, Irazabal MV, Eirin A, et al. Efficacy of rituximab and plasma exchange in antineutrophil cytoplasmic antibody-associated vasculitis with severe kidney disease. J Am Soc Nephrol. 2020;31:2688–2704.
- Pepper RJ, Chanouzas D, Tarzi R, et al. Intravenous cyclophosphamide and plasmapheresis in dialysis-dependent ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2013;8:219–224.
- Cheng L, Gou S-J. Whether the addition of high-dosage methylprednisolone to plasma exchange was more effective than plasma exchange in the treatment for severe ANCA-associated vasculitis? Kidney Int. 2022;101(3):647–648.
- Gulati K, Edwards H, Prendecki M, et al. The authors reply. Kidney Int.2022;101(3):648–649.
- Morel P, Karras A, Porcher R, et al. Management of severe renal disease in anti-neutrophil-cytoplasmic-antibody-associated vasculitis: place of rituximab and plasma exchange? Rheumatology (Oxford). 2022;61(10): 4056–4064 .
- Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21(10): 1628–1636.
- Brix SR, Noriega M, Tennstedt P, et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. 2018;94(6): 1177–1188.
- Hilhorst M, Wilde B, van Breda Vriesman P, et al. Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol. 2013;24:1371–1375.
- Nezam D, Porcher R, Grolleau F, et al. Kidney histopathology can predict kidney function in ANCA-associated vasculitides with acute kidney injury treated with plasma exchanges. J Am Soc Nephrol. 2022;33:628–637.
- Klemmer PJ, Chalermskulrat W, Reif MS, et al. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am J Kidney Dis. 2003;42:1149–1153.
- de Luna G, Chauveau D, Aniort J, et al. Plasma exchanges for the treatment of severe systemic necrotizing vasculitides in clinical daily practice: data from the French vasculitis study group. J Autoimmun. 2015;65:49–55.
- Uechi E, Okada M, Fushimi K. Effect of plasma exchange on in-hospital mortality in patients with pulmonary hemorrhage secondary to antineutrophil cytoplasmic antibody-associated vasculitis: a propensity-matched analysis using a nationwide administrative database. PLoS One. 2018;13:e0196009.
- Cartin-Ceba R, Diaz-Caballero L, Al-Qadi MO, et al. Diffuse alveolar hemorrhage secondary to antineutrophil cytoplasmic antibody-associated vasculitis: predictors of respiratory failure and clinical outcomes. Arthritis Rheumatol. 2016;68:1467–1476.
- Quartuccio L, Bond M, Isola M, et al. Alveolar haemorrhage in ANCA-associated vasculitis: long-term outcome and mortality predictors. J Autoimmun. 2020;108:102397.
- Jayne DRW, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18(7): 2180–2188.
- Rutgers A, Slot M, van Paassen P, et al. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am J Kidney Dis. 2005;46:253–262.
- Levy JB, Hammad T, Coulthart A, et al. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66:1535–1540.
- McAdoo SP, Tanna A, Hrušková Z, et al. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92:693–702.
- Hu X, Shen C, Meng T, et al. Clinical features and prognosis of MPO-ANCA and anti-GBM double-seropositive patients. Front Immunol. 2022;13:991469.
- Walters GD, Willis NS, Cooper TE, et al. Interventions for renal vasculitis in adults. Cochrane Database Syst Rev. 2020;1(1):CD003232.
- Walsh M, Catapano F, Szpirt W, et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta-analysis. Am J Kidney Dis. 2011;57:566–574.
- Rifle G, Chalopin JM, Zech P, et al. Treatment of idiopathic acute crescentic glomerulonephritis by immunodepression and plasma-exchanges. A prospective randomised study. Proc Eur Dial Transplant Assoc. 1981;18:493–502.
- Mauri JM. Therapeutic plasma exchange in the treatment of rapidly progressive glomerulonephritis. Plasma Ther Transfus Technol. 1985;6:587–591.
- Guillevin L, Cevallos R, Durand-Gasselin B, et al. Treatment of glomerulonephritis in microscopic polyangiitis and Churg-Strauss syndrome. Indications of plasma exchanges, Meta-analysis of 2 randomized studies on 140 patients, 32 with glomerulonephritis. Ann Med Internet (Paris). 1997;148:198–204.
- Zäuner I, Bach D, Braun N, et al. Predictive value of initial histology and effect of plasmapheresis on long-term prognosis of rapidly progressive glomerulonephritis. Am J Kidney Diseases. 2002;39:28–35.
- Chung SA, Langford CA, Maz M, et al. American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Care Res (Hoboken). 2021;2021(73):1088–1105.
- Zeng L, Walsh M, Guyatt GH, et al. Plasma exchange and glucocorticoid dosing for patients with ANCA-associated vasculitis: a clinical practice guideline. BMJ. 2022;376:e064597.
- Kaplan A, Szpirt W. Should we still use therapeutic plasma exchange for rapidly progressive glomerulonephritis in ANCA associated vasculitis? Kidney and Dialysis. 2022;2(3):399–406.
- Collister D, Farrar M, Farrar L, et al. Plasma exchange for ANCA-associated vasculitis: an international survey of patient preferences. Kidney Med. 2023;5(3):100595.
- Furuta S, Nakagomi D, Kobayashi Y, et al. Effect of reduced-dose vs high-dose glucocorticoids added to rituximab on remission induction in ANCA-associated vasculitis: a randomized clinical trial [Internet]. JAMA. 2021;325:2178–2187. Available from
- Shin JI, Geetha D, WM S, et al. Anti-glomerular basement membrane disease (Goodpasture disease): from pathogenesis to plasma exchange to IdeS. Ther Apher Dial. 2022;26:24–31.
- Soveri I, Mölne J, Uhlin F, et al. The IgG-degrading enzyme of Streptococcus pyogenes causes rapid clearance of anti-glomerular basement membrane antibodies in patients with refractory anti-glomerular basement membrane disease. Kidney Int. 2019;96:1234–1238.