Abstract
Mate choice by females is influenced by male size, resource provisioning and other proxies for male quality. In decapod crustaceans, mating dynamics are complicated by size-dependent sperm limitation associated with unusually low sperm :egg ratios. We explored how mate size influences mating dynamics in the spotted spiny lobster (Panulirus guttatus), a philopatric species that dwells on shallow and often isolated coral reefs in the Caribbean where choice of mates can be limited. We varied the availability and size of male and female lobsters in a series of laboratory experiments and then quantified courtship behaviour, mate choice and fertilization success of each mating. We found that large males initiated most interactions with other males and won 99% of those encounters. Large males were also more successful in garnering mates, but males of all sizes attempted to mate with all sizes of females. Females nearly always (92% of trials) chose males larger than themselves. However, if large males were unavailable, females mated with smaller males, which resulted in reduced fertilization success. Thus, for species like P. guttatus that dwell in patchy habitats with limited mate availability, the optimal strategy for mate choice is context-dependent, although not without cost to the largest females.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
In sexually reproducing species that pair to mate, mate choice can profoundly influence reproductive success and thus their inclusive fitness (Trivers Citation1972). Mate selection or ‘choosiness’ depends on variance in mate quality, the cost of breeding (e.g. gamete production, risk of injury during mating or searching for mates, and care of offspring), and the consequences of delayed mate choice relative to the benefits (Gibson & Langen Citation1996; Kokko & Monaghan Citation2001). Competition for mates may also ensue and is influenced primarily by operational sex ratio and variability in mate quality (Kokko & Monaghan Citation2001). Differences between the sexes in these attributes can result in conflicting optimal strategies for mating and thus complex mating dynamics.
Typically, inequalities in gamete investment or care of offspring results in females being more selective in their choice of mates than their male counterparts (Watt et al. Citation1986; Dugatkin Citation1992; Reynolds Citation1996; Censky Citation1997; Drickamer et al. Citation2000). However, the costs to males of female selection is not trivial and can be detrimental to male health and reproductive success (Dewsbury Citation1982; see also the review by Wedell et al. Citation2002). Reflecting this cost, the reproductive success of males can become limited by the production of sperm (Nakatsuru & Kramer Citation1982; Wedell et al. Citation2002; Dunn et al. Citation2006; Sato et al. Citation2006; Sato & Goshima Citation2006). In such instances, males too become choosy when selecting mates, depending on the operational sex ratio and female promiscuity (Dewsbury Citation1982; Wedell et al. Citation2002). Mating dynamics can be further complicated by habitat patchiness or size-selective harvesting by humans, factors that change local demographics and thereby influence operational sex ratios and the availability of quality mates (Sato et al. Citation2006; Sato & Goshima Citation2006; Robertson & Butler Citation2009).
Many of these dynamics play out in the mating systems of decapod crustaceans (i.e. shrimps, crabs, and lobsters). Although decapod mating systems vary considerably in their detail, they share two important attributes: males and females must couple to mate and sperm : egg ratios are typically low (Lipcius Citation1985; Jivoff Citation1997; Baeza Citation2007; Butler et al. Citation2011). Fisheries for economically valuable decapods, many of which target larger individuals, not only diminish the reproductive capacity of exploited populations but also fundamentally alter their mating systems (DeMartini et al. Citation1993; MacDiarmid & Butler Citation1999; Kendall et al. Citation2002). The loss of large females is detrimental to the reproductive capacity of decapod populations subject to fishing because of the exponential relationship between female size and fecundity (MacDiarmid & Sainte-Marie Citation2006). Often under-appreciated is the importance of large males in ensuring the reproductive success of decapod crustaceans: large males dominate matings, have greater sperm stores, and can replenish those sperm stores faster than can small males (Jivoff Citation1997; MacDiarmid & Butler Citation1999; Kendall et al. Citation2001). Thus, human exploitation can disrupt the delicate balance of decapod mating systems by altering population size structure and therefore fertilization success. Similar effects have also been observed for populations in fragmented or patchy habitats where individuals can be few and size structure unpredictable (Robertson & Butler Citation2009).
Such is the case for Panulirus guttatus (Latreille, 1804), the spotted spiny lobster, which occurs in shallow coral reef habitats throughout the Caribbean, Florida and Bermuda (Holthius Citation1991). Postlarvae of this strongly philopatric species settle directly on reefs and as adults rarely leave the reef areas on which they dwell (Sharp et al. Citation1997; Lozano-Alvarez et al. Citation2002; Robertson & Butler Citation2009). As a result of sporadic settlement, populations of P. guttatus on isolated patch reefs can develop widely varying size structures and sex ratios, resulting in more variable reproductive success among small, fragmented populations (Robertson & Butler Citation2009).
Here we investigate the potential mechanisms that underlie differences in reproductive success among small populations of P. guttatus by detailing the mating dynamics of males and females of various sizes. Where mates are readily available, we hypothesized that female P. guttatus would choose the largest male available to ensure high fertilization success. Where large males are not available, we predicted that females would nonetheless mate with smaller males, but would suffer lower fertilization success as a result of sperm limitation.
Methods
Our laboratory experiments were conducted in the Florida Keys, Florida (USA) from March to June 1997–2000, which is the peak reproductive season for Panulirus guttatus in Florida. Lobsters were haphazardly collected by divers from nearby reefs and were returned to those reefs after experimentation. We captured new lobsters each year for our experiments and released them at the end of the reproductive season. In all we utilized over 120 lobsters (approximately equal numbers of males and females) for our experiments spanning four reproductive seasons. Lobsters were used for a season and mixed among experiments, although never more than once in a particular experiment. All experiments were conducted in round, polyurethane tanks (1.5 m diameter, 1.2 m deep) equipped with aeration and flow-through seawater. The tanks were outdoor, so seawater temperature (16–31°C during study) and photoperiod varied naturally. Carapace length (CL), measured to the closest mm from the rostrum edge between the eyes to the junction of the cephalothorax and abdomen, was used as a standard gauge of lobster size. Panulirus guttatus males and females exhibit different sizes at maturity and different maximum sizes. Females mature at 32 mm CL, whereas males do not mature until 38 mm CL (Robertson & Butler Citation2003). Fecundity for females varies with size, increasing from about 40,000 eggs per clutch at first maturity to over 100,000 eggs per clutch at maximum size (Robertson & Butler Citation2003). Maximum size for males is near 70 mm CL, whereas females reach only about 60 mm CL. Thus for our experiments, male and female lobsters were grouped into small, medium and large size classes based on size at maturity. For females the size groupings were small (S; 30–45 mm CL), medium (M; 45.1–55 mm CL) and large (L; 55.1+mm CL) and for males the size groupings were small (S; 40–50 mm CL), medium (M; 50.1–60 mm CL) and large (L; 60.1+mm CL).
Mating constraint experiment
To test the hypothesis that the formation of mating pairs is not physically constrained by male : female size differences, we placed a single small, medium or large female lobster in each tank with either a single small or large male. Unmated females release unfertilized eggs that fail to attach to their pleopods, so we used this as evidence that females were unable to mate with the male present. We checked the females each day for the presence of a spermatophore (i.e. an externally visible sperm packet) or attached eggs for evidence of successful mating. If a spermatophore or eggs were present, the size of the male with which the female mated was recorded, and the egg mass was removed once eyespots within the eggs were visible (~10–14 days after extrusion). Egg masses were removed by securing each female ventral side up on a foam-covered board and gently scraping the eggs off the female's pleopods with a scalpel. To determine fecundity, the entire egg mass was weighed to the nearest 0.0001 g, then the number of eggs in three samples, each weighing 0.02–0.04g, were counted using a dissecting microscope. For each sample the total number of eggs was calculated as:
The mean fecundity was then calculated for all three clutch samples to arrive at a single estimate per female.
Competition, courtship, and mate choice experiments
We evaluated male competition for mates, courtship behaviour and mate choice in laboratory experiments using three different experimental scenarios manipulating adult lobster size: (1) small females and large males, (2) small males and large females and (3) one small, one medium and one large individual of each sex. We used scenarios 1 and 2 (i.e. skewed population size structure) to test for mate choice and male–male competition when mate sizes were suboptimal. In scenario 3, male and female sizes were evenly distributed (one S, M, and L individual per sex per experimental tank); therefore, the test was of courtship preference by both males and females, male competition, and mate choice when a range of sizes were available for each sex. In preliminary trials, we attempted to eliminate the effect of male competition on female mate choice by tethering males to separate shelters, but no courtship or mating occurred so males were not tethered in the formal trials. Instead, we collected data on male competitive behaviour in addition to data collected on courtship and mating.
For each trial, three females were placed into a single tank each morning and at nightfall; we added three males and observed their behaviour. We used different lobsters each day in different population size-structure scenarios (i.e. scenario 1, 2 or 3) to ensure independence of the trials. Observations were recorded in 5-min intervals and continued until no mating interactions had occurred for 0.5 h. At the end of the experiment all animals were removed from the experimental tank and placed into separate-sex holding tanks. During the experiment we recorded: (1) the number of competitive (i.e. aggressive) interactions and frontal approaches (i.e. courtship); (2) which lobster courted and which was approached; (3) which lobster terminated a courtship event; and (4) mating. We also used underwater time-lapse video at night using infrared illumination to record mating behaviour over 1–3-day periods utilizing the same population size-structure treatments. The same data (noted above) were recorded for the live and videotape observations.
Contingency table analyses utilizing raw values were used to test for differences in competitive behaviour, courtship and mate choice. To test for male competition (3×2 contingency table), we examined the independence of male size (three size classes: S, M, L) versus the outcome of male–male interactions, based on whether a male ‘won’ or ‘lost’ the interaction they initiated. The ‘loser’ was the lobster that backed away from the encounter first. We also conducted two separate analyses of courtship: male preference of female size, and female response to male courtship. Again, lobsters of three size classes (S, M, L) were examined, and we tested whether male courtship (i.e. frontal approach; Lipcius Citation1985) of females was independent of male and female size (3×3 contingency table). We examined female response to courtship by males by testing (2×3 contingency table) for differences in female response (female retreats, male retreats, mating) by size class (S, M, L). Finally, by dividing both males and females into size classes (S, M, L) we tested for female preference for male mate size (3×3 contingency table).
Mate choice
To more closely examine mate choice under uniform size distributions, we placed three males (small 40–50 mm CL, medium 50.1–60 mm CL, and large 60.1 + mm CL) and three females (small 30–45 mm CL, medium 45.1–55 mm CL, and large 55.1 + mm CL) into a tank and determined mate choice from the pairings. Although mate choice can be determined directly from visual observations of mating events, matings are rare enough that this requires many hours of observations. We used an indirect but more efficient means of assessing mate choice. Using quick-dry marine epoxy, we blocked the right gonopore of small males, the left gonopore of medium males, and neither gonopore of the large males. All females were checked for the presence of a spermatophore each morning and, based on the position of the spermatophore deposited on the female, the male with whom she mated could be identified. Mated females were placed in individual holding tanks until eyespots appeared on their eggs and fecundity determined as described above. We combined the results of this experiment with the previous data on mate choice and analysed these data as described above.
Sperm limitation
To examine the potential for sperm limitation of fertilization success in Panulirus guttatus, we conducted mating experiments with males that were at least 20 mm CL smaller than the females with whom they mated. We compared the results of these matings with those where males were larger. Each day the females were checked for the presence of a spermatophore or eggs. Once a spermatophore was deposited, the female was removed from the tank and the resulting egg mass was removed, weighed and counted as described above to estimate the number of fertilized eggs. Similar data from matings of various male and female sizes derived from the experiments described earlier (mate constraints, mate competition, mate choice) were also used in this analysis. To test for the effect of both male and female size on fecundity we used male size class and female size class as factors and the number of eggs fertilized as the dependent variable in a 3×3 model I ANOVA.
Results
Mating constraints
Size posed no constraint to mating among adult male and female Panulirus guttatus. All combinations of small, medium and large males and females coupled successfully and those couplings resulted in the transfer of a spermatophore and production of an egg mass. However, patterns of courtship and the resultant fecundity of these matings differed among size combinations, as described below.
Male competition
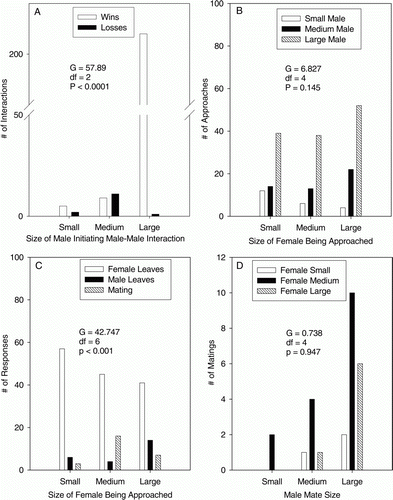
We investigated competitive hierarchies among males by testing for differences between the size of the male initiating a male–male interaction and whether the male lost or won the interaction by retreating or not. Large males initiated significantly more (88%) competitive interactions with other males and won significantly more of those interactions (99%) (A; G=57.89; df=2; P<0.0001).
Figure 1. The four panels summarize the results from male-male competitive interactions, male-female courtship interactions, and mating by Panulirus guttatus. The G-statistic, degrees of freedom (df) and p-value for each corresponding contingency test are displayed within each panel. (A) Results from a male–male competition experiment testing whether the winner of the contest was independent of male size. (B) Results of courtship interactions testing whether courtship by males differed among males and females of different sizes. (C) Results of female response to courtship by males of different sizes. (D) Results of mate choice testing whether different size classes of females preferred to mate with males of a particular size.
Courtship
We found no relationship between the size of the male initiating courtship and the size of the female being courted (B; G=6.827; df=4; P=0.145). Large males initiated 64.5% of the courtships, but did not preferentially court females of a particular size.
Mate choice
However, female response to courtship varied significantly depending on female size (C; G=27.150, df=4, P<0.0001). As female size increased, they were less likely to flee from courting males and drove off more males. Females in the medium size class mated more often (61.5%) than either large or small females. However, females of all sizes mated significantly more often with larger males (D; G=8.462, df=2, P=0.015). Nearly 70% of the matings were with large males and in 92% of all matings, the male was larger (1–20 mm CL) than the female.
Sperm limitation
The number of eggs fertilized per brood differed significantly with female size and male size (, ). Male size did not impact the fecundity of all females, but lower levels of fertilization were observed when large females (≥55 mm CL) mated with small males (≤45 mm CL; ).
Figure 2. The effect of female size and male size on fecundity (no. eggs/clutch) of Panulirus guttatus. ANOVA results indicate that fecundity depends on both male and female size; specifically, matings between large females and small males produced significantly fewer eggs.
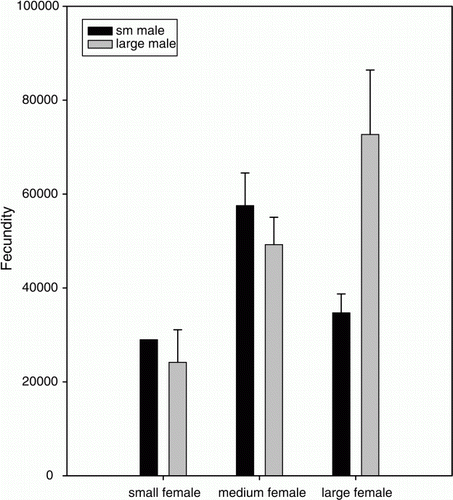
Table I. Two-factor model I ANOVA testing the effect of female size and male size on single-clutch fecundity of Panulirus guttatus. Individuals were combined into size classes based on size at reproduction for analysis.
Discussion
Our results suggest that male size and the potential for sperm limitation strongly influence female choice in Panulirus guttatus, although females can and will mate with small males if need be. This is presumably an adaptive strategy useful in ensuring some measure of reproductive success even when females are constrained within small, isolated wild populations where their choice of males can be limited. In our experiments, the largest available males typically initiated the most aggressive interactions with other males and courtship attempts with females, but this did not entirely prevent courtship and mating by smaller males. Females preferred larger males as mates, but not always the largest male available. Although we found no constraints to mating between individuals of disparate sizes, the cost to large females who mated with small males was a reduction in fecundity.
The literature on spiny lobsters is contradictory with respect to the presence of male choice of females based on their size, even within the same species. McKoy (Citation1979) and MacDiarmid (Citation1989) conducted field studies of Jasus edwardsii in New Zealand and reported that males establish a dominance hierarchy that determines who courts and mates; which is usually the largest male present. The same has been reported in laboratory studies of P. argus (Lipcius Citation1985; Butler et al. Citation2011). Other studies of J. edwardsii (Kensler Citation1967), P. cygnus (Morgan Citation1972), P. marginatus (DeMartini et al. Citation1993) and P. argus (Izquierdo et al. Citation1987; MacDiarmid & Butler Citation1999; Butler et al. Citation2011) found that males are not particularly choosy with respect to female size. In these species, males appear to gain greater reproductive success simply by increasing the number of matings. Our results for P. guttatus are similar. We found that male courtship of female P. guttatus was independent of female size (B). Large, male P. guttatus were significantly more aggressive than small males (A) and dominated the total number of matings, but they did not prefer one size of female over another (D). Only the smallest males favoured small females (B).
The inconsistencies in male mate preference results in the literature, both within and among species, is likely due to differences in test conditions. Some studies were conducted in laboratory tanks (e.g. Lipcius Citation1985; our study) where small males and females cannot emigrate to avoid aggression by large males. Under conditions where mating populations are isolated, as in laboratory tanks or patches of habitat in the wild (e.g. isolated coral patch reefs where P. guttatus often occurs), sexual conflict may be exacerbated. Indeed, recent theoretical work indicates that in closed populations aggression is strongly correlated with mating success because small males are repeatedly bullied and females cannot disperse (Eldaker et al. Citation2009). However, laboratory studies and studies conducted within marine reserves provide information on natural mating systems in ‘intact’ lobster populations whose size structure has not been truncated by fishing. However, few natural populations of spiny lobster remain because of intense fishing on these commercially valuable species, and mating dynamics in most present-day spiny lobster populations therefore differ, as revealed in studies of their mating dynamics (MacDiarmid & Sainte Marie Citation2006; Butler et al. Citation2011). Populations impacted by fishing contain few large males or females, and are dominated by size classes of individuals just below the minimum legal size, at least half or more of which are not mature because most minimum sizes are set at the size at which 50% are believed to be reproductive.
The present study of P. guttatus conducted under laboratory conditions included mature males and females across the full range of sizes, although of equal proportion. Subordinate individuals of course could not emigrate from the tank, but neither do they do so on small patch reefs, where they can admittedly move away and hide on the reef to curtail direct conflict. In the Florida Keys where our study took place, P. guttatus is not fished as it is elsewhere, so our results are more likely to reflect mating conditions in ‘natural’ populations of P. guttatus and probably not those indicative of heavily fished areas.
Female P. guttatus took an active role in the mating dynamics that we observed in our experiments. Females were often approached by males of all sizes and, in response, the females usually backed away, terminating the interaction. Females never mated with the first male to approach. Successful matings were commonly preceded by multiple approaches by courting males, suggesting that females actively choose mates from those available. The largest male in the tank was usually the most successful, and the choice of females in 69.2% of all the matings we observed. This suggests that sperm limitation may be a factor in the choice of mates by female P. guttatus.
Sperm limitation often results from sperm depletion, a consequence of repeated mating over short periods of time (Nakatsuru & Kramer Citation1982; Wedell et al. Citation2002; Dunn et al. Citation2006). We did not test for sperm depletion per se. Once mating occurred in our experiments, all males were removed from the tank and replaced with new, untested males. Still, our study revealed that sperm limited fertilization success occurs during a single mating in P. guttatus when small males with apparently insufficient sperm stores mated with large females. Like P. argus (MacDiarmid & Butler Citation1999), female P. guttatus of a given size extrude a more or less consistent number of eggs per clutch and only those fertilized are retained in the female's clutch. Female spiny lobsters mate only once per clutch (Butler et al. Citation2011), so sperm accumulation from several matings is not possible. Low sperm : egg ratios in decapod crustaceans, including spiny lobsters, make them susceptible to sperm limitation, particularly when large females are mated by small males. This result appears robust across decapod taxa (MacDiarmid & Butler Citation1999; Kendall et al. Citation2001; Sato & Goshima Citation2006; Sato et al. Citation2006), and now includes data on P. guttatus.
Spermatophore size and female size were uncorrelated when females were mated with larger males, indicating that male P. guttatus do not allocate sperm based on female size (unpubl. data), a phenomenon demonstrated by large male P. argus and J. edwardsii (MacDiarmid & Butler Citation1999; Mauger Citation2001) and in other taxa (Wedell et al. Citation2002). Given that small male P. guttatus cannot fertilize the entire egg clutch produced by large females, sperm abundance can limit reproductive success for at least a portion of the population. It is curious then that large males do not partition their sperm stores. We hypothesize that the nearly year-round mating season of P. guttatus (Sharp et al. Citation1997; Briones-Fourzan & Contreras-Ortiz Citation1999; Robertson & Butler Citation2009), compared to the narrow seasonal mating duration of P. argus and J. edwardsii, may preclude the need for multiple matings during a short period of time and thus diminish selection for sperm allocation per mating.
Our findings about mating dynamics and the potential for sperm limitation in P. guttatus also have implications for local fisheries on P. guttatus, which are increasing in the Caribbean as fisheries for P. argus, its congener in the region, decline (Ehrhardt et al. Citation2010). As in other fisheries, the abundance of larger P. guttatus would be expected to decline as fishing increases, but only if fishing pressure were sex-biased, targeting large males, would one expect sperm limitation of reproductive success. However, there is remarkably little information on or management of the largely artisinal P. guttatus fisheries in the Caribbean.
Lobster research in the Caribbean is dominated by studies of the Caribbean spiny lobster P. argus the target of major fisheries in the region (Ehrhardt et al. Citation2010; Chávez Citation2009). Yet, as our understanding of the mating dynamics and ecology of P. guttatus grows, what is emerging is a picture of a species quite unique from its better known congenor. Panulirus guttatus is a highly residential species that settles and remains on individual patch reefs for long periods of time, perhaps their entire lives (Sharp et al. Citation1997; Robertson & Butler Citation2009). The size structure of P. guttatus populations on individual reefs is highly dependent on postlarval supply and varies among reefs, creating equally variable patterns of reproductive success, especially on small isolated patch reefs (Robertson & Butler Citation2009). It is under these conditions – small populations with unpredictable size structures and sex ratios – that context-dependent mate selection has evolved and serves to minimize disruption of reproductive success and the potential for Allee effects.
Editorial responsibility: Franz Uiblein
Acknowledgements
We would like to thank our colleagues C. Acosta, J. Schratwieser, D. Behringer, W. Sharp, and R. Bertelsen for their assistance with the field work, along with A. MacDiarmid and B. Herrnkind for advice. This study was supported by awards to M. Butler from the US National Science Foundation (OCE-9730195), NOAA National Undersea Research Program (9913), and Florida Sea Grant (R/LR-B-38).
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Baeza , JA. 2007 . Sex allocation in a simultaneously hermaphroditic marine shrimp . Evolution , 61 : 2360 – 73 .
- Briones-Fourzan , P and Contreras-Ortiz , C. 1999 . Reproduction of the spiny lobster Panulirus guttatus (Decapoda:Palinuridae) on the Caribbean coast of Mexico . Journal of Crustacean Biology , 19 : 171 – 79 .
- Butler , MJ IV , Heisig-Mitchell , J , MacDiarmid , AB and Swanson , J. 2011 . The effect of male size and spermatophore characteristics on reproduction in the Caribbean spiny lobster, Panulirus argus . New Frontiers in Crustacean Biology , 15 : 69 – 84 .
- Chávez , E. 2009 . Potential production of the Caribbean spiny lobster (Decapoda, Palinura) fisheries . Crustaceana , 82 : 1393 – 412 .
- Censky , EJ. 1997 . Female mate choice in the non-territorial lizard Ameiva plei (Teiidae) . Behavioral Ecology and Sociobiology , 40 : 221 – 25 .
- DeMartini , ED , Ellis , DM and Honda , VA. 1993 . Comparisons of spiny lobster Panulirus marginatus fecundity, egg size, and spawning frequency before and after exploitation . Fishery Bulletin US , 91 : 1 – 7 .
- Dewsbury , DA. 1982 . Ejaculate cost and male choice . The American Naturalist , 119 : 601 – 10 .
- Drickamer , LC , Gowaty , PA and Holmes , CM. 2000 . Free female mate choice in house mice affects reproductive success and offspring viability and performance . Animal Behavior , 59 : 371 – 78 .
- Dugatkin , LA. 1992 . Sexual selection and imitation: Females copy the mate choice of others . The American Naturalist , 139 : 1384 – 89 .
- Dunn , AM , Andrews , T , Ingrey , H , Riley , J and Wedell , N. 2006 . Strategic sperm allocation under parasitic sex-ratio distortion . Biology Letters , 2 : 78 – 80 .
- Eldakar , OT , Dlugos , MJ , Pepper , JW and Wilson , DS. 2009 . Population structure mediates sexual conflict in water striders . Science , 326 : 816
- Ehrhardt , NM , Puga , R and Butler , MJ IV. 2010 . “ Large ecosystem dynamics and fishery management concepts: The Caribbean spiny lobster, Panulirus argus, fisheries ” . In Towards Marine Ecosystem-Based Management in the Wider Caribbean , Edited by: Fanning , L , Mahon , R and McConne , P . 157 – 75 . Amsterdam : Amsterdam University Press .
- Gibson , RM and Langen , TA. 1996 . How do animals choose their mates? . Trends in Ecology and Evolution , 11 : 468 – 70 .
- Holthuis , LB. 1991 . Marine lobsters of the world: An annotated and illustrated catalogue of species of interest to fisheries known to date , Vol. 13 , 292 Rome : FAO . FAO Fish Symposium no. 125
- Izquierdo RC , Alvarez JAB , Iglesias ED , Perez RB , Diaz CG , Aviles WB , et al. 1987 . Biologico-perquero de la langosta en el archipielago Cubano . Atlas 1 – 125 .
- Jivoff , P. 1997 . The relative roles of predation and sperm competition on the duration of the post-copulatory association between the sexes in the blue crab, Callinectes sapidus . Behavoral Ecology and Sociobiology , 40 : 175 – 85 .
- Kendall , MS , Wolcott , DL , Wolcott , TB and Hines , AH. 2001 . Reproductive potential of individual male blue crabs, Callinectes sapidus, in a fished population: Depletion and recovery of sperm-number and seminal fluid . Canadian Journal of Fisheries and Aquatic Sciences , 58 : 1168 – 77 .
- Kendall , MS , Wolcott , DL , Wolcott , TG and Hines , AE. 2002 . Influence of male size and mating history on sperm content of ejaculates of the blue crab, Callinectes sapidus . Marine Ecology Progress Series , 230 : 235 – 40 .
- Kensler , CB. 1967 . Fecundity in the marine spiny lobster Jasus verreauxi (H. Milne Edwards) (Crustacea: Decapoda: Palinuridae) . New Zealand Journal of Marine Freshwater Research , 2 : 143 – 55 .
- Kokko , H and Monaghan , P. 2001 . Predicting the direction of sexual selection . Ecology Letters , 4 : 159 – 65 .
- Lipcius , RN. 1985 . “ Size-dependent reproduction and molting in spiny lobsters and other long-lived decapods ” . In Crustacean Issues 3: Factors in Adult Growth , Edited by: Wenner , AM . 129 – 48 . Boston , MA : AA Balkema Press .
- Lozano-Alvarez , E , Carrasco-Zanini , G and Briones-Fourzan , P. 2002 . Homing and orientation in the spotted spiny lobster, Panulirus guttatus (Latreille, 1804), towards a subtidal coral reef habitat . Crustaceana , 75 : 859 – 73 .
- MacDiarmid , AB. 1989 . Size at onset of maturity and size-dependent reproductive output of female and male spiny lobsters Jasus edwardsii (Hutton)(Decapoda, Palinuridae) in northern New Zealand . Journal of Experimental Marine Biology and Ecology , 127 : 229 – 43 .
- MacDiarmid , AB and Butler , MJ IV. 1999 . Sperm economy and limitation in spiny lobsters . Behavioral Ecology and Sociobiology , 146 : 14 – 24 .
- MacDiarmid , AB and Sainte-Marie , B. 2006 . “ Reproduction ” . In Lobsters: Biology, Management, Aquaculture and Fisheries , Edited by: Phillips , B . 45 – 77 . Oxford : Blackwell Publishing .
- Mauger JW. 2001 . Sperm depletion and regeneration in the spiny lobster Jasus edwardsii . MSc thesis , University of Auckland , New Zealand . 87 pages .
- McKoy , JL. 1979 . Mating behaviour and egg laying in captive rock lobster, Jasus edwardsii (Crustacea: Decapoda: Palinuridae) . New Zealand Journal of Marine Freshwater Research , 13 : 407 – 13 .
- Morgan , GR. 1972 . Fecundity in the Western Rock Lobster Panulirus longipes cygnus (George)(Crustacea: Decapoda: Palinuridae) . Australian Journal of Marine and Freshwater Research , 23 : 133 – 41 .
- Nakatsuru , K and Kramer , DL. 1982 . Is sperm cheap? Limited male fertility and female choice in lemon tetras (Pisces, Characidae) . Science , 216 : 753 – 55 .
- Reynolds , JD. 1996 . Animal breeding systems . Trends in Ecology and Evolution , 11 : 68 – 72 .
- Robertson , DN and Butler , MJ IV. 2003 . Growth and size at maturity in the spotted spiny lobster, Panulirus guttatus . Journal of Crustacean Biology , 23 : 265 – 72 .
- Robertson , DR and Butler , MJ IV. 2009 . Variable reproductive success in isolated populations of lobster . Journal of Experimental Marine Biology and Ecology , 377 : 84 – 92 .
- Sato , T , Ashidate , M , Jinbo , T and Goshima , S. 2006 . Variation of sperm allocation with male size and recovery rate of sperm numbers in spiny king crab Paralithodes brevipes . Marine Ecology Progress Series , 312 : 189 – 99 .
- Sato , T and Goshima , S. 2006 . Impacts of male-only fishing and sperm limitation in manipulated populations of an unfished crab, Hapalogaster dentate . Marine Ecology Progress Series , 313 : 193 – 204 .
- Sharp , WC , Hunt , JH and Lyons , WG. 1997 . Life history of the spotted spiny lobster, Panulirus guttatus, an obligate reef-dweller . Marine Freshwater Research , 48 : 687 – 98 .
- Trivers , RL. 1972 . “ Parental investment and sexual selection ” . In Sexual Selection and the Descent of Man 1871–1971 , Edited by: Campbell , B . 136 – 79 . Chicago , IL : Aldine .
- Watt , WB , Carter , PA and Donohue , K. 1986 . Females’ choice of ‘Good Genotypes’ as mates is promoted by an insect mating system . Science , 233 : 1187 – 90 .
- Wedell , N , Gage , MJG and Parker , GA. 2002 . Sperm competition, male prudence and sperm-limited females . Trends in Ecology and Evolution , 17 : 313 – 20 .