Abstract
The role of microbial heterotrophs in deep-ocean sediment communities is explored using a simplified model of the cycling of carbon within a generic, size-based food web. Measurements of potential rates of respiration and growth of bacteria appear to be low, given the high concentrations of both microbial biomass and dissolved organic matter (DOM) in pore waters. This enigma can be explained theoretically by assuming that much of the microbial biomass is restricted in function at high pressure and low temperature, that a large fraction of the dissolved organic carbon (DOC) is refractory, and that the small fraction of the DOM that is labile and thus available must be liberated from the particulate pool by extracellular enzyme activity, viral infection or through the feeding processes of the metazoans. Free-living heterotrophic microbes in the sediments thus play a minor role in metazoan food webs, providing only a small fraction of the nourishment of metazoans. On the other hand, microbial heterotrophs appear to be responsible in part for the high levels of refractory DOM observed to accumulate in the deep ocean.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Quantification of the cycling of organic detritus in surficial deep-sea sediments has been made possible through the combined use of in-situ incubations (Smith & Hinga Citation1983; Smith Citation1992; Rowe et al. Citation2008a), particle flux estimates (Tietjen et al. Citation1989; Dunne et al. Citation2007), pore water profiles (Reimers et al. Citation1984) and time-based sediment accumulation rate measurements (Yeager et al. Citation2004). Although some of the terminology varies, both geochemists (Wenzhofer & Glud 2002; Burdige Citation2006) and ecologists (Smith & Hinga Citation1983; Pfannkucke et al. Citation1983) have estimated rates of early diagenesis that are in general agreement (Andersson et al. Citation2004). However, the specific mechanisms responsible for organic detritus remineralization remain unclear. Few approaches have emerged that can clearly resolve food web pathways in deep-sea sediments.
A seemingly promising approach has been to measure respiration and growth of sized-based components of benthic boundary-layer communities directly. If this approach could be accomplished at the same time that system-level carbon flux measurements are being made, then in theory the sum of the respiration and growth of each size or functional component of the community (microbes, meiofauna, macrofauna and megafauna) should equal the total utilization of organic matter, as measured by any or all of the methods listed above. Indeed, individual organisms incubated in situ and aboard ship have provided reasonable respiration rates for some fish and megafauna. These incubation-based data have been summarized in a set of general equations, or allometric models, that allow estimating respiration rates based on sample depth, mean animal size and temperature (Mahaut et al. 1995), and this allometric model-based approach is now widely applied (Heip et al. Citation2001; Rowe et al. Citation2003, Citation2008b). In addition, small-volume sediment samples have been incubated with radiolabelled substrates to measure potential microbial respiration and growth at in-situ pressure and temperature (Deming & Colwell Citation1982, Citation1985; Rowe & Deming Citation1985; Relexans et al. Citation1996). Thus the sum of these individual rate measurements should theoretically add up to the total oxygen consumption within the benthic boundary layer at any given location and time (Rowe et al. Citation2008b; Soetaert & van Oevelen Citation2009).
One prominent conundrum is that measured microbial rates reported in the literature are in general substantially lower than might be expected for this quantitatively dominant group, leading some authors (ourselves included) attempting to budget carbon flow to ignore the measured rates. The assumption has long been that microbes ‘condition’ the detritus to make it palatable (richer in nitrogen content via bacterial cells: Newell et al. Citation1984) for the deposit feeders that dominate metazoan life in deep-sea sediments (Richardson & Young Citation1987), even though most experimental evidence, to the contrary, suggests that free-living microbial heterotrophs contribute only a small fraction of the carbon demand of the infaunal metazoans (Cammen Citation1989). Alternatively, some authors resort to solving for the microbial rates by comparing microsensor profiles of oxygen with total benthic fluxes obtained from chamber measurements (e.g. Glud et al. Citation1994), or by subtracting the sum of the metazoan rates (based on direct measurements of the larger fauna in combination with allometric models for the smaller forms) from measurements of the total carbon input (estimated from sediment traps, sediment accumulation, pore water profiles, and in-situ chamber incubations). We are now suggesting that this latter approach be revised.
Our first microbial respiration rates for deep-sea sediments under simulated in situ conditions (based on utilization of glutamic acid; Rowe & Deming Citation1985) were too low to fit the paradigm of heterotrophic microbes as the base of a sediment detrital food web. Recognizing that rates based on a single amino acid tracer might well have underestimated consumption of the range of dissolved organic compounds likely available, we applied a factor of five in an attempt to reflect consumption of the pool of total dissolved free amino acids (DFAAs; glutamic acid represented ∼20% of DFAAs). The resulting rates were consistent with a more important role for sediment-dwelling bacteria. In a similar study off Greenland, we improved the approach by using a natural mix of phytoplankton-derived DFAAs as the radiolabelled substrates, yet obtained low rates, implying a limited contribution of the bacteria to total community carbon cycling (Rowe et al. Citation1997). The very cold temperatures of this seafloor seemed a reasonable explanation for reduced rates (Pomeroy et al. Citation1991). In the Goban Spur in the NE Atlantic, Heip et al. (Citation2001) estimated the microbial component of sediment community oxygen consumption (SCOC) by difference, using the Mahaut et al. (1995) allometric model, and thus concluded that a considerable fraction of the SCOC was attributable to the microbes. On transects between Svalbard and Norway in the North Atlantic, Piepenburg et al. (Citation1995) estimated the microbial component of respiration by subtracting the meiofauna and macrofauna respiration rates from SCOC, thus attributing a large fraction of the total community carbon cycling to microbial respiration. Most recently, in the Gulf of Mexico, Deming & Carpenter (Citation2008) measured microbial rates of respiration using radiolabelled DFAA and growth using tritiated thymidine in pressurized whole-core incubations (evaluated by Relexans et al. Citation1996, as most likely to resemble in-situ rates). When these rates were compared to estimates made from a food web carbon budget constructed for the same locations (Rowe et al. Citation2008b), the model-estimated microbial rates were again higher, by an order of magnitude, than the measured microbial rates in Deming & Carpenter (Citation2008). We suggest that relying only on mechanistic models can be misleading when actual measurements have been made. ‘Models are always wrong, but some of them are useful’ (anon.). Thus, we have attempted to construct a theoretical revision of a deep-benthic food web to resolve the described disparities and set the stage for further refinement.
Our hypothesis is that motile metazoans at the sediment water interface consume settling particulate organic matter before it becomes available to the microbial populations within the sediments. In their seemingly endless searching for food, metazoans move to newly settled fecal pellets, molts, aggregates and cells as soon as the material arrives on the seafloor (Witte et al. Citation2003). The metazoans then facilitate the production and release of dissolved organic matter (DOM) in their various acts of consuming, digesting, and egesting the particles, all done ‘sloppily’. Within the sediments, the heterotrophic microbes contribute to DOM production through extracellular enzyme activity (EEA) (Smith et al. Citation1992; Boetius & Damm Citation1998; Vetter et al. Citation1998). These free-living microbes also fall prey to viral infection that returns their cellular material to the DOM pool (CitationFurman 1999; Danovaro et al. Citation2008). Thus the free-living cells dwelling in the sediments take advantage of the ‘leftovers’ but are not, in this scheme, a major supporting step in the food web. This idea also appears consistent with the carbon in the free-living microbial community representing only a small fraction (ca. 1%) of the total detrital organic carbon concentration. This sediment community thus mimics the planktonic food web, with a microbial loop that consumes DOM after it is liberated from living phytoplankton and zooplankton (Deming & Baross Citation1993). A critical if often overlooked distinction in the deep benthic scenario is that deposit-feeding metazoans harbour extensive and often highly active gut microflora in their digestive tracts (Deming & Colwell Citation1982), where extracellular enzymes can generate food in the form of DOM for themselves, their hosts (to absorb across the gut wall) and their free-living counterparts in the sediment (to consume from pore waters). Whereas others have recognized the importance of metazoans accessing freshly deposited detritus rapidly (Witte et al. Citation2003), the role of animal-associated microbial activity (Plante et al. Citation1990) has not been fully appreciated.
Methods
We have constructed a hypothetical alternative food web network () to illustrate a revised role for free-living microbes in sediment food webs. This model is a set of boxes, representing standing stocks or concentrations, connected by arrows, representing fluxes between boxes. The units in the hypothetical model are mg C m−2 for the stocks and mg C m−2 d−1 for the fluxes. The model is meant to illustrate that the role of sediment-inhabiting microbes in deep-water sediment food webs is more complicated than that presented in previous food web models and carbon budgets. Rather than conditioning particulate organic carbon (POC) that has settled to the seafloor, the microbial populations consume labile DOC (LDOC) after it has been made available by a suite of possible processes: hydrolysis of POC by extracellular enzyme activity (EEA) (Boetius & Damm Citation1998), bacterial cell lysis due to infection by viruses (Danovaro et al. Citation2008) and alteration of POC associated with metazoan feeding, including passage through digestive tracts and the gauntlet of extracellular hydrolytic enzymes produced by the metazoans and their gut flora. These three pathways are illustrated explicitly in the food web model (). This alternative view diminishes the importance of free-living heterotrophic microbes as a food source to metazoans in mass-balance budgets, but recognizes the potential importance of populations living in the guts of metazoans (Deming & Colwell Citation1982).
Figure 1. Theoretical model illustrating the flow of carbon compounds through a surface sediment food web at abyssal plain depths, given data from the deep (3.6 km) Gulf of Mexico, supplemented with missing information from other abyssal sites ( and ). The boxes are stocks or concentrations measured in mg C m−2 to a depth of 10 cm and the arrows are fluxes or transformations measured in mg C m−2 d−1. The top four stocks (boxes) are in the surface 10 cm layer whereas the bottom two stocks are in a suboxic/anoxic layer 10 cm beneath the surface. Populations of chemoautotrophs exist along the chemical gradients in the boundary between the two layers. The stocks and fluxes correspond to values in and .
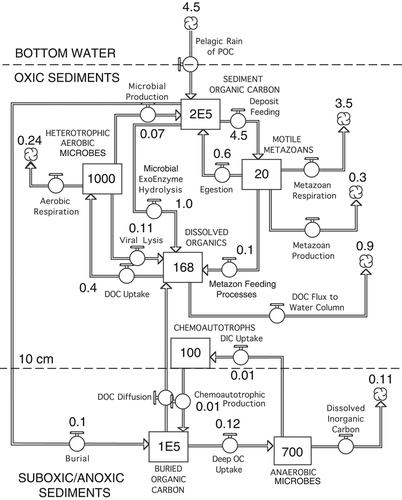
As a first test of this new model, we have relied primarily upon data from the deep Gulf of Mexico (GoM) (Deming & Carpenter Citation2008; Rowe et al. Citation2008b). The available data for the stocks of the food web have been entered into . lists the fluxes that link the stocks. When data from the deep GoM are lacking, we have substituted published information from other abyssal plain or continental rise depths, as indicated. Given the data available, we constructed a differential equation for each stock. At steady state, the fluxes into each stock must equal the fluxes exiting each stock. This system of interdependent coupled equations was then solved simultaneously for steady state, making minor adjustments in rates and stocks as needed to achieve steady state. Thus, represents stock values at a ‘steady state’ solution for the origin and fate of carbon on the Sigsbee Abyssal Plain of the central Gulf of Mexico.
Table I. Stocks and concentrations of food web components in sediments at a ‘general’ abyssal plain/abyssal rise site.
Table II. Rates of exchanges between stocks in food web (); values listed as ‘model-based’ were calculated by solving the model equations for steady state.
Our model is confined to the surface 10 cm of sediment (), but assumes there are exchanges of dissolved and particulate carbon at the sediment–water interface and at the lower boundary 10 cm below the surface. The 10 cm surface layer is assumed to be oxic (Rowe et al. Citation2008a), to contain both aerobic bacteria and metazoans, and to be fairly well mixed by biological activity (Yeager et al. Citation2004). A lower 10 cm boundary layer is assumed to be suboxic or anoxic and to contain no metazoans, but to be populated by anaerobic heterotrophs. Populations of chemoautotrophs are assumed to occupy the boundary layer between the oxic and anoxic sediments. Most biogeochemical models of transformations within a sediment column partition the vertical axis on millimeter to centimeter scales, and include diffusion and mixing (Boudreau Citation1997; Soetaert & van Oevelen Citation2009), but our emphasis is on the relationships between biomass and biological processes rather than physical transfers because our intention is to illustrate how organic matter is consumed, not recreate vertical profiles of concentrations.
The carbon food web model (, and )
Our generic abyssal plain food web ‘location’ is the central Sigsbee Abyssal Plain in the Gulf of Mexico. Five sites have been sampled at depths ranging from approximately 3.4 to 3.65 km. Of these, a central site (JSSD1 or S1) at 3.65 km depth was chosen as our ‘model’ location. Standing stocks (the boxes) of the interacting components, with source citations, were the following: organic carbon in the surface 10 cm: 200,000 mg C m−2 (Yeager et al. Citation2004; Morse & Beazley Citation2008, Santschi & Rowe Citation2008); motile metazoans: 20 mg C m−2 (Rowe et al. Citation2008b); and free-living heterotrophic microbes in the surface 10 cm: 1000 mg C m−2 (Deming & Carpenter Citation2008). We have assumed that viruses are present, but they are not explicitly represented.
We have assumed that the microbes below the surface 10 cm layer include anaerobic heterotrophs and that chemoautotrophs occupy the boundary layer within the chemical gradients between them, as indicated in the model (). However, we cannot accurately estimate the biomasses of the assumed chemoautotrophic functional groups.
The dissolved organic carbon in the model (DOC=168 mg C m−2 in the 0–10 cm layer) is taken from Burdige et al. (Citation1999) at similar water depths in the NE Pacific. This concentration is based on an assumed porosity of 0.7. This value is at the lower end of the range of total DOC values observed at eight locations along the California continental margin (Burdige et al. Citation1999; Burdige Citation2006; see Discussion).
Average SCOC at the GoM abyssal sites was 3.9 mg C m−2 d−1 (Rowe et al. Citation2008a). Burial rates were ca. 0.12 mg C m−2 d−1 (Yeager et al. Citation2004; Santschi & Rowe Citation2008). Microbial respiration was estimated to be ca. 0.242 mg C m−2 d−1, whereas microbial production was 0.075 mg C m−2 d−1 (Deming & Carpenter Citation2008).
Given SCOC and microbial respiration in the surface layer, we estimated by subtraction that the metazoan respiration would be 3.5 mg C m−2 d−1 at steady state. Secondary production of the metazoans was assumed to be 10% of the respiration or 0.3 mg C m−2 d−1. It was assumed that the microbial populations were supported by LDOC that was released from the three sources illustrated in (EEA, viral infection and metazoan feeding) with a total rate of 0.4 mg C m−2 d−1. The DOC would be susceptible to diffusion and mixing along concentration gradients and this would transfer refractory DOC (RDOC) vertically along concentration gradients out of the sediment into the bottom water (Burdige et al. Citation1999; Burdige Citation2007).
The POC flux necessary to support all food web respiration, production and burial was estimated to be ca. 4.5 mg C m−2 d−1. By difference, the feeding rate of the metazoans necessary to support their respiration and growth, plus release of DOC, would be ca. 4.55 mg C m−2 d−1. The deposit feeding rate is higher than the total input value because a portion of the mud is recycled as faeces back into the organic carbon stock. While we assume that the deeper (>10 cm) populations of heterotrophic microbes were active at some level (Deming & Colwell Citation1985), we believe this rate was relatively low compared to the surface layer. We base this assumption on the absence of reduced metabolic end products such as sulfide, reduced Mn or reduced Fe (Rowe et al. Citation2008a) in these abyssal plain sediments. On the other hand, substantial concentrations of ammonium (Christensen & Rowe Citation1984; Rowe et al. Citation2003) have been shown to continue to increase gradually with depth in abyssal plain sediments and oxygen disappeared below 10–20 cm in the pore water (Rowe et al. Citation2003, Citation2008a), indicating that heterotrophic catabolic processes were continuing at depth in the sediment, but at low rates. The downward ‘diffusive flux’ of oxygen, estimated from oxygen profiles, is thought to be a function of sub-surface organic matter consumption by microbes (Glud et al. Citation1994; Wenzhöfer & Glud Citation2002). At our sites on the continental margin, the rates of oxygen consumption based on oxygen profiles (measured ex situ aboard ship on recovered cores) averaged ca. 15% (std. dev. 11%) of the total SCOC (Rowe et al. Citation2008a). The assumption that sub-surface heterotrophy is important is reinforced by observed increases in dissolved inorganic carbon (DIC) concentrations in abyssal sediment pore waters (Burdige et al. Citation1999; Burdige Citation2007). This deep DIC flux thus also corresponds to the ‘diffusive flux’ estimated by Wenzhöfer & Glud (Citation2002), who found that the ratio of total SCOC to diffusive oxygen flux ranged from 3 to 4 in productive waters at shallow sites down to 1 under extremely oligotrophic conditions at deep sites.
The liberation of labile and refractory DOC (, column 1) is set in the model to balance the measured demand of DOC by microbial respiration and growth (0.387 mg C m−2 day−1; Deming & Carpenter Citation2008), as well as the release of RDOC into the bottom water. This production of RDOC and LDOC is divided among the three sources we have suggested: EEA, viral infections and metazoan feeding. The relative importance of the three cannot be estimated at this point.
Discussion
The theoretical deterministic model we have presented suggests that microbial heterotrophs play a more complicated role in the cycling of organic matter in deep-sea sediments than previously portrayed. Deming & Yager (Citation1992) found that microbial DOC utilization responded to POC input as follows: DOC utilization=.0235 + 0.17 (POC Input), r 2=0.88 (n=6). This relationship would predict that microbial carbon utilization in our model would be ca. 0.77 mg C m−2 d−1, or just less than 20% of the total POC utilization. This is about double the measured estimate of total potential heterotrophic carbon utilization at our generic site on the Gulf of Mexico abyssal plain (Deming & Carpenter Citation2008). Likewise, our new rendition here includes deeper utilization based on oxygen penetration (0.12 mg C m−2 d−1), following the reasoning that this represents both chemolithotrophic and heterotrophic microbial oxygen utilization (Glud et al. Citation1994; Wenzhöfer & Glud Citation2002; Rowe et al. Citation2008a). That is, the alternative food web we propose is possible, but we may be underestimating utilization by measuring only DFAA uptake. Regardless of the precise forms of DOC utilized by the microbes, our model demonstrates how the influx of POC can be mobilized in dissolved form, as required for microbial consumption by one or all of the three processes we illustrate. A ‘two-step’ process for organic matter decomposition in sediments has been proposed before by Gaillard & Rabouille (Citation1992), who suggested that complex POC is hydrolysed in the first step to form DOC, making it available for microbial remineralization. Furthermore, this pathway implies that the measurements that have been made of potential microbial respiration and growth are not severe underestimates. Inclusion of other labile compounds in the DOC pool (Khripounoff & Rowe Citation1985; Burdige Citation2006) may increase the value of this pathway. The bound and free amino acids make up about 20–30% of the total DOC at other deep-ocean locations ().
Traditional biogeochemical models of surficial sediments include terms for the physical mixing of solids and the mixing and diffusion of pore water, implying that these mechanisms limit diagenetic processes (Boudreau Citation1997). Our ecological model demonstrates that rates of processes are controlled by the input of particulate organic matter. A strength of our approach is that a mass balance is established between a fairly well-constrained rate-limiting input term and the presumed biological fates of that input, independent of the vertical transfers on a scale of centimeters. Models that include vertical physical transfers by mixing and diffusion are much better than ours at reproducing the variations in concentrations on vertical scales of centimeters (Soetaert et al. Citation2002). However, our mass balance illustrates the biological processes that are responsible for the variations.
A natural puzzle is why the biomass of the microbes is so high, but their potential to remineralize organic carbon remains relatively low. One possibility is that a large fraction of the cells encountered living freely in the sediments or adhering to sediment (clay) particles have been transported there on settling particulates (Deming Citation1985; Turley & Mackie Citation1995), or laterally from the continental margin (Santschi & Rowe Citation2008), and are thus greatly slowed in their activities or inhibited by high pressure and low temperature in this alien environment. Although counts of cells on sinking particles can be low (Vanucci et al. Citation2001), leaving such inactive cells to accumulate slowly, they can also be high (Deming Citation1985). In a static sedimentary layer, these alien bacteria may define the maximum carrying capacity of that environment (Schmidt et al. Citation1998; Vetter et al. Citation1998), preventing displacement by adapted bacteria until the layer is physically disturbed by metazoans or erosional forces. The highest microbial activity will be found, we contend, among the microbial residents in the dynamic gut environment of the deposit feeders (Deming & Colwell Citation1982).
A puzzling feature of depauperate abyssal plain habitats is the high concentration of total DOC in the pore waters, reaching 100 to more than 300 µmol l−1 in the surface 10 cm or so (Burdige et al. Citation1999; Burdige Citation2006), compared to the deep-water column, where concentrations are on the order of 40 µmol l−1 (Williams & Druffel Citation1987). In fact, Burdige et al. (Citation1999) present evidence that the sediment is a significant source of RDOC diffusing back into the deep-water column, based on both DOC profiles in the pore waters and measured fluxes into incubation chambers on the sea floor. Burdige (Citation2006) and others (Hedges Citation1992; Carlson et al. Citation2010) have pointed out that DOC in deep water is one of the largest pools of organic carbon globally. Our model has dealt with labile compounds (LDOC) by definition, an accommodation reflected stoichiometrically by the return of remineralized metabolic byproducts (DIC) into the bottom water, but we have also incorporated a significant flux of RDOC back into the water column based on the measurements of Burdige et al. (Citation1999). It is worth noting that the flux of DOC back into the deep-water column is significant, at ca. 20% of the original POC input. The model thus illustrates potential biological mechanisms that control or contribute to high levels of RDOC accumulating in deep water.
Dissolved free amino acids (DFAAs) are a relatively modest fraction of LDOC in the pore water. Precise profiles of DFFAs have maxima just below the surface few centimeters, but then decline rapidly with depth and toward the sediment–water interface (Macko Citation1992; Pantoja & Lee Citation2003), indicating rapid and complete utilization within the pore water. This pattern differs from the profiles of total DOC in Burdige et al. (Citation1999), which continue to increase, albeit slowly, at depth, indicating little or no further remineralization with depth or time. Much of the total DOC thus appears to be refractory (RDOC) and unavailable to the heterotrophs (Nagata Citation2008), allowing the RDOC fraction (Hedges & Keil Citation1995) to become a component of a slowly increasing deep water DOC reservoir.
Models of food webs can be used to illustrate that the interacting interdependent components of the system are understood. In our case, however, we also want this model to illustrate what is left unknown. If we have underestimated heterotrophic ‘Aerobic Respiration’ in the surface layer because it is based only on DFAA incubations, then the measured ‘DOC Uptake’ would be an underestimate and ‘DOC Flux to the Water Column’ would be an overestimate. Our estimate of DOC flux to the water column (0.9 mg C m−2 d−1) is close to that measured by Burdige et al. (Citation1999), however. Is the ‘diffusive oxygen flux’ (Wenzhöfer & Glud Citation2002; Seiter et al. Citation2005) maintained only by sub-surface metabolism, or is it a better estimate of microbial activity? If the within-sediment consumption of labile DOC in general is a good estimate of the total consumption in surficial sediments, then the mechanisms that release RDOC have been identified. The relative importance of the three mechanisms that liberate the DOC remains speculative, as does the relationship between total oxygen consumption versus the diffusive oxygen flux (DOF). The latter ratio is critical because the DOF alone has been used in estimates of global organic matter remineralization (Seiter et al. Citation2005). Only further study will resolve lurking unknowns in this hypothetical model and its importance to the global carbon cycle.
Editorial responsibility: Tom Fenchel
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Andersson J , Wijsman J , Herman P , Middelburg J , Soetaert J , Heip C 2004 . Respiration patterns in the deep ocean. Geophysical Research Letters 31 , LO3304, 4 pages .
- Boetius , A and Damm , E. 1998 . Benthic oxygen uptake, hydrolytic potentials and microbial biomass at the Arctic continental slope . Deep-Sea Research I , 45 : 239 – 75 .
- Boudreau B. 1997. Diagenetic Models and Their Implementation. Heidelberg: Springer-Verlag. 414 pages.
- Burdige D. 2006. Geochemistry of Marine Sediments. Princeton, NJ: Princeton University Press. 609 pages
- Burdige , D. 2007 . Preservation of organic matter in marine sediments: Controls, mechanisms and an imbalance in sediment organic carbon budgets . Chemical Reviews , 107 : 467 – 85 .
- Burdige , D , Berelson , W , Coale , K , McManus , J and Johnson , K. 1999 . Fluxes of dissolved organic carbon from California continental margin sediments . Geochimica et Cosmochimica Acta , 63 : 1507 – 15 .
- Cammen L. 1989. The relationship between ingestion rate of deposit feeders and sediment nutritional value, In: Lopez G, Taghon G, Levinton J, editors. Ecology of Marine Deposit Feeders, Lecture Notes on Coastal and Estuarine Studies. Berlin: Springer-Verlag, p 200–22
- Carlson , C , Hansell , D , Nelson , N , Siegel , D , Smethie , W Khatiwala , S . 2010 . Dissolved organic carbon export and subsequent remineralization in the mesopelagic and bathypelagic realms of the North Atlantic basin . Deep-Sea Research II , 57 : 1433 – 45 .
- Christensen , JP and Rowe , G. 1984 . Nitrification and oxygen consumption in Northwest Atlantic deep-sea sediments . Journal of Marine Research , 42 : 1099 – 116 .
- Danovaro , R , Dell'Anno , A , Corinaldesi , C , Magagnini , M , Noble , R Tamburini , C . 2008 . Major viral impact on the functioning of benthic deep-sea ecosystems . Nature , 454 : 1084 – 87 .
- Deming , JW. 1985 . Bacterial growth in deep-sea sediment trap and box core samples . Marine Ecology Progress Series , 25 : 305 – 12 .
- Deming , JW and Colwell , RR. 1982 . Barophilic bacteria associated with the digestive tracts of abyssal holothurians . Applied and Environmental Microbiology , 44 : 1222 – 30 .
- Deming , JW and Colwell , RR. 1985 . Observations of barophilic microbial activity in samples of sediment and intercepted particulates from the Demerara Abyssal plain . Applied and Environmental Microbiology , 50 : 1002 – 06 .
- Deming JW, Yager PL. 1992. Natural bacterial assemblages in deep-sea sediments: Towards a global view. In: Rowe G, Pariente V, editors. Deep-Sea Food Chains and the Global Carbon Cycle. Dordrecht: Kluwer, p 11–27
- Deming JW, Baross JA. 1993. The early diagenesis of organic matter: Microbial activity. In: Engel MH, Macko SA, editors. Organic Geochemistry, Vol. 6, Topics in Geobiology. New York, NY: Plenum Press, p 119–44
- Deming , JW and Carpenter , S. 2008 . Factors influencing benthic bacterial abundance, biomass, and activity on the northern continental margin and deep basin of the Gulf of Mexico . Deep-Sea Research II , 55 : 2597 – 606 .
- Dunne J , Sarmiento J , Gnanadesikan A . 2007 . A synthesis of global particle export from the surface ocean and cycling through the ocean interior and on the sea floor . Global Biogeochemical Cycles 21, GB4006. 16 pages .
- Furman , J. 1999 . Marine viruses: Biogeochemical and ecological effects . Nature , 399 : 541 – 48 .
- Gaillard J-F, Rabouille C. 1992. Using Monod kinetics in geochemical models of organic carbon mineralization in deep-sea surficial sediments. In: Rowe G, Pariente V, editors. Deep-Sea Food Chains and the Global Carbon Cycle. Dordrecht: Kluwer, p 309–24
- Glud , R , Gundersen , J , Jorgensen , B , Revsbech , N and Schulz , H. 1994 . Diffusive and total oxygen uptake of deep-sea sediments in the eastern South Atlantic Ocean: In situ and laboratory measurements . Deep-Sea Research , 41 : 1767 – 88 .
- Hedges , J. 1992 . Global biogeochemical cycles: Progress and problems . Marine Chemistry , 39 : 67 – 93 .
- Hedges , JI and Keil , RG. 1995 . Sedimentary organic matter preservation: An assessment and speculative synthesis . Marine Chemistry , 49 : 81 – 115 .
- Heip , C , Duineveld , G , Flach , E , Graf , G , Helder , W Herman , P . 2001 . The role of the benthic biota in sedimentary metabolism and sediment–water exchange processes in the Goban Spur area (NE Atlantic) . Deep-Sea Research II , 48 : 3223 – 43 .
- Khripounoff , A and Rowe , G. 1985 . Les apports organiques et leur transformation en milieu abyssal a l'interface eau–sediment dans 1'Ocean Atlantique tropical . Oceanologica Acta , 8 : 293 – 301 .
- Macko S. 1992. The characterization of organic matter in abyssal sediments, pore waters and sediment traps. In: Rowe G, Pariente V, editors. Deep-Sea Food Chains and the Global Carbon Cycle. Dordrecht: Kluwer Academic Publishers, p 325–38
- Mahaut , M , Sibuet , M and Shirayama , Y. 1995 . Weight-dependent respiration rates in deep-sea organisms . Deep-Sea Research I , 42 : 1575 – 82 .
- Morse , J and Beazley , M. 2008 . Organic matter in deepwater sediments of the Northern Gulf of Mexico and its relationship to the distribution of benthic organism . Deep-Sea Research II , 55 : 2563 – 71 .
- Nagata T. 2008. Organic matter-bacteria interactions in seawater. In: Kirchman D, editor. Microbial Ecology of the Oceans, 2nd edn, New York, NY: Wiley, p 207–41
- Newell , S , Fell , J , Statzell-Tallman , A , Miller , C and Cefalu , R. 1984 . Carbon and nitrogen dynamics in decomposing leaves of three coastal marine vascular plants of the subtropics . Aquatic Botany , 19 : 183 – 92 .
- Pantoja , S and Lee , C. 2003 . Amino acid remineralization and organic matter lability in Chilean coastal sediments . Organic Geochemistry , 34 : 1047 – 56 .
- Pfannkuche , O , Theeg , R and Thiel , H. 1983 . Benthos activity, abundance and biomass under an area of low upwelling off Morocco, Northwest Africa . ‘Meteor’ Forschung-Ergebnisse , 36 : 85 – 96 .
- Piepenburg , D , Blackburn , T , von Dorrien , C , Gutt , J , Hall , P Hulth , S . 1995 . Partitioning of benthic community respiration in the Arctic (northwest Barents Sea) . Marine Ecology Progress Series , 118 : 199 – 213 .
- Plante , CJ , Jumars , PA and Baross , JA. 1990 . Digestive associations between marine detritivores and bacteria . Annual Review of Ecology and Systematics , 21 : 93 – 127 .
- Pomeroy , L , Wiebe , W , Deibel , D , Thompson , R , Rowe , G and Pakulski , JD. 1991 . Bacterial responses to temperature and substrate concentration during the Newfoundland spring bloom . Marine Ecology Progress Series , 75 : 143 – 59 .
- Reimers , C , Kalhorn , S , Emerson , S and Nealson , K. 1984 . Oxygen consumption rates in pelagic sediments from the central Pacific: First estimates from microelectrode profiles . Geochimica et Cosmochimica Acta , 48 : 903 – 10 .
- Relexans , J-C , Deming , J , Dinet , A , Gaillard , J-F and Sibuet , M. 1996 . Sedimentary organic matter and micro-meiobenthos with relation to trophic conditions in the tropical northeast Atlantic . Deep-Sea Research I , 43 : 1343 – 68 .
- Richardson , M and Young , D. 1987 . Abyssal benthos of the Venezuela Basin, Caribbean Sea: Standing stock consideration . Deep-Sea Research , 34 : 145 – 64 .
- Rowe , G and Deming , J. 1985 . The role of bacteria in the turnover of organic carbon in deep-sea sediments . Journal of Marine Research , 43 : 925 – 50 .
- Rowe , G , Boland , G , Escobar Briones , EG , Cruz-Kaegi , ME , Newton , A Piepenburg , D . 1997 . Sediment community biomass and respiration in the northeast water polynya, Greenland: A numerical simulation of benthic lander and spade core data . Journal of Marine Systems , 10 : 497 – 515 .
- Rowe G, Lohse A, Fain Hubbard G, Boland G, Escobar Briones E, Deming J. 2003. Preliminary trophodynamic carbon budget for the Sigsbee Deep Benthos, Northern Gulf of Mexico. In Stanley D, Scarborough-Bull A, editors. Fisheries, Reefs and Offshore Development, American Fisheries Society Symposium 36. Bethesda, MD: American Fisheries Society, p 225–38
- Rowe , G , Morse , J , Nunnally , C and Boland , G. 2008a . Sediment community oxygen consumption in the deep Gulf of Mexico . Deep-Sea Research II , 55 : 2686 – 91 .
- Rowe , G , Wei , C , Nunnally , C , Haedrich , R , Montagna , P Baguley , J . 2008b . Comparative biomass structure and estimated carbon flow in food webs in the deep Gulf of Mexico . Deep-Sea Research II , 55 : 2699 – 711 .
- Santschi , P and Rowe , G. 2008 . Radiocarbon-derived sedimentation rates in the Gulf of Mexico . Deep-Sea Research II , 55 : 2572 – 76 .
- Schmidt , JL , Deming , JW , Jumars , PA and Keil , RG. 1998 . Constancy of bacterial abundance in surficial marine sediments . Limnology and Oceanography , 43 : 976 – 82 .
- Seiter K , Hensen C , Zabel M . 2005 . Benthic carbon mineralization on a global scale . Global Biogeochemical Cycles 19 , GB1010. 26 pages .
- Smith , D , Simon , M , Alldredge , A and Azam , F. 1992 . Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution . Nature , 359 : 139 – 42 .
- Smith , KL Jr. 1992 . Benthic boundary layer communities and carbon cycling at abyssal depths in the central North Pacific . Limnology and Oceanography , 37 : 1034 – 56 .
- Smith KL Jr, Hinga K. 1983. Sediment community respiration in the deep sea. In: Rowe G, editor. The Sea, vol. 8, Deep-Sea Biology. New York, NY: Wiley, p 331–70
- Soetaert , K and van Oevelen , D. 2009 . Modeling food web interactions in benthic deep-sea ecosystems: A practical guide . Oceanography , 22 : 128 – 43 .
- Soetaert , K , Declippele , V and Herman , P. 2002 . FEMME, a flexible environment for mathematically modeling the environment . Ecological Modelling , 151 : 177 – 93 .
- Tietjen , J , Deming , JW , Rowe , G , Macko , S and Wilke , R. 1989 . Meiobenthos of the Hatteras Abyssal Plain and Puerto Rico Trench: Abundance, biomass and associations with bacteria and particulate fluxes . Deep-Sea Research , 36 : 1567 – 77 .
- Turley , C and Mackie , P. 1995 . Bacterial and cyanobacterial flux to the deep NE Atlantic ocean on sedimenting particles . Deep-Sea Research I , 42 : 1453 – 74 .
- Vannuci , S , Dell'Anno , A , Pusceddu , A , Fabiano , M , Lampitt , R and Danovaro , R. 2001 . Microbial assemblages associated with sinking particles in the Porcupine Abyssal Plain (NE Atlantic Ocean) . Progress in Oceanography , 50 : 105 – 21 .
- Vetter , Y-A , Deming , JW , Jumars , PA and Krieger-Brockett , BB. 1998 . A predictive model of bacterial foraging by means of freely-released extracellular enzymes . Microbial Ecology , 36 : 75 – 92 .
- Wenzhöfer , F and Glud , R. 2002 . Benthic carbon mineralization in the Atlantic: A synthesis based on in situ data from the last decade . Deep-Sea Research I , 49 : 1255 – 79 .
- Williams , P and Druffel , E. 1987 . Radiocarbon in dissolved organic matter in the central North Pacific Ocean . Nature , 330 : 246 – 48 .
- Witte , U , Wenzhöfer , F , Sommer , S , Boetius , A , Heinz , P Aberle , N . 2003 . In situ experimental evidence of the fate of a phytodetritus pulse at the abyssal sea floor . Nature , 424 : 763 – 66 .
- Yeager , KM , Santschi , PH and Rowe , G. 2004 . Sediment accumulation and radionuclide inventories (239,240Pu, 210Pb and 234Th) in the northern Gulf of Mexico, as influenced by organic matter and macrofaunal density . Marine Chemistry , 91 : 1 – 14 .