Abstract
Holothurians are among the most species-rich taxa in the megabenthos on the northern Mid-Atlantic Ridge at depths of 2200–3700 m. Extensive new collections of 32 holothurian species were made in 2007–2010 in the Charlie Gibbs Fracture Zone area as part of the ECOMAR project. New material includes samples taken using a trawl and the ROV Isis. Samples and in situ observations from the ROV were of particular value because the morphological details of a number of holothurian species could be clarified. Many of these species are gelatinous and fragile and were damaged in trawls. Three species of elasipodid holothurians are described as new to science. An annotated check-list of all species of deep-sea holothurians collected in the Charlie Gibbs Fracture Zone area is provided. The checklist includes synonyms, distribution data and morphological descriptions as well as photographs taken in situ and in vivo. Ecological remarks are given for some species.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
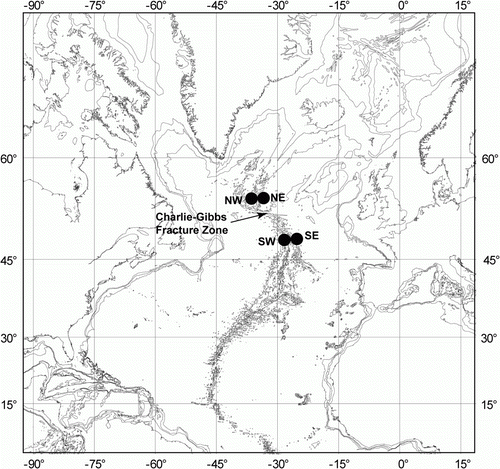
Over the last decade special attention has been given to the benthic fauna at the Mid-Atlantic Ridge (MAR). Several cruises were organized by the MAR-ECO project focusing on the ridge segment between Iceland and the Azores (Bergstad & Godø Citation2003, http://www.mar-eco.no/). These studies have resulted in a number of papers dealing with the ecology and the taxonomy of benthic fauna at the MAR (Dilman Citation2008; Mironov Citation2008; Tabachnik & Collins Citation2008; Gebruk et al. Citation2010, etc.). Between 2007 and 2010 the ECOMAR programme (http://www.oceanlab.abdn.ac.uk/ecomar/), a UK-funded field project contributing to MAR-ECO, conducted three cruises to the Charlie Gibbs Fracture Zone (CGFZ) area of the MAR onboard the RRS James Cook. Four target sites were investigated on either sides of the MAR north and south of the CGFZ and the sub-polar front, at a target depth of around 2500 m (). The ECOMAR project is aimed at investigation of the MAR ecosystem and its controlling physical factors. The main goals of the cruises were to investigate the benthic megafauna – species composition, biomass and abundance and distribution of species and communities. The benthic fauna was sampled using a trawl while an ROV provided images and physical samples. Holothurians obtained formed the basis for the present work.
Holothurians are one of the most species-rich megafaunal taxa at the northern MAR (Gebruk Citation2008; Gebruk et al. Citation2010). Within the ECOMAR they often dominated the trawl catches in numbers and biomass, and were very abundant in the video footage (C. Alt, unpubl. data). They were common on sedimented plains and slopes at all four ECOMAR sites.
Deep-sea holothurians are often very fragile and/or gelatinous and are therefore easily damaged by conventional sampling methods. Hence, characters of external morphology remain unknown in many species despite their importance for species identifications. Although underwater observation techniques are becoming more common in deep-sea research, video and photography surveys are rarely used to complement taxonomic work. Most deep-sea holothurians cannot be recognized to species or even generic level on photographs. Images and videos obtained using the ROV Isis on the ECOMAR cruises provided unique data on the external appearance and also the biology and ecology of deep-sea holothurians.
The present article reports on the taxonomic composition of holothurians of the CGFZ area of the MAR, with comments on their distribution, ecology and biology. Three new species are described.
Material and methods
Holothurian samples were obtained from a total of 13 trawls taken in 2007 (RRS James Cook cruise JC011), 2009 (RRS James Cook cruise JC037), and 2010 (RRS James Cook cruise JC048) (). Samples were also obtained on the latter cruise using the ROV Isis. Additional samples were collected on test dive 158 of the ROV on JC048 west of the northwest ECOMAR site, at a depth of 3500 m. Full station data and a cruise report can be found on the ECOMAR website (see above). On cruises JC011 and JC037 benthic fauna were collected using a semi-balloon otter trawl (OTSB 14, 13.7 m headline, fished on a single wire). Trawl samples were preserved in 10% formalin and were transferred to 80% ethanol for long-term preservation. Selected specimens were preserved in 80% ethanol for genetics. Two species were obtained from the megacorer samples in JC048. The megacorer was deployed with eight larger core tubes (internal diameter 100 mm) that were used for sampling macrofauna. Individual cores were sliced at 0–2, 2–6 and 6–10 cm intervals. All cores from a megacorer drop were mixed into a single sample. Sediment from deeper than 10 cm below the surface was investigated for evidence of deep burrowing macrofauna and megafauna. All core slices were washed through a net of 500- and 300-µm mesh sieves with filtered sea water. The sieved sediment was washed into 500-ml Nalgene™ bottles, and fixed with 10% buffered formalin solution.
Table I. Data on stations of ECOMAR cruises
Individual specimens were collected by the ROV Isis. The ROV was equipped with two high-definition video cameras each hosting a pair (red and green) of laser lights for determining the size of viewed objects. The ROV also had four HMI lights and a Scorpio digital still camera with flash unit. Observations on benthic fauna were made during video transect dives and on dives dedicated to specimen collections (). On collection dives each specimen was recorded on video and photographed prior to sampling. Sampling was conducted using a suction sampler (slurp-gun) flowing into a carousel with six transparent chambers with video control. Larger specimens were sampled using the two ROV manipulators. The samples were sorted and processed in a temperature controlled room at 4–6°C onboard and then preserved in 80% ethanol. Some specimens were photographed in the cold room prior to preservation.
Holothurians were identified after the cruise by examining external and internal taxonomic characteristics with a dissecting microscope. The morphology of calcareous elements (ossicles in the skin and internal organs and the structure of the calcareous ring) was studied on temporary and permanent preparations using a compound microscope. To extract ossicles, pieces of tissue were dissolved in chlorinated bleach solution.
Terms and abbreviations
C-shaped ossicles, small ossicles, less than 0.1 mm in size, occurring in the elpidiid genera Amperima, Ellipinion, Protelpidia and Scotoplanes. C-shaped ossicles are usually not taxonomically useful at the species level.
Deflection angle, the angle between the arm or apophysis and the vertical axis in ossicles (A).
Mouth tube, that part of the body with tentacles anterior to the velum, present in many elpidiids.
NHM, Natural History Museum, London.
NOCS, National Oceanography Centre Southampton, UK
OTSB, semi-balloon Otter trawl
Peniagone-type ossicles, ossicles consisting of a central stem with four usually bent arms and 0–4 apophyses on the arms or stem.
Taxonomy
Order Dendrochirotida Grube, Citation1840
Family Cucumariidae Ludwig, Citation1894
Staurocucumis abyssorum (Théel, 1886)
(A)
Cucumaria abyssorum Théel, 1886a: 66–67, plates 4(6), 16(6); von Marenzeller Citation1893: 14; Ludwig Citation1894: 122–125, plates 9(28–29), 13(1–5); Grieg Citation1921: 11, text figure 9; Ludwig & Heding Citation1935: 179; Cherbonnier Citation1941: 93–96, 101, figures 1, 3(j,n–p).
Staurocucumis abyssorum Ekman Citation1927: 385–387; Clark & Deichmann Citation1936: 566; Hansen Citation1988: 302–303, figure 1.
Abyssocucumis abyssorum Heding Citation1942: 33–35, text figures 34–36; Gage et al. Citation1985: 191.
Cucumaria abyssorum var. grandis Théel, 1886a: 67–68, plate 5(1).
Cucumaria abyssorum var. hyalina Théel, 1886a: 68–69, plate 4(7).
Cucumaria sluiteri Ohshima, Citation1915: 263, 263, plate 10(21a,b).
Cucumaria ingolfi Deichmann in Mortensen, Citation1927: 396.
Staurocucumis ingolfi Clark & Deichmann Citation1936: 567.
Cucumaria albatrossi Cherbonnier, Citation1941: 96–101, 103, figures 2, 3(a–i,k–m).
Material examined
St. JC011/17 (1 ind.), St. JC011/23 (60 ind.), St. JC037/15 (131 ind.), St. JC037/19 (127 ind.), St. JC037/27 (102 ind.). This species was observed in situ at stations JC048/33 Dive 170 (3 ind.), JC048/43 Dive 174 (1 ind.), JC048/48 Dive 176 (22 ind.), JC048/36 Dive 171 (4 ind.) and JC048/51 Dive 177 (1 ind.).
Distribution
Cosmopolitan species, known from all oceans except the Arctic. In the Atlantic Staurocucumis abyssorum occurs in the central and western parts. Depth 1210–4636 m.
Order Aspidochirotida Grube, Citation1840
Family Synallactidae Ludwig, Citation1894
Bathyplotes natans (M. Sars, Citation1868)
Holothuria natans M. Sars, 1868: 20.
Bathyplotes natans Östergren Citation1896: 352–353, plate 18(27–35); Citation1902: 6; Ludwig Citation1901: 137–138; Grieg Citation1921: 7; Mortensen Citation1927: 384–385, text figures 228(2), 229; Deichmann Citation1930: 100–102, plate 9(1–2, 9); Citation1954: 386; Heding Citation1942: 10–12, text figures 10, 11(1–10), 12(1–2); Pawson Citation1963b: 90–94, plate 7(1–7); 1965: 16–18, figure 4; 1970: 51; Gage et al. Citation1985: 194; Harvey et al. Citation1988: 183; Miller & Pawson Citation1990: 4; Madsen & Hansen Citation1994: 79–82, figures 48–50; Rowe & Gates Citation1995: 328; Liao Citation1997: 73–74, figure 39.
Stichopus natans G. O. Sars Citation1872: 30; M. Sars Citation1877: 58, plate 7(18–41); Théel 1886a: 193; Bell Citation1892: 51.
Stichopus tizardi Théel, Citation1882b: 696; 1886a: 193; Bell Citation1892: 51; Koehler Citation1896: 108–11, figures 33–35.
Bathyplotes tizardi Östergren Citation1896: 354; Ludwig Citation1901: 138; Perrier Citation1902: 350; Mitsukuri Citation1912: 35–39, text figure 8; Grieg Citation1921: 4.
Bathyplotes fallax Östergren, Citation1896: 355, plate 18(44).
Herpysidia reptans Perrier, Citation1898: 247–248.
Bathyplotes reptans Perrier Citation1902: 352–358, plates 12(3–4), 18(1–9); Mortensen Citation1927: 384.
Bathyplotes assimilis Koehler & Vaney, Citation1905: 25–26, plates 3(3), 10(1–3).
Bathyplotes papillosus Koehler & Vaney, Citation1905: 28–29, plate 10(21–24).
Bathyplotes patagiatus Fisher, Citation1907: 688–690, plate 72(1).
Bathyplotes östergreni Ohshima, Citation1915: 225–226, plate 8(3a–d).
Bathyplotes heterostylides Heding, Citation1942: 12–13, text figures 12(3–5), 13(1–15).
Material examined
St. JC011/23 (3 ind.), St. JC037/19 (1 ind.) and St. JC037/75 (3 ind.).
Distribution
North Atlantic: off Norway, Rockall Trough, Faroe-Shetland Channel, west off Ireland, south off Iceland, MAR south of the CGFZ, Gulf of Mexico. Also known from the Pacific (New Zealand and East China Sea). Depth 193–2750 m. Our new records are the deepest for this species.
Benthothuria funebris R. Perrier, Citation1898
(B, B)
Benthothuria funebris Perrier, Citation1898: 1665; 1899: 248; 1902: 365–371; Deichmann Citation1930: 91; Heding Citation1940: 363–364; 1942: 6; Gage et al. Citation1985: 194–195; Harvey et al. Citation1988: 183.
Material examined
St. JC011/23 (8 ind.), St. JC011/75 (2 ind.), St. JC011/101 (12 ind.), St. JC037/15 (15 ind.), St. JC037/19 (26 ind.), St. JC037/27 (53 ind.) and St. JC037/61 (2 ind.). This species was observed in situ at stations JC048/15 Dive 161 (1 ind.), JC048/29 Dive 169 (1 ind.), JC048/36 Dive 171 (1 ind.), JC048/48 Dive 176 (4 ind.) and JC048/54 Dive 179 (1 ind.).
Distribution
North Atlantic. Depth 782–3757 m.
Remarks
Common species in the CGFZ area, both north and south from the fracture zone. Gelatinous consistency in this species suggests the ability to swim.
Hansenothuria sp.
(, C)
Material examined
St. JC048/43 Dive 174 (1 fragment). This species was observed in situ at stations JC048/40 Dive 173 (several ind.) and JC048/53 Dive 178 (1 ind.).
Remarks
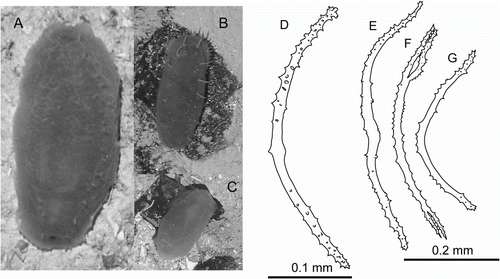
The specimen was about 18 cm long and was referred to Hansenothuria based on the following characters: broad ventrolateral brim, two rows of dorsal papillae, absence of midventral tube feet, dorsal anus, absence of ossicles in the body wall skin, resemblance of ossicles in papillae to those of Hansenothuria benti Miller and Pawson, Citation1989. However, our specimen differs in its pinkish colour of the body wall and the papillae. In H. benti the body colour ranges from light blue to pale purple, and the papillae colouration ranges from translucent to black.
The skin was thick and very gelatinous with a lot of mucus. Ossicles were found only in the papillae (D–G) and tube feet. They were rod-shaped, up to 0.5 mm long, ranging from almost straight to bent or curved forming a C or ring shape; ossicles spinous over most of its length; some ossicles with a central swelling, and some had additional processes or bifurcations at the ends. Tentacles were not preserved. Ossicles were absent in the gonad.
The specimen was observed swimming in the slurp-gun chamber after sampling. When the ROV was retrieved on deck, only fragments of this specimen were left. This species was observed on cliffs and talus slopes south of the CGFZ on JC048.
Mesothuria cathedralis Heding, Citation1940
Mesothuria (Allantis) cathedralis Heding, Citation1940: 336–338, text figure 5, figures 1–10.
Mesothuria (Penichrothuria) cathedralis Heding Citation1942: 8–9, text figure 8(1–5).
Mesothuria cathedralis Gage et al. Citation1985: 196; Gebruk et al. Citation2012: 284–289, figure 7.
Material examined
St. JC011/17 (6 ind.), St. JC011/23 (11 ind.), St. JC011/75 (4 ind.), St. JC011/101 (1 ind.), St. JC037/15 (8 ind.), St. JC037/19 (35 ind.), St. JC037/27 (30 ind.), St. JC037/70 (3 ind.) and St. JC048/16 Dive 162 (1 ind.).
Distribution
North Atlantic, including Porcupine Abyssal Plain, the Gulf of Guinea, off Morocco, Irminger Basin off southern Greenland to southeast of Iceland, MAR and the Gulf of Mexico. Depth 1292–4930 m.
Remarks
Epibenthic species. The skin is covered with globigerina shells, sponge ossicles and particles of sand and detritus.
Mesothuria maroccana R. Perrier, Citation1898
(D)
Mesothuria maroccana Perrier, Citation1898: 1665; 1899: 245; 1902: 312–317, plate 16(32–35); Hérouard Citation1923: 17; Deichmann Citation1930: 97, plate 7(2–7); Citation1940: 191; Citation1954: 385; Grieg Citation1921: 4; Hansen Citation1956: 46, figure 14a; Gebruk et al. Citation2012: 301–303, figure 9(a–b).
Mesothuria (Mesothuria) maroccana Heding Citation1940: 333; 1942: 8.
Holothuria murrayi (var.?) Théel, 1886: 187, plate 9(3).
Holothuria intestinalis var. verrilli Hérouard, Citation1896: 163.
Material examined
St. JC011/17 (10 ind.), St. JC011/75 (5 ind.), St. JC037/15 (6 ind.), St. JC037/19 (1 ind.), St. JC037/27 (2 ind.) and St. JC048/40 Dive 173 (1 ind.).
Distribution
Common in the North Atlantic deep sea. Known from the Moroccan coast, the Azores, south of Iceland, Jamaica, eastern Gulf of Mexico, east of Florida, MAR. Depth 700–3120 m.
Remarks
This epibenthic species was observed on cliffs directly on hard rock substrate. Specimen was bright white in situ as a result of pteropod shells covering most of the body (D). These shells were lost during sampling. The specimen collected by ROV was covered with scattered globigerina shells and mineral particles only. The body colour was brownish, changing to dark purple in the tentacles; the hind gut and the skin around anus were dark purple. The specimen had very long filiform dorsal tube feet attached to rock in situ.
Molpadiodemas violaceus (Théel, 1886)
Pseudostichopus villosus var. violaceus Théel, 1886a: 172, plate 10(6b).
Molpadiodemas violaceus O'Loughlin & Ahearn Citation2005: 165, figures 1(e,i), 2(f), 7(g–i), 8(u–x).
Pseudostichopus villosus Théel, 1886a: 170–171 (partim).
Material examined
St. JC011/75 (1 ind.).
Distribution
West and central North Atlantic, South Atlantic, Antarctic, South Indian Ocean, South Pacific. Depth 2196–6354 m.
Paelopatides grisea R. Perrier, Citation1899
(A)
Paelopatides grisea Perrier, 1899: 248; 1902: 361–365; Mortensen Citation1927: 388; Heding Citation1940: 351; Billett et al. Citation1985: 407; Gage et al. Citation1985: 195–196; Harvey et al. Citation1988: 183; Miller & Pawson Citation1990: 5.
Paelopatides gigantea Deichmann Citation1930: 104–106 (partim); Sibuet Citation1977: 554; Miller & Pawson Citation1990: 35. (non Verrill, Citation1884)
Material examined
St. JC011/23 (1 ind.), St. JC011/101 (38 ind.), St. JC011/106 (13 ind.), St. JC011/111 (18 ind.), St. JC037/15 (1 ind.), St. JC037/19 (1 ind.) and St. JC037/61 (1 ind.). This species was observed in situ at stations JC048/6 Dive 159 (1 ind.) and JC048/15 Dive 161 (9 ind.).
Distribution
North Atlantic: Canary Islands, between the Azores and Portugal, Porcupine Abyssal Plain, Rockall Trough, Bahama Islands, Caribbean, MAR. Depth 1695–4060 m.
Pseudostichopus peripatus (Sluiter, 1901)
(D)
Meseres peripatus Sluiter, Citation1901a: 10–11; 1901b: 48–49, plates 5(5), 8(7); Perrier Citation1902: 359; Rowe & Gates Citation1995: 285.
Pseudostichopus peripatus O'Loughlin & Ahearn Citation2005: 174–175, figure 1(10–12). (non Marenzeller, Citation1893 )
Pseudostichopus occultatus Hérouard Citation1902: 14–15, plate 2(4–14) (partim). (non von Marenzeller, 1893)
Pseudostichopus occultatus var. plicatus Koehler & Vaney, Citation1905: 9–10, plates 3(8), 9(1–3); Heding Citation1940: 353.
Plicastichopus plicatus Heding Citation1940: 354–359; 1942: 6.
Pseudostichopus propinquus Fisher, Citation1907: 691–693, plates 71(3), 72(2), 73(3), 74(1), 76(3); Imaoka Citation1978: 382.
Pseudostichopus (Trachostichopus) propinquus Heding Citation1940: 357; Imaoka Citation1978: table 1–1; 1990: 148, 152.
Meseres propinquus O'Loughlin Citation2002: 309.
Pseudostichopus aleutianus Ohshima, Citation1915: 228, plate 8(5a–c); Imaoka Citation1978: 380.
Pseudostichopus (Trachostichopus) aleutianus Heding Citation1940: 353–359; Imaoka Citation1978, table 1–2.
Pseudostichopus unguiculatus Ohshima, Citation1915: 230–231, plate 8(7a–c); Imaoka Citation1978: 384; Rowe & Gates Citation1995: 285.
Pseudostichopus (Pseudostichopus) unguiculatus Heding Citation1940: 353–360; Imaoka, Citation1978: table 1–1; 1990: 152; Thandar Citation1992: 167.
Pseudostichopus marenzelleri Hérouard, Citation1923: 25; Mortensen Citation1927: 287–288; Deichmann Citation1930: 90.
Pseudostichopus (Pseudostichopus) marenzelleri Heding Citation1940: 353–359; Imaoka Citation1978: table 1–1; Thandar Citation1992: 167.
Pseudostichopus lapidus Hérouard, Citation1923: 26–28, plate 4(5).
Pseudostichopus (Pseudostichopus) lapidus Heding Citation1940: 353–360.
Plicastichopus ingolfi Heding, Citation1942: 5–6, text figures 4–5, plate 1(4–5).
Meseres ingolfi Rowe & Gates Citation1995: 285.
Pseudostichopus (Trachostichopus) tuberculatus Imaoka, Citation1990: 149–152, figures pp. 149, 151.
Material examined
St. JC011/23 (7 ind.), St. JC011/75 (6 ind.), St. JC011/101, St. JC011/106, St. JC037/15 (23 ind.), St. JC037/19 (2 ind.), St. JC037/27 (11 ind.), St. JC037/61 (2 ind.), St. JC037/67 (10 ind.), St. JC037/70 (4 ind.) and St. JC048/24 Dive 165 (several ind.). Many specimens of this species were observed in situ at stations JC048/6 Dive 159, JC048/15 Dive 161, JC048/16 Dive 162, JC048/26 Dive 167, JC048/33 Dive 170, JC048/36 Dive 171 and JC048/51 Dive 177.
Distribution
Cosmopolitan eurybathic species known from the Indo-Pacific, North and South Pacific Ocean, North and South Atlantic Ocean, Southern Ocean. Depth 134–5453 m.
Remarks
In the ECOMAR area this species had a very characteristic in situ appearance: its dorsal side was covered with many of the large (up to 2 cm diameter) disc-like foraminiferan Discospirina tenuissima which were usually lost during sampling.
Synallactes aff . crucifera
(, J)
Material examined
St. JC037/15 (3 ind.), St. JC011/23 (3 ind.) and St. JC048/16 Dive 162 (1 ind.). This species was observed in situ at the Station JC048/36 Dive 171 (3 ind.).
Description
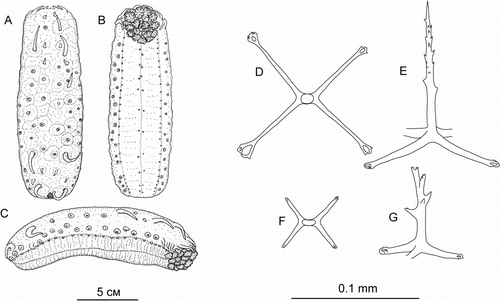
Colour whitish, in situ light pink. Skin thick, non-transparent, rough owing to dense layer of ossicles. Body elongated (A–C), up to 80 mm in length in fresh specimen, 60 mm in ethanol. Dorsal papillae in 6 rows, retractable. Tentacles 18. Ventrolateral tube feet about 25 pairs. Mid-ventral tube feet about 10 pairs, scarcely distributed. Anus dorsal. Ossicles spatulated crosses only, each with long spinous apophysis. Dorsal crosses (D,E): arms up to 0.1 mm long, central apophysis ~0.15 mm with prominent spines; ends of arms spatulated, each with a large hole; 1–3 small holes sometimes present; small ossicles may have only one hole or lack holes completely. Apophyses not perforated. Ventrally very small crosses sparsely distributed; arms 0.03–0.05 mm (F,G).
Remarks
Examined specimens resemble Synallactes crucifera R. Perrier, Citation1898 in external morphology: body shape, skin colour, arrangement of dorsal papillae, ventrolateral and midventral tube feet. Their ossicles are also similar in size, number of perforations on arms and spinous apophysis lacking projections. However, ossicles with perforated central apophyses described by Perrier were not found.
This species was frequently observed on steep slopes – on rocky outcrops and sediment pockets between them. It is likely that this species has the ability to swim.
Synallactidae gen. et sp. indet.
(, C)
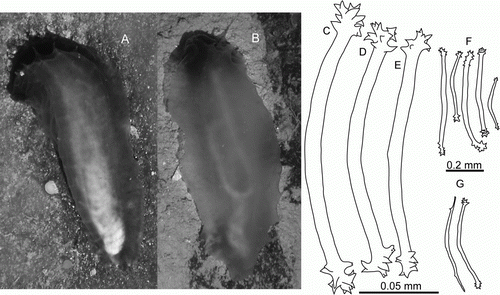
Figure 4. Synallactidae gen. et sp. indet. (A,B) Underwater photographs: (A) St. JC048/40 Dive 173; (B) St. JC048/43 Dive 174. (C–E) St. JC048/43 Dive 174, ossicles of papillae, scale 0.05 mm; (F) St. JC048/43 Dive 174, tentacle ossicles, scale 0.2 mm; (G) St. JC048/43 Dive 174, gonad ossicles, scale 0.2 mm.
Material examined
St. JC048/43 Dive 174 (one very damaged specimen). This species was also observed in situ at the station JC048/40 Dive 173 (several specimens).
Description
Body about 20 cm in length, anterior end wide, posterior end tapered, ventral side flat, dorsal side convex. Skin thick, gelatinous, semi-transparent ranging in colour from light to deep purple; tentacles and skin around mouth the most pigmented. Dorsal papillae in 2 rows, longest papillae in the anterior one-third of dorsum, other papillae minute and slender, almost not visible. Mid-ventral tube feet in double row. Ventrolateral brim wide, widest in anterior half. Ossicles curved rods (C–G), present in papillae, mid-ventral tube feet, tentacles and gonads; rods more or less of the same type; rods in papillae and tube feet up to 0.18 mm in length with enlarged ends covered by large spines; in tentacles rods up to 0.55 mm in length, more spinous and varying in shape compared to rods in papillae and tube feet; rods in gonad up to 0.5 mm in length, more slender than those in external skin.
Remarks
The specimen swam actively in the slurp-gun chamber soon after sampling. By the time the specimen could be preserved it had become badly damaged, owing to its very gelatinous consistency.
The species was observed on cliffs and talus slopes south of the CGFZ, often together with Hansenothuria sp.
Order Elasipodida Théel, 1882
Family Deimatidae Théel, Citation1879
Deima validum validum Théel, Citation1879
(E)
Deima validum Théel, 1879: 5, figures 36–38; 1882a: 68–70, plates XVIII, XIX, XXXI(4–9), XXXVI(4), XXXVII(8), XLIII(7), XLIV(13), XLVI(5); Sluiter Citation1901b: 60.
Deima validum validum Hansen Citation1967: 488–490, figure 5.
Deima fastosum Théel, 1879: 5–6, figures 1–3; 1882a: 71–73, plates XX, XXI(1), XXXI(10–13), XXXV(7–10), XXXVI(7), XXXVII(3), XLIII(2–3, 5), XLVI(8).
Deima blakei Théel, Citation1886b: 1–2, figures 1–2; Koehler & Vaney Citation1905: 55–57, plate XI(13–15); Hérouard Citation1923: 40–41, plates V(7), VI(5); Deichmann Citation1930: 115–116, plates X(7–11), XI(1–3); Citation1936: 9; Citation1940: 198–199.
Deima atlanticum Hérouard, Citation1898: 88–89, figures 1–2; 1902: 32–35, plates III(3), IV(18), V(1–5), VIII(26–29); Grieg Citation1921: 4, plate I(2–3).
Deima mosaicum Ohshima, Citation1915: 233–234.
Material examined
St. JC011/23 (2 ind.). This species was observed in situ at stations JC048/36 Dive 171 (1 ind.) and JC048/43 Dive 174 (1 ind.).
Distribution
Cosmopolitan species, occurring from tropical to moderate latitudes. Depths 724–5424 m, in the North Atlantic 1049–4360 m.
Family Laetmogonidae Ekman, Citation1926
Laetmogone billetti sp. nov. Rogacheva & Gebruk
(, , H, I, K, G—I)
Holotype
NHM 2012.3, RRS James Cook, St. JC048/24, ROV Isis Dive 165, 54°01.08′N, 34°09.46′W, 2501–2398 m, 9–10 June 2010, specimen 50 mm long in preserved state.
Paratypes
NHM 2012.4-5, RRS James Cook, St. JC048/16, ROV Isis Dive 162, 53°59.39′N, 36°11.66′W, 2450–2272 m, 6 June 2010, 2 ind., 80 and 40 mm in length in preserved state; NHM 2012.6, RRS James Cook, St. JC048/56, ROV Isis Dive 180, 49°01.18′N, 27°42.33′W, 2758 m, 29 June 2010, 1 ind., 25 mm long.
Additional material
This species was observed in situ at the Station JC048/6 Dive 159.
Type locality
North Atlantic, MAR, CGFZ area. Depth 2272–2758 m.
Diagnosis
Tentacles 15, large. Tube feet small and slender, up to 32 pairs. Dorsal papillae conspicuous, up to 12 pairs. Ossicles of the body wall wheels only; dorsal wheels of two types: large wheels (0.1–0.2 mm in diameter) with 5 or 4 central rays and central holes smaller than peripheral ones, and small wheels (0.05–0.1 mm) with central primary crosses and central holes larger than peripheral ones; ventral wheels small (0.05–0.06 mm) with 3 or 4 central rays.
Description
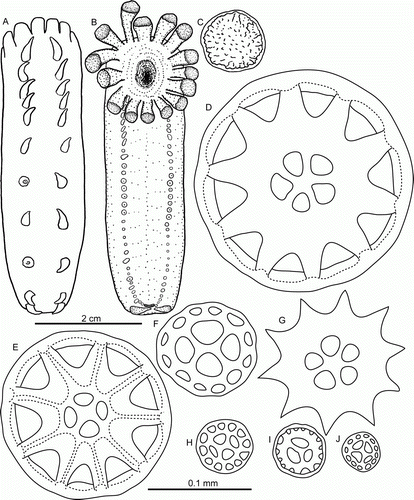
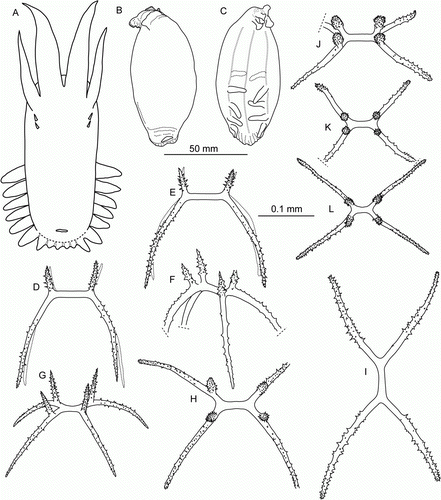
Colour in-vivo light purple (H,I, K, G—I). Dorsal side convex, ventral flat. Skin semi-transparent. Body elongated, length up to 80 mm in fresh specimens, 50 mm in ethanol preserved specimens. Tentacles 15, large; tentacle discs with small knobs (C). Number of papillae and tube feet increased with body size. Two rows of conspicuous dorsal papillae (A); 12 dorsal papillae in one row in the largest specimen. Tube feet small in preserved specimens, long and slender in live specimens, up to 32 pairs. Ventrolateral brim absent.
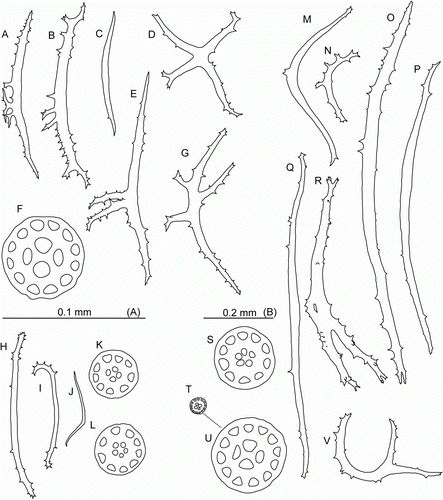
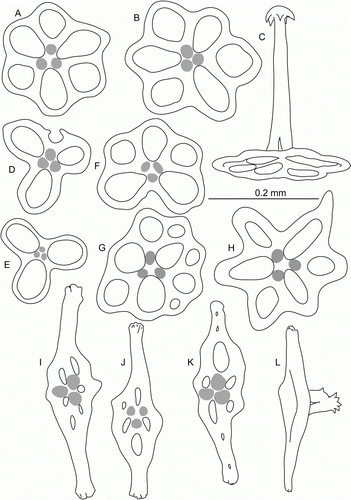
Wheel-type ossicles numerous both on dorsum and ventrum. Dorsal wheels of two size types: large (>0.1 mm) and small (<0.1 mm). Large wheels predominant (D,E), 0.10–0.22 mm in diameter, spokes 9–10 in most wheels, rarely 8 or 11 spokes; central rays usually 5, rarely 4 (primary crosses) or 6 rays. Stellate ossicles (ossicles with no rim) (G) rarely occur, apparently these are undeveloped wheels. Small wheels (F,H) 0.05–0.1 mm in diameter with central primary crosses and 4 central holes, central holes larger than peripheral ones. Small wheels may represent an early stage of development of wheel-type ossicles. Ventral ossicles (I,J) small wheels 0.05–0.06 mm in diameter, centrally primary cross or 3 rays; ventral ossicles resemble dorsal wheels of small type. Wheels in tube feet small (F), 0.04–0.06 mm in diameter, with central primary crosses and 4 central holes larger than peripheral ones. Slightly curved rods also present, up to 0.65 mm in length with small and large spines, large spines often bifurcated and bearing smaller spines (A,B); small rods can bear few little spines; some rods with additional processes (E,G). One more type of ossicles on ventrum flattened crosses with spinous arms (D). In papillae wheels large (K,L) and small, similar in size and shape to those in dorsal skin, however small wheels dominant; rods also present, up to 0.4 mm in length, slightly curved (H,J) or with curved ends (I); spines mainly on rod ends. Ossicles in tentacles very variable (M–V): numerous small and large wheels as on dorsum, small wheels being more numerous, rods up to 1.1 mm long, from straight to curved or irregular, most of rods with numerous spines of different size, large spines sometime bifurcate and bear additional spines.
Remarks
Observed mainly on steep slopes with patches of detritus. Possibly able to swim.
Relationships
Laetmogone billetti sp. nov. is similar to species with two types of wheels and slender tube feet: L. fimbriata (Sluiter, 1901) and L. biserialis Fisher, Citation1907 . Laetmogone billetti differs from both these species by having large wheels with 5 central rays dominating on the dorsum. In L. fimbriata and L. biserialis large wheels have 6 central rays.
Distribution
Known only from the type locality.
Etymology
The species is named after Dr David S. M. Billett, renowned deep-sea biologist and expert on deep-sea holothurians.
Family Psychropotidae Théel, 1882
Benthodytes gosarsi Gebruk, Citation2008
(E; online supplement 2)
Benthodytes gosarsi Gebruk, Citation2008: 49–50, 52, figures 1(a), 2, 3.
Material examined
St. JC011/23 (39 ind.), St. JC011/75 (21 ind.), St. JC011/101 (1 ind.), St. JC037/15 (15 ind.), St. JC037/19 (42 ind.), St. JC037/27 (40 ind.), St. JC037/61 (56 ind.), St. JC037/67 (23 ind.) and St. JC037/70 (23 ind.). This species was observed in situ at stations JC048/3 Dive 158 (1 ind.), JC048/6 Dive 159 (2 ind.), JC048/15 Dive 161 (9 ind.), JC048/26 Dive 167 (1 ind.), JC048/29 Dive 169 (1 ind.), JC048/33 Dive 170 (14 ind.), JC048/36 Dive 171 (7 ind.), JC048/48 Dive 176 (11 ind.) and JC048/51 Dive 177 (2 ind.).
Remarks
Benthodytes gosarsi was described based on material collected at two stations on the MAR: north of the Azores and south of the CGFZ. Later this species was found off Iceland and in the Whittard Canyon (original data): BIOICE (SBS) Sample Nr 2862, cruise B-13-95, St. 734, 30 August 1995, 61.168–61.170°N, 18.008–18.026°W, 2399 m; BIOICE (SBS) Sample Nr 3572, cruise B-4-03, St. 391, 5 September 2003, 63.502–63.500°N, 29.637–29.667°W, depth 2238–2242 m; RRS James Cook, Cruise JC36, St. JC36/005 ROV Isis Dive 99, 48°9.078′N, 10°34.607′W–48°8.590′N, 10°34.119′W, depth 3647–3680 m.
Benthodytes gosarsi was observed also on the Isis test dive (Dive 158), west from the ECOMAR northwest site (base of the MAR slope) at 3500 m depth.
Specimens were bright green in situ, which is the colour of their external skin layer. Specimens from trawl catches often lacked the external skin layer and their colour ranged from greenish-brown to purple.
Benthodytes gosarsi was one of the dominant holothurian species both in number and biomass at the northwest ECOMAR site and was also very abundant at the southeast site (original data, will be published separately). The species occurred only on flat areas. A young specimen of B. gosarsi was observed swimming on Dive 168 (online supplement 2).
Distribution
Eastern and central North Atlantic. Depth 2238–3680 m.
Benthodytes lingua R. Perrier, Citation1896
(B)
Benthodytes lingua Perrier, Citation1896: 902; 1902: 456–461, plates XII(1–2), XXI(1–9); Deichmann Citation1930: 124–125; Citation1940: 200–201, plate XXXV(3–4); Citation1954: 384; Heding Citation1942: 15.
Benthodytes janthina Grieg Citation1921: 11; Heding Citation1942: 15. (non von Marenzeller, Citation1892)
Pannychia glutinosa Hérouard, Citation1902: 32, plate IV(17).
Material examined
St. JC037/27 (2 ind.), and St. JC048/43 Dive 174 (1 ind.). This species was observed in situ at stations JC048/33 Dive 170 (7 ind.), JC048/36 Dive 171 (2 ind.) and JC048/48 Dive 176 (1 ind.).
Remarks
Benthodytes lingua was common at the southern sites. This species is benthopelagic.
Distribution
North and South Atlantic. Depth 860–4700 m.
Benthodytes sanguinolenta Théel, 1882
Benthodytes sanguinolenta Théel, Citation1882a: 104–105, plates XXIII, XL(4–5), XLII(6); Ludwig Citation1894: 53–60, plate I(1–8); Koehler & Vaney Citation1905: 72; Clark Citation1913: 233; Ohshima Citation1915: 245; CitationClark 192: 142; Clark Citation1923a: 162; Citation1923b: 420; Heding Citation1940: p. 367; Hansen Citation1956: 44–45; 1975: 94–96, plates III–VI, IX(6–7), XII(4–5); Carney & Carey Citation1976: 69; Pawson Citation1982: 129–145; Bluhm & Gebruk Citation1999: 175, figure 3(d); Rogacheva et al. Citation2009: 463, figure 2.
Material examined
St. JC037/19 (1 ind.).
Distribution
Cosmopolitan eurybathic species. Recorded in the Atlantic (MAR, Peru Basin, off Bahama Islands), Pacific (Tasman Sea, Kermadec Trench, off Panama isthmus, Japan and Cascadia Basin), Indo-Pacific, South Africa and the Bay of Bengal and off the Crozet Islands. Depths 768–7250 m.
Psychropotes depressa (Théel, 1882)
(F,G)
Euphronides depressa Théel, Citation1882a: 93–96, plates XXVI, XXX(5–6), XL(7), XLVI(4); Ohshima Citation1915: 244–245, figure 1.
Psychropotes depressa Hansen Citation1975: 106–111, figures 43–44.
Euphronides depressa var. minor Théel, Citation1886b: 2.
Euphronides cornuta Verrill, Citation1884: 217; Citation1885: 518, 538, figures 32–33; Deichmann Citation1930: 127–128; Heding Citation1940: 368.
Euphronides tanneri Ludwig, Citation1894: 39–44, plates III(7), IV, V(17–19).
Euphronides auriculata Perrier, Citation1896: 901–902; 1902: 434–438, plates XIII(1–2), XX(12–13); Grieg Citation1921: 8–9.
Euphronides violacea Perrier, Citation1896: 902; 1902: 438–441, plate XX(14), Deichmann Citation1930: 128–129; Citation1940: 201–202; Citation1954: 384; Heding Citation1942: 15–16; Madsen Citation1947: 16.
Euphronides talismani Perrier, Citation1896: 902; 1902: 441–444, plate XX(15); Hérouard Citation1902: 30–31, plate II(19–22); Deichmann Citation1930: 129; Heding Citation1942: 15, text figure 15.
Benthodytes assimilis Théel, Citation1886b: 2–3.
Material examined
St. JC011/23 (2 ind.), St. JC011/101 (5 ind.), St. JC037/15 (2 ind.), St. JC037/19 (2 ind.), St. JC037/27 (13 ind.), St. JC037/61 (10 ind.), St. JC037/67 (5 ind.) and St. JC037/70 (1 ind.). This species was observed in situ at stations JC048/15 Dive 161 (2 ind.), JC048/17 Dive 163 (1 ind.), JC048/24 Dive 165 (1 ind.), JC048/26 Dive 167 (8 ind.), JC048/33 Dive 170 (1 ind.), JC048/43 Dive 174 (1 ind.), JC048/48 Dive 176 (1 ind.), JC048/54 Dive 179 (2 ind.) and JC048/56 Dive 180 (1 ind.).
Distribution
Psychropotes depressa is known from the North and South Atlantic, and Pacific: off Japan, Gulf of Panama and Chile. One of the common species in the ECOMAR area. Depth 957–4200 m.
Family Elpidiidae Théel, Citation1879
Amperima furcata (Hérouard, Citation1899)
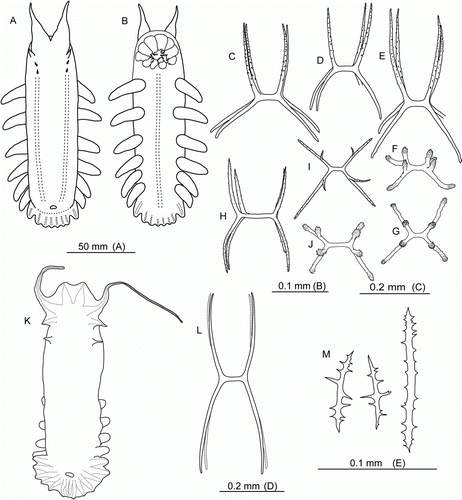
(A–E, K, M,N, C)
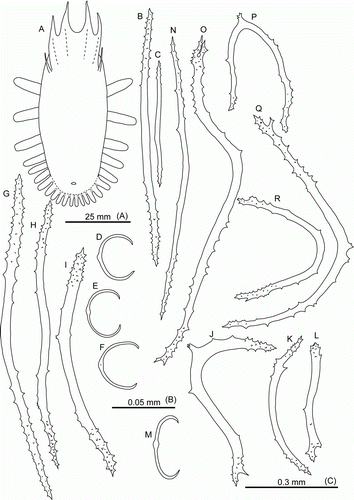
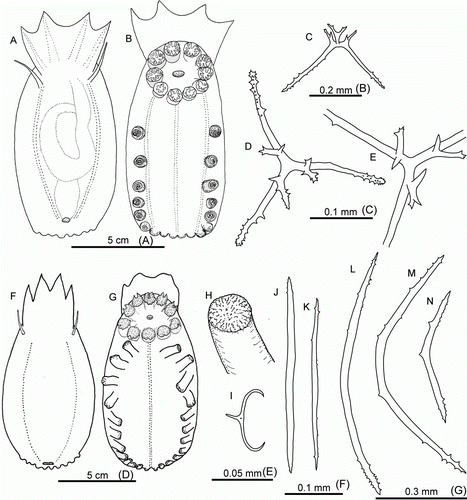
Figure 7. (A–E) Amperima furcata (Hérouard, Citation1899). (A,B) St. JC048/43 Dive 174, dorsal and ventral view, scale A; (C–E) dorsal ossicles, St. JC048/54, Dive 179: (C) scale B; (D,E) scale C. (F–N) Ellipinion delagei (Hérouard, Citation1896), St. JC048/24 Dive 165. (F,G) Dorsal and ventral view, scale D; (H) distal part of tube foot, magnified; (I) ventral C-shaped ossicle, scale E; (J,K) ventral rods, scale F; (L–N) tube foot ossicles, scale G.
Kolga furcata Hérouard, 1899: 171, figure 2; 1902: 40–41, plates III(7), VI(4–10), VIII(17).
Amperima furcata Hansen Citation1975: 159, figure 75; Gebruk Citation1990: 141–142, figure 61(7–9).
Periamma furcata Hérouard Citation1923: 91.
Material examined
St. JC011/23 (120 ind.); St. JC037/15 (90 ind.); St. JC048/43 Dive 174 (4 ind.) and St. JC048/54 Dive 179 (1 ind.). This species was observed in situ at stations: JC048/33 Dive 170 (88 ind.), JC048/36 Dive 171 (30 ind.) and JC048/56 Dive 180 (1 ind.).
The ECOMAR specimens collected by the ROV were in excellent condition, allowing the external morphological characters to be clarified.
Description
Colour in vivo pinkish, tentacle discs light pink; colour in ethanol whitish (K, M,N, C). Skin semi-transparent, full of ossicles. Body ovoid. Length up to 65 mm in vivo, in preserved specimens up to 35 mm. Velum composed of two pairs of papillae fused almost over their whole length (A–E). One pair of long papillae posteriorly to velum. Tube feet 8 pairs, located in the posterior two-thirds of the body, crowded posteriorly, forming a narrow brim.
Ossicle morphology corresponds well with the diagnosis. Dorsal ossicles (C–E) with arms curved downwards, arms up to 0.35 mm in length. Apophyses bifurcated (rarely not); length of apophyses up to 0.12 mm. Ventral ossicles with arms straight or slightly curved downwards, arms up to 0.33 mm in length, distal one-third of arms may be curved upwards; apophyses bifurcated, up to 0.07 mm long.
Remarks
Very common species at the southern ECOMAR sites. Benthopelagic species.
Distribution
Northeast and central Atlantic (off the Azores, Bay of Biscay, MAR), northeast Pacific. Depth 1846–4700 m.
Ellipinion delagei (Hérouard, Citation1896)
(F–N, K)
Scotoplanes delagei Hérouard, Citation1896: 167–168, figure 3; 1902: 39–40, plates VI(1–3), VIII(8–9).
Ellipinion delagei Hérouard Citation1923: 90–91; Hansen Citation1975: 163; Harvey et al. Citation1988: 185–186; Gebruk Citation1990: 133, figure 58.
Material examined
St. JC037/15 (20 ind.), St. JC037/19 (1 ind.) and St. JC048/24 Dive 165 (1 ind.).
Remarks
Occurred on flat areas and cliffs. Benthopelagic species. (Also see remarks for Ellipinion alani sp. nov.)
Distribution
Central and northeast Atlantic. Depth 1165–2750 m.
Ellipinion alani sp. nov. Rogacheva & Gebruk
(, N, F)
Holotype
NHM 2012.7, RRS James Cook, St. JC048/24, ROV Isis Dive 165, 54°01.08′N, 34°09.46′W, 2501–2398 m, 9–10 June 2010, specimen 70 mm long.
Paratypes
NHM 2012.8, RRS James Cook, St. JC048/24, ROV Isis Dive 165, 54°01.08′N, 34°09.46′ W, 2501–2398 m, 9–10 June 2010 (1 ind., 70 mm long) and NHM 2012.9, St. JC048/43, ROV Isis Dive 174, 48°43.87′–48°44.23′N, 28°39.01′–28°39.57′W, 2620–2547 m, 22 June 2010 (1 ind., 100 mm long).
In situ observations
This species was observed in situ at the Station JC048/33 Dive 170 (16 ind.).
Type locality
North Atlantic, MAR, CGFZ area. Depth 2398–2620 m.
Diagnosis
Skin semi-transparent, light orange. Velum consists of two pairs of papillae; one pair of papillae posteriorly from velum. Tube feet 11–12 pairs, posterior 6 pairs fused at the base. Ossicles absent in dorsal skin. Ventral ossicles rods up to 0.85 mm in length and C-shaped elements up to 0.05 mm.
Description
Body ovoid. Length in vivo 110–130 mm, in ethanol 65–100 mm. Skin semi-transparent, colour in vivo light orange (N, F). Tentacles and tips of tube feet are more pigmented than the body skin. Velum consists of two pairs of papillae fused over half to two-thirds of their length. One pair of free papillae located posteriorly to velum. Tube feet 11–12 pairs; posterior 6 pairs of tube feet partly fused forming brim. Tentacles 10. Ossicles lacking in dorsum; in dorsal papillae numerous rods occur in tips. On ventrum sparsely distributed rods (B,C), up to 0.85 mm long, straight or slightly curved, with spinous ends and rare C-shaped ossicles (D–F) 0.03–0.05 mm in size, usually with central swelling; C-shaped ossicles more numerous than rods, or approximately in equal proportion with rods. In tube feet mostly rods (G–L) up to 1.1 mm in length, curved or irregular, rarely straight, with spinous ends and usually smooth central part; C-shaped ossicles 0.04–0.07 mm in size also present (M). In tentacles numerous rods of different size and shape (N–R), curved, irregular or rarely straight, with pointed or blunt spinous ends; spines more numerous in distal parts of rods, on some rods spines present in the middle; large spines bear additional small spines, additional processes sometime develop; C-shaped ossicles about 0.04 mm also present, less numerous than in tube feet.
Remarks
Tips of tube feet are enlarged and form discs, their surface knobbed and variously pigmented. A similar feature was observed in E. delagei (H). This character is not known in other elpidiids. The function of the discs is unclear.
Ellipinion alani is the second species of the genus occurring in the Atlantic (E. delagei also occurs in the Atlantic). Ellipinion alani is a benthopelagic species; it has been observed on flat areas and talluses. It was one of the most common species at the southwest site.
Relationships
Ellipinion alani is most closely related to E. delagei. Both species lack dorsal ossicles and have C-shaped ossicles and rods on the venter. Ellipinion alani differs from E. delagei in the form of C-shaped ossicles: these lack processes in the middle part in the new species.
Distribution
Known only from the type locality.
Etymology
The species is named after Dr J. Alan Hughes for his great help in this work and his contribution to benthic studies in the ECOMAR project.
Kolga nana (Théel, Citation1879)
(J)
Elpidia nana Théel, 1879: 15–16, figures 20–22.
Kolga nana Théel Citation1882a: 39–42, plates II(3–4), XXXIII(1–2); XXXIV(5); XXXVI(25), XLII(5, 8); Rogacheva Citation2012: 1186–1190, figures 4–7.
Kolga hyalina Hansen Citation1975: 170–171 (partim); Billett & Hansen Citation1982: 804–806, figures 2(1–9), 4–6; Gage et al. Citation1985: 200; Harvey et al. Citation1988: 185; Gebruk Citation1990: 121–122 (partim). (non Danielssen & Koren, 1879)
Kolga sp. Gebruk Citation2008: 50, 51, 52, 58, figure 1(c).
Material examined
St. JC048/24 Dive 165 (5 ind.).
Remarks
Description of this material was published earlier (Rogacheva Citation2012).
Extensive aggregations of Kolga nana were observed at the northeast site. The density of the aggregations was in tens of specimens per m2. A number of specimens were seen inside the CGFZ on the ROV Isis Dive 169. K. nana is a benthopelagic species.
Distribution
The North Atlantic and Antarctic. Depth 1484–6235 m.
Peniagone azorica von Marenzeller, Citation1893
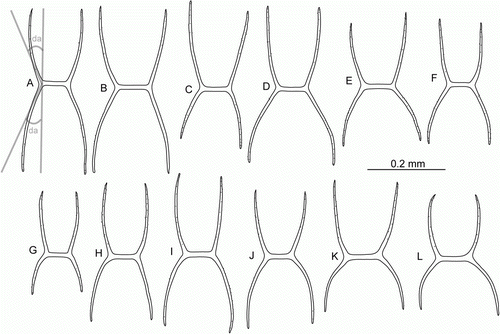
(, H,I, J, D)
Figure 9. Peniagone azorica von Marenzeller, 1893, dorsal ossicles, side view, ha. (A–D) St. JC048/24 Dive 165; (E–G) St. JC011/101; (H–L) BIOICE Sample No. 2852. (da) Deflection angle.
Peniagone azorica von Marenzeller, Citation1893: 12–13, plates 1(4), 2(5); Hérouard Citation1902: 42–43, plate VI(21–26); 1923: 87–88; Grieg Citation1921: 8, figure 4; Heding Citation1942: 20; Hansen Citation1975: 138–142 [partim: figure 63(5–9)]; Gage et al. Citation1985: 199; Tyler et al. Citation1985: 71–81; Gebruk Citation1990: 110–111, figure 45 (partim).
Material examined
St. JC011/101 (33 ind.), St. JC011/106 (6 ind.), St. JC011/111 (64 ind.), St. JC037/79 (29 ind.) and St. JC048/24 Dive 165 (1 ind.). This species was observed in situ at stations JC048/15 Dive 161 (1 ind.) and JC048/26 Dive 167 (36 ind.).
Additional material
BIOICE, south of Iceland, the Reykjanes Ridge, Sample Nr 2862, Cruise B-13-95, St. 734, Agassiz trawl, 30 August 1995, 61.17°N, 18.01–18.03°W, 2399 m, 40 ind.; Sample Nr 2863, B-13-95, St. 734, Agassiz trawl, 30 August 1995, 61.17°N, 18.04–18.05°W, 2400 m, 27 ind. RV Akademik Kurchatov, St. 916, Scotia Sea, 56°29′S, 50°51′W, 4660–5380 m, 2 ind.
Remarks
Peniagone azorica was described from the northeast Atlantic based on the Princess-Alice (Hirondelle II) collection. Hansen (Citation1975) refered P. vedeli Hansen, Citation1956 to this species as well as a number of specimens from the Kermadec Trench (South Pacific). Gebruk (Citation1990) re-established P. vedeli and referred most of the Kermadec specimens of Hansen to P. vedeli and P. affinis Théel, 1882. Gebruk (Citation1990) also reported P. azorica from the Antarctic.
In the ECOMAR material, Peniagone azorica was identified mainly based on the morphology of its dorsal ossicles. It was a common holothurian species in the ECOMAR area. A close similarity was found between specimens of P. azorica and P. islandica Deichmann, Citation1930, the latter another common ECOMAR species. Peniagone islandica was described originally from the Reykjanes Ridge in 1930 (Deichmann Citation1930) but had never been collected since. The two species have a very similar external morphology and are indistinguishable in situ. Differences were found in ossicle morphology, the most important taxonomic feature in Peniagone. However, ossicles intermediate between those typical for P. azorica and P. islandica were also present. This is an important aspect in support of synonymizing the two species, in which case P. islandica would be a younger synonym. However, we prefer to accept P. azorica and P. islandica as separate species until new evidence becomes available. The taxonomy of these species requires further investigation, including the examination of the type specimens and additional material from the Atlantic.
The following is a comparative description of taxonomic characters in P. azorica and P. islandica.
| i. | External morphology: Body elongate, live specimens up to 110 mm long in P. azorica and up to 150 mm long in P. islandica. Skin semi-transparent, light orange in vivo, whitish in ethanol-preserved specimens. Velum about 1/3 of the body length, with 2 pairs of papillae, the anterior pair the largest, fused at the proximal 1/3, posterior pair minute. Two pairs of minute small papillae posteriorly from velum. Tube feet 9–10 pairs, arranged in posterior 3/4 of the body, 4 or 5 posteriormost pairs fused forming a wide brim. | ||||
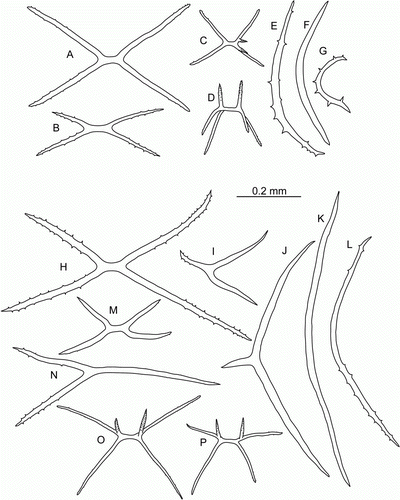
| ii. | Dorsal ossicles. In both species of the Peniagone-type, with curved arms and usually 4 (or 3, very rarely 2) apophyses rising from the arms; arms and apophyses with small serrations more conspicuous on apophyses (C–E,H,I). In P. azorica (, H,I) length of arms 0.10–0.24 mm, usually 0.17–0.22 mm; arms and apophyses approximately equal in length, sometimes arms longer than apophyses, rarely shorter. Deflection angle 13–28° in apophyses, 13–35° in arms. The most common type of dorsal ossicles in P. azorica (the ‘azorica’ type) with apophyses slightly shorter than arms or approximately equal in length; deflection angle in apophyses 15–25°, in arms 25–30° (A,B,H–J). In P. islandica ( and ) dorsal ossicles are more variable, the arm length 0.15–0.42 mm, usually 0.17–0.25 mm; arms shorter than apophyses, rarely equal in length or longer. Deflection angle in apophyses 10–32°, in arms 30–78°. The most common type of dorsal ossicles in P. islandica (the ‘islandica’ type) with arms widely spaced, shorter than apophyses; distal part of arms curved downwards, deflection angle in arms 35–45°, in apophyses 20–23° (A–D, A–C,F–H,L). However, many variations of the ‘islandica’ type were found, e.g. ossicles with widely spaced arms, deflection angle in arms >50° (F–H), or ossicles with exceptionally long narowly spaced apophyses and arms (J). Ossicles of the ‘azorica’ type prevail in P. azorica, while ossicles of the ‘islandica’ type dominate in P. islandica. However, in both P. azorica and P. islandica ossicles of both types can be mixed, or ossicles of intermediate types occur (C–G,K,L and D,E,I). Overall the variability in the shape of dorsal ossicles in these two species is much higher than in other species of Peniagone. | ||||
| iii. | Ventral ossicles. In both species flattened crosses with 4 short spinous apophyses and spinous enlarged arm ends (F,G,J). | ||||
Figure 10. Peniagone islandica Deichmann, Citation1930, dorsal ossicles, side view. (A–D) St. JC048/43 Dive 174; (E–G) St. JC037/15; (H,I) St. JC048/54 Dive 179.
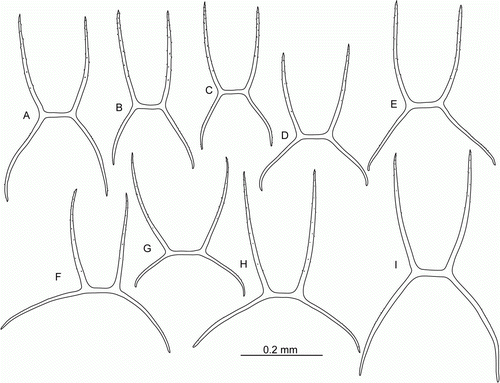
Figure 11. Peniagone islandica Deichmann, Citation1930, dorsal ossicles, side view. (A–E) St. JC048/53 Dive 178; (F–J,K–M) two specimens from St. JC037/15.
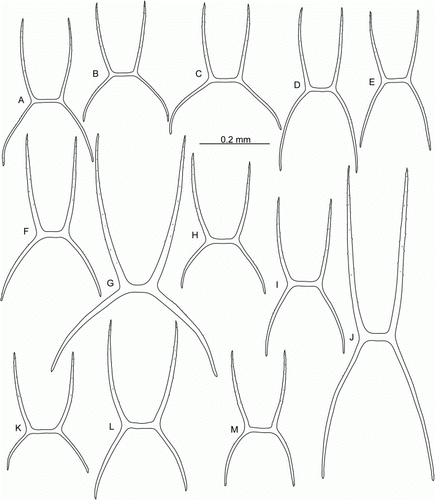
Figure 12. (A–G) Peniagone islandica Deichmann, Citation1930, St. JC048/53 Dive 178. (A,B) Dorsal and ventral view, scale A; (C–E) dorsal ossicles, scale C; (F,G) ventral ossicles, scale B. (H–J) Peniagone azorica von Marenzeller, 1893, St. JC011/101. (H,I) Dorsal ossicles, scale C; (J) ventral ossicles, scale B. (K–M) Peniagone longipapillata Gebruk, Citation2008. (K) Dorsal view, St. JC048/16 Dive 162, scale A; (L) dorsal ossicle of Peniagone-type, St. JC048/24 Dive 165, scale D; (M) dorsal rods, St. JC048/24 Dive 165, scale E.
The genetic relationship between P. islandica and P. azorica was examined based on sequences of four gene segments isolated from the ECOMAR material: 16S mtDNA, 18S and 28S rRNA and histone 3 H3 (C. Alt unpubl. data). Only H3 showed a clear separation of these species. On the consensus tree P. islandica and P. azorica grouped in one clade, but with low support (0.86). This may indicate that they are diverging species. The high degree of variability and overlap in the morphology of the dorsal ossicles in the two species supports this hypothesis.
Records show P. azorica and P. islandica are separated by the sub-polar front at the ECOMAR study site: P. azorica occurred north of the front, while P. islandica to south of it. Both species were relatively common at the MAR and are benthopelagic.
Specimens from the Scotia Sea (Akademik Kurchatov, St. 916) referred to P. azorica by Gebruk (Citation1990) were re-examined and found to belong to a separate species, possibly a new one.
Distribution
Reliable records come from the central and eastern North Atlantic. Depth 1385–4020 m.
Peniagone islandica Deichmann, Citation1930
(, , A-G M, L)
Peniagone islandicus Deichmann, Citation1930: 137; Heding Citation1942: 20–21, text figure 19.
Peniagone islandica Hansen Citation1975: 150; Gebruk Citation1990: 95–96, figure 35.
Material examined
St. JC011/23 (103 ind.), St. JC037/15 (49 ind.), St. JC037/19 (20 ind.), St. JC037/27 (20 ind.), St. JC037/79 (1 ind.); St. JC048/43 Dive 174 (1 ind.) and St. JC048/54 Dive 179 (1 ind.). This species was observed in situ at stations JC048/33 Dive 170 (11 ind.), JC048/36 Dive 171 (10 ind.), JC048/40 Dive 173 (numerous), JC048/53 Dive 178 (numerous) and JC048/56 Dive 180 (numerous).
Remarks
An important character that distinguishes this species, and is contained in the original description, is the number of tentacles which totals 8. This number is unusual for Peniagone and the wider Elpidiidae family: most elpidiids have 10 tentacles, with the exception of the genus Achlyonice which has 10–12 tentacles. Eight tentacles were present in the two (and only) type specimens upon which the original description of P. islandica was based (Deichmann Citation1930). The 8 tentacles were later confirmed in the two type specimens by Heding (Citation1942) and Hansen (Citation1975).
All of the specimens of P. islandica and P. azorica examined in this study had 10 tentacles. Numerous specimens of Peniagone identified as P. azorica and collected in the type locality of P. islandica (south-west of Iceland) by the BIOICE programme were re-examined. In all these specimens the number of tentacles was also 10.
Also, see ‘Remarks’ for P. azorica.
Distribution
Northern MAR, from the CGFZ area to Iceland. Depth 2137–2758 m.
Peniagone longipapillata Gebruk, Citation2008
(K–M, O, F,G,P)
Peniagone longipapillata Gebruk, Citation2008: 56–58, figures 1(b), 9, 10.
Material examined
St. JC011/111 (1 ind.), St. JC048/16 Dive 162 (1 ind. fixed in formalin) and St. JC048/24 Dive 165 (1 ind.).
Peniagone longipapillata was described based on incomplete damaged specimens that had been collected by the MAR-ECO cruise at several stations on the MAR between the Azores and the CGFZ. The description included one in situ image showing a dark red specimen with two very long papillae extending from the velum (Gebruk Citation2008, figure 1B). The ECOMAR specimens collected by the ROV were in excellent condition allowing the external morphological characters to be clarified, in particular the structure of the velum.
Diagnosis
(After Gebruk Citation2008, and clarified by the present material.) Body elongated and flattened. Colour reddish to dark pink. Skin gelatinous. Tube feet 10–13 pairs, in posterior half of the body; posteriormost tube feet fused forming wide lobe. Velum composed of 3 pairs of papillae; first anteriormost pair short, fully fused; second pair extremely long papillae fused proximally, exceeding body in length; third pair very short. One pair of short dorsal papillae posterior of velum. Ossicles dorsally with 4 curved arms, 0.3–0.4 mm long, and 4 apophyses 0.15–0.25 mm long; both arms and apophyses spinous; ossicles of deeper skin layers spinous rods up to 0.12 mm long. Ventral ossicles primary crosses, with arms 0.10–0.15 mm long, slightly bent; short apophysis on each arm.
Description
Body in vivo up to 160 mm in length (without long velum papillae). Skin thick, gelatinous, semi-transparent, reddish. Mouth tube long, comprising one third of the body length. Velum consists of three pairs of papillae (K): the anteriormost papilae short, completely fused forming a lobe; papillae in the second pair exceptionally long, may exceeded twice the body length (G); papillae of the third pair are the smallest, they have appearance of a small triangular lobe at the base of papillae of the second pair. One additional pair of short papillae posterior to the velum. Tube feet 10–13 pairs; 6 posteriormost pairs of tube feet fused forming posterior swimming lobe. Unlike the type specimens in the ECOMAR specimen the ossicles of the ‘Peniagone-type’ (L) were very scarce in the skin. Ossicles in deeper skin layers numerous spinous rods up to 0.12 mm long (M), not noted in the type specimens. Ventral ossicles only numerous rods in deeper skin layers, similar to those on dorsum, no primary crosses present in the type series.
Remarks
Despite some differences in the ossicle composition, we refer the specimens to Peniagone longipapilata, mainly because the main type of dorsal ossicles corresponds well to the original description. Also, this species has very characteristic unusually long papillae and a reddish colour, which are characters rarely found in Peniagone. Differences in ossicles may arise from natural variability or be the result of differences in preservation. More material is required to clarify this issue.
Peniagone longipapillata is a benthopelagic species.
Distribution
Central and northeast Atlantic. Apart from the ECOMAR study area this species was observed in situ in the Whittard Canyon, northeast Atlantic, at depths around 3500 m (RRS James Cook cruise JC036). It is also known from the Porcupine Seabight (D. Billett, pers. comm.). Depth 2272–3500 m.
Peniagone coccinea sp. nov. Rogacheva & Gebruk
(, , H,I, O, E; online supplement 1)
Holotype
NHM 2012.10, RRS James Cook, St. JC048/38, ROV Isis Dive 172, 48°43.63′N, 28°28.83′W, 2600 m, 19–20 June 2010, specimen 70 mm long.
Paratypes
NHM 2012.11, RRS James Cook, St. JC048/43, ROV Isis Dive 174, 48°43.87′–48°44.23′N, 28°39.01′–28°39.57′W, 2620–2547 m, 22 June 2010 (1 ind., 65 mm long) and NHM 2012.12, St. JC037/27, 48°57.34′–49°13.55′N, 27°49.83′–27°50.92′W, 2700 m, 18 August 2009 (1 ind., ~35 mm long, originally preserved in 10% formalin).
Additional material
St. JC037/15 (15 ind.), St. JC037/19 (14 ind.) and JC037/27 (10 ind.). This species was observed in situ at stations: JC048/33 Dive 170 (24 ind.), JC048/36 Dive 171 (9 ind.) and JC048/40 Dive 173 (numerous).
Type locality
North Atlantic, MAR, to south of the CGFZ. Depths 2600–2750 m.
Diagnosis
Body elongated, posterior end broad. Colour bright red, skin transparent. Velum with 2 pairs of long and wide papillae fused at the base. Tube feet 9 pairs, posterior pairs forming brim. Ossicles of Peniagone-type, scattered; dorsal ossicles with spinous curved arms up to 0.2 mm long, each arm with one spinous apophysis up to 0.07 mm long.
Description
Length 70–90 mm in vivo, reducing to 50 mm in ethanol preserved specimens. Body length about 2.5× the width. Posterior end wide (more round in specimens caught by trawls; B,C). Colour from light red to scarlet (H,I, E). Skin thick, transparent, consistency gelatinous; skin may be not transparent in specimens sampled by trawls or preserved in ethanol. Velum (A) large, may reach in length 2/3 of the body length; 2 pairs of wide papillae fused at the very base; the first pair longer than the second. Velum almost lacking, reduced to small protrusion in specimens caught by trawls (B). Two pairs of small papillae arranged posteriorly of velum, not preserved in specimens caught by trawl. Tube feet 9 pairs; posterior 2 pairs small; posterior 3 pairs fused forming narrow brim. Ossicles of Peniagone-type scarcely distributed in skin; some specimens from trawl catches lacked skin with ossicles. Dorsal ossicles ( D–H) with arms up to 0.2 mm long, strongly curved downward, spinous; apophyses 4, up to 0.07 mm long, covered by small spines, pointed; ossicles of deeper skin layers on dorsum flattened crosses with spinous arms about 0.2 mm in length (I). Ventral ossicles (J–L) flattened crosses with highly spinous arms bearing 4, rarely 3, short blunt spinous apophyses; arms up to 0.15 mm, apophyses 0.05 mm, ossicles in deeper skin layers lacking. Tube feet ossicles flattened crosses with 0–2 apophyses (A–C), crosses with arms curved downward with 1–2 apophyses (D), and various rods up to 0.5 mm in length, often spinous (E–G). In tentacles flattened crosses and crosses with arms curved downward, with 0–2 apophyses ( H,M,O,P), usually apophyses absent; various rods (J–L,N) and ossicles of irregular shape also present (I).
Remarks
This benthopelagic species is common at both southern ECOMAR sites. Commensal polynoid polychaetes attached to the ventral side were found in one specimen (online supplement 1).
Relationships
Dorsal ossicles in Peniagone coccinea resemble those in P. porcella R. Perrier, Citation1896, but in the former they are smaller and less robust (dorsal ossicles in P. porcella have arms 0.2–0.3 mm long and apophyses of about 0.1 mm long). Peniagone coccinea has a very characteristic appearance, recognizable on in situ images and videos: the velum consists of 2 long and wide papillae that are free over almost their whole length, and bright red colour of body that is rare in Peniagone.
Distribution
Known only from the type locality.
Etymology
The species name coccinea means ‘scarlet’, corresponding to the colour of this holothurian.
Penilpidia midatlantica Gebruk, Citation2008
Penilpidia midatlantica Gebruk, Citation2008: 52–54, figures 4–6.
Material examined
St. JC037/19 (1 ind.).
Distribution
Penilpidia midatlantica is known only from the MAR: north of the Azores and to southeast of CGFZ. Depth 2078–2430 m.
Family Pelagothuriidae Ludwig, Citation1894
Enypniastes eximia Théel, 1882
Enypniastes eximia Théel, Citation1882a: 56–57, plate VIII(6–7); Sluiter Citation1901b: 77–79, plates II(8–9), V(5); Mitsukuri Citation1912: 215–218, plate VII(59–60); Heding Citation1950: 118; Pawson Citation1976: 289, plate I(a); 1982: 132, figure 3(a–c); Ohta Citation1985: 127–128; Gebruk Citation1989: 61–63, figure 2(6–7); Miller & Pawson Citation1990: 10–13, figures 1(c,d), 4; Bluhm & Gebruk Citation1999: 181, figure 6(d).
Enypniastes decipiens Koehler & Vaney, Citation1910: 95–96, plate III(1).
Enypniastes globosa Hansen & Madsen, Citation1956: 58–59, plate I(4–5).
Peniagone ecalcarea Sluiter, Citation1901a: 74–75, plate X(2).
Enypniastes ecalcarea Heding Citation1950: 118, figures 1–4.
Euryplastes obscura Koehler & Vaney, Citation1905: 71–72, plate IV(7–9).
Pelagothuria bouvieri Hérouard, Citation1906: 1–6, figure 2; 1923: 94–99, plate VI(1).
Planktothuria diaphana Gilchrist, Citation1920: 373–381, figures 1–4.
Enypniastes diaphana Billett et al. Citation1985: 400–403, figure 2.
Material examined
Only visual observation at station JC048/43 Dive 174 (1 ind.).
Distribution
Cosmopolitan species, occurring in tropical and moderate latitudes at depth range of 0–5433 m (Gebruk Citation1989).
Order Molpadiida Haeckel, Citation1896
Family Molpadiidae Müller, Citation1850
Molpadia musculus Risso, Citation1826
Molpadia musculus Risso, Citation1826: 293; Clark Citation1908: 165, plates 22(4–9), 23(4–7); Deichmann Citation1940: 225, plate 40(1–15); Citation1954: 405; Pawson Citation1965: 11, figure 3; Citation1977: 100, figures 1–4; Cherbonnier Citation1965: 17, plates 7(i–q), 8(a–j); Tortonese Citation1965: 98, figure 42; Pawson et al. Citation2001: 317–318, figure 2(a–c).
Molpadia violacea Studer, Citation1876: 464; Pawson Citation1963a: 15–16, plate 3(4–8); 1965: 12–13.
Trochostoma violaceum Théel Citation1886a: 42, plate 2(6); Lampert Citation1889: 842; Perrier Citation1905: 65.
Haplodactyla violacea Heding Citation1931: 280.
Eumolpadia violacea Heding Citation1935: 42, text figure 8(7–10), plates 5(10), 7(3), 8(4); Ludwig & Heding Citation1935: 144–145, text figure 11.
Eumolpadia asaphes Heding, Citation1935: 42–44, text figure 9, plates 5(9–10), 7(2).
Ankyroderma loricatum Perrier, Citation1898: 1666; 1902: 535, plate 22(23–28); Hérouard Citation1923: 133.
Ankyroderma danielsseni Théel, Citation1886a: 39, plate 2(6).
Material examined
St. JC011/17 (20 ind.), St. JC011/23 (39 ind.), St. JC011/101 (2 ind.), St. JC011/106 (1 ind.), St. JC011/111 (1 ind.), St. JC037/19 (66 ind.), St. JC037/27 (204 ind.) and St. JC037/61 (1 ind.).
Remarks
This infaunal species was common at all three ECOMAR sites that were trawled.
Distribution
Cosmopolitan species, unknown only from the Arctic. Depth 35–5205 m.
Molpadia aff. blakei (Théel, 1886)
()
Material examined
St. JC011/23 (11 ind.), St. JC011/75 (3 ind.), St. JC011/101 (3 ind.), St. JC011/106 (2 ind.), St. JC037/15 (2 ind.), St. JC037/19 (21 ind.), St. JC037/27 (32 ind.), St. JC037/61 (5 ind.), St. JC037/67 (5 ind.) and St. JC037/70 (4 ind.).
Description
Body length 9–38 mm. Skin brownish-light violet. Anterior part separated by constriction behind calcareous ring. Tentacles 15, discs with 2 processes. Body wall ossicles (A–H) tables with three-pillared spires; most tables with 6 holes; tables with 3–5 holes also present. Phosphatic deposits absent. Tail with fusiform ossicles with three-pillared spire and few holes of different size (I–L).
Remarks
In the ECOMAR specimens, discs in the body wall ossicles had mostly 6 holes that were different from prevailing discs with three holes in the original description and descriptions of Deichmann (Citation1930, Citation1940) and Pawson et al. (Citation2001). The ECOMAR specimens were referred to as Molpadia blakei based on the composition of ossicles. The variation of ossicles in M. blakei is not well known. Examination of the type material of M. blakei and additional material from the north Atlantic is required for more reliable identification.
This infaunal species was common in trawl catches at three ECOMAR sites: both northern sites and the southeast site.
Order Apodida Brandt, Citation1835
Family Myriotrochidae Théel, Citation1877
Myriotrochus clarki Gage & Billett, Citation1986
(L)
Myriotrochus clarki Gage & Billett, Citation1986: 247, figures 7(d), 9(d), 13–17, 18(a); Harvey et al. Citation1988: 190; Smirnov Citation1999: 17.
Myriotrochus vitreus Cherbonnier Citation1970: 1269. (non M. Sars, 1866)
Myriotrochus sp. Gage et al. Citation1985: 203.
Material examined
St. JC011/075 (3 ind.), St. JC011/101 (2 ind.), St. JC037/15 (1 ind.), St. JC037/19 (6 ind.), St. JC037/27 (5 ind.), St. JC037/61 (4 ind.), St. JC037/67 (5 ind.), St. JC037/70 (6 ind.), St. JC048/49 (2 anterior fragments), St. JC048/50 (1 anterior fragment), St. JC048/52 (1 anterior fragment).
Remarks
Although this species is infaunal, it was observed in situ at the sediment surface at the station JC048/24 Dive 165. Skin in the specimen in vivo was transparent and light greenish in colour.
Distribution
The northeast Atlantic (Rockall Trough and the Bay of Biscay) and the Mid-Atlantic Ridge. Depth 480–2907 m.
Family Synaptidae Östergren, Citation1898
Labidoplax sp.
(A,B)
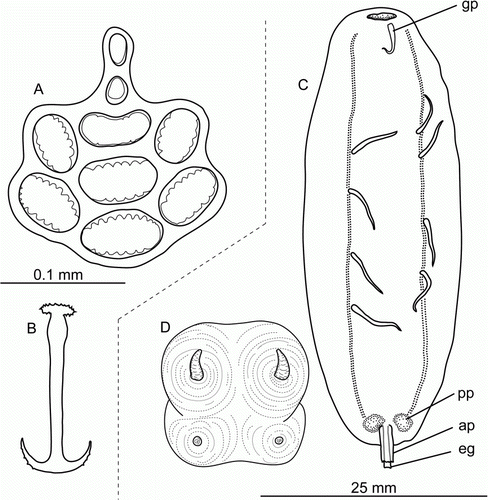
Figure 16. (A,B) Labidoplax sp., St. JC037/15, body wall ossicles. (C,D) Gephyrothuria alcocki Koehler & Vaney, Citation1905. St. JC037/19. (C) Dorsal view, scale 25 mm; (D) tentacle disc, magnified. ap, anal papilla; gp, genital papilla; eg, endgut; pp, posterior protuberance.
Material examined
St. JC011/75 (a fragment of body wall skin), St. JC048/34 (1 full ind., 1 anterior and 2 posterior fragments), St. JC048/22 (1 anterior fragment), St. JC048/27 (fragments), St. JC048/42 (1 ind.), St. JC048/49 (1 ind. and 1 anterior fragment), St. JC048/50 (1 anterior fragment), St. JC048/52 (1 ind.).
Description
Specimens 4–20 mm long. Skin whitish with dense layer of ossicles. Tentacles 11, each with 1 terminal and 2 lateral digits. Calcareous ring consists of 11 pieces. Ossicles anchors and anchor plates (A,B). Anchors 0.13–0.20 mm long, narrower than anchor plates, outer margin of arms with few serrations; shaft swollen posteriorly. Anchor plates 0.15–0.20 mm in length, 0.12–0.18 mm wide; anterior part with 7 serrate holes, rarely serration absent; posterior hole bean-shaped, rarely rounded; handle tapered, with 2 smooth holes, the posteriormost hole the biggest, sometimes holes fused into long narrow hole.
Remarks
The morphology of the anchor and the anchor plate ossicles is the most characteristic feature in the taxonomy of Labidoplax. Labidoplax sp. from the ECOMAR area is similar to L. georgii Smirnov, Citation1997 in the number of tentacles and the ossicle morphology. There are some minor differences in the shape of the handle, which is not narrowed in L. georgii. Ossicles of our specimens also resemble L. media Östergren Citation1905, the species with 12 tentacles. Labidoplax georgii is known based on three records off New Caledonia from a depth range 570–1675 m. Labidoplax media is distributed at sublittoral depths at Norwegian, Scottish and Irish coasts and in the North and Mediterranean Seas.
Only two species of Labidoplax are known in the deep sea: L. southwardorum and L. similimedia. Both species were described by Gage (Citation1985) from the Rockall Trough in the northeast Atlantic, from depths between 1000 and 2950 m.
Only a fragment of one specimen was found in OTSB catches. Other specimens were collected with a megacorer. Specimens of Labidoplax sp. were found mainly in the upper 0–2 cm sediment slices. However, two specimens also were found in 2–6 cm slices. At stations JC048/49, JC048/50 and JC048/52, Labidoplax sp. was found together with Myriotrochus clarki but in different sediment layers: 0–2 and 2–6 cm, respectively.
Incertae familiae
Family Gephyrothuriidae Koehler & Vaney, Citation1905
Gephyrothuria alcocki Koehler & Vaney, Citation1905
(C,D, L)
Figure 17. Underwater images. (A) Staurocucumis abyssorum, St. JC048/43 Dive 174; (B) Benthothuria funebris, St. JC048/29 Dive 169; (C) Hansenothuria sp., St. JC048/40 Dive 173; (D) Mesothuria maroccana, St. JC048/40 Dive 173; (E) Benthodytes gosarsi, St. JC048/3 Dive 158; (F) Psychropotes depressa, St. JC048/43 Dive 174; (G) Psychropotes depressa, St. JC048/54 Dive 179; (H) Laetmogone billetti sp. nov., St. JC048/24 Dive 165; (I) Laetmogone billetti sp. nov., St. JC048/16 Dive 162; (J) Peniagone azorica, St. JC048/24 Dive 165; (K) Amperima furcata, St. JC048/43 Dive 174; (L) Myriotrochus clarki, St. JC048/24 Dive 165; (M) Peniagone islandica and Amperima furcata, St. JC048/43 Dive 174; (N) Ellipinion alani sp. nov., St. JC048/24 Dive 165; (O) Peniagone longipapillata, St. JC048/24 Dive 165.
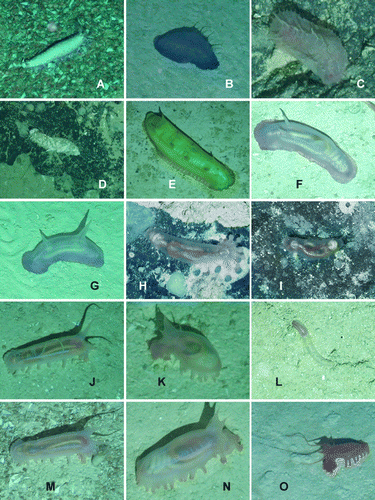
Figure 18. (A–J) Underwater images; (K–P) freshly caught specimens from ROV, photos courtesy: David Shale. (A) Paelopatides grisea, St. JC048/6 Dive 159; (B) Benthodytes lingua, St. JC048/43 Dive 174; (C) Synallactidae gen. et sp. indet., St. JC048/40 Dive 173; (D) Pseudostichopus peripatus, St. JC048/24 Dive 165; (E) Deima validum validum, St. JC048/43 Dive 174; (F,G) Peniagone longipapillata, St. JC048/24 Dive 165; (H,I) Peniagone coccinea sp. nov., St. JC048/54 Dive 179; (J) Synallactes aff. crucifera, St. JC048/16 Dive 162; (K) Laetmogone billetti sp. nov., St. JC048/56, Dive 180; (L) Peniagone islandica, St. JC048/54 Dive 179; (M,N) Amperima furcata, St. JC048/54 Dive 179; (O) Peniagone coccinea sp. nov., St. JC048/54 Dive 179; (P) Peniagone longipapillata, St. JC048/16 Dive 162.
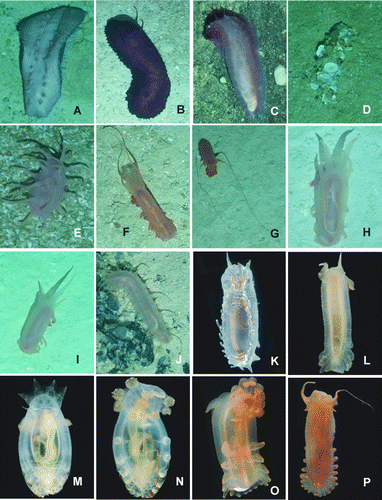
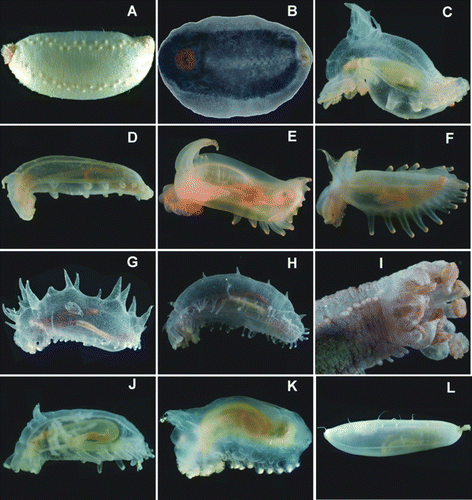
Figure 19. Freshly caught specimens from trawls and ROV, photos courtesy: David Shale. (A) Abyssocucumis abyssorum, St. JC037/15; (B) Benthothuria funebris, St. JC037/15; (C) Amperima furcata, St. JC048/54 Dive 179; (D) Peniagone azorica, St. JC048/24 Dive 165; (E) Peniagone coccinea sp. nov., St. JC048/54 Dive 179; (F) Ellipinion alani sp. nov., St. JC048/24 Dive 165; (G) Laetmogone billetti sp. nov., St. JC048/56, Dive 180; (H,I) Laetmogone billetti, St. JC048/24 Dive 165; (J) Kolga nana, St. JC048/24 Dive 165; (K) Ellipinion delagei, St. JC048/24 Dive 165; (L) Gephyrothuria alcocki, St. JC037/61.
Gephyrothuria alcocki Koehler & Vaney, Citation1905: 78–80, figures 6–8; Clark Citation1908: 22, 186; Hérouard Citation1923: 33; Deichmann Citation1930: 202; Citation1940: 358; Heding Citation1935: 78; O'Loughlin Citation1998: 495–496, figure 1.
Himasthlephora glauca Clark, Citation1908: 22, 40–41, 185, plate XIII(1–4); Heding Citation1935: 78.
Gephyrothuria glauca Hérouard Citation1923: 33; Deichmann Citation1930: 202–203; Citation1940: 209–211; Heding Citation1940: 358; Hansen Citation1956: 48; Pawson et al. Citation2001: 315.
Gephyrothuria europeensis Hérouard, Citation1923: 30–33, plate IX(10).
Material examined
St. JC011/17 (1 ind.), St. JC011/23 (33 ind.), St. JC011/75 (34 ind.), St. JC011/101 (7 ind.), St. JC011/106 (6 ind.), St. JC011/111 (1 ind.), St. JC037/15 (4 ind.), St. JC037/19 (89 ind.), St. JC037/ 27 (118 ind.), St. JC037/61 (12 ind.), St. JC037/67 (9 ind.) and St. JC037/70 (7 ind.).
Description
Body cylindrical, posterior end narrowed (C). Skin thin, smooth (in fresh specimens), whitish, non-transparent or semi-transparent, naked or completely covered with sand particles. Mouth terminal. Anus subdorsal, opened on tip of anal papillae, skin of anal papillae thin and damaged by intestine protruding out in all observed specimens; posterior end pointed upward. Papillae 6–9, filiform, paired and unpaired; paired papillae mainly arranged obliquely. Tentacles 15; tentacle discs with 2 short appendices on the outer margin (D). Genital papillae if present located close to the anterior end of dorsal interradius. Gonad in single tuft.
Remarks
The ECOMAR specimens were referred to as G. alcocki according to the last revision of Gephyrothuriidae by O'Loughlin (Citation1998). In that work, G. europeensis and G. glauca are considered as young synonyms of G. alcocki. However, Pawson et al. (Citation2001) referred the northwest Atlantic Gephyrothuria to G. glauca, not commenting on its relationship to G. alcocki.
This infaunal species was common in trawl catches at the following three ECOMAR sites: both northern sites and the southeast site.
Distribution
Cosmopolitan in tropical and moderate latitudes. Depths 732–5379 m.
Faunal composition
In total, 32 species of Holothuroidea were identified in the ECOMAR area including the 3 species new to science: Laetmogone billetti sp. nov., Ellipinion alani sp. nov. and Peniagone coccinea sp. nov. The two species Hansenothuria sp. and Labidoplax sp., might also be new to science, but for their reliable identification more data are required on the variation of the ossicles. One species was identified only to family level (Synallactidae gen. sp.). The species belong to five orders: Dendrochirotida (1 species), Aspidochirotida (10 species), Elasipodida (16 species), Molpadiida (2 species) and Apodida (2 species). One species, Gephyrothuria alcocki, belongs to the family Gephyrothuriidae, a group for which the taxonomic position is uncertain (Solís-Marín Citation2003), currently placed within the Molpadiida (O'Loughlin Citation1998; Pawson et al. Citation2001). New data on the molecular phylogeny of Gephyrothuriidae suggest that it constitutes a separate order from the Molpadiida (C. Alt unpubl. data).
Elasipodida, in particular the family Elpidiidae, appeared the most species-rich taxon at the CGFZ area (Gebruk et al. Citation2010). This was expected, as elpidiids are very common in the deep sea at lower bathyal and especially abyssal depths.
The deep North Atlantic is one of the best-explored regions of the deep ocean. About 55 holothurian species are known from the northern MAR from 20°N to Iceland. All, with the exception of the three new species, were known from the North Atlantic. Many of these species were reported earlier by the MAR-ECO project (Gebruk Citation2008). The holothurians in MAR-ECO were sampled using the otter-trawl (the gear similar to that used in ECOMAR) at 9 stations: 5 southeast and 4 northwest of the CGFZ, in the depth range 1237–3527 m. At these stations 24 species were identified. From these species, 17 were found in the ECOMAR material and of those Peniagone longipapillata, Penilpidia midatlantica and Benthodytes gosarsi, are species that were described by the MAR-ECO expedition (Gebruk Citation2008). Twelve of the ECOMAR species were reported by MAR-ECO north of the Azores, while several species reported by MAR-ECO were not found by ECOMAR. Some of them, such as Psychropotes longicauda, probably occur at greater depth than was sampled within ECOMAR, and others occur at shallower depths (e.g. Thyone sp.). Paroriza pallens and Peniagone diaphana, reported by MAR-ECO northwest of the CGFZ, also were absent from the ECOMAR samples. Overall, despite the narrower depth range sampled in ECOMAR, more holothurian species were found in the CGFZ area compared to MAR-ECO: 32 and 24 species, respectively. This is a result of more intensive benthos sampling in ECOMAR together with the use of the ROV.
Sampling using ROV and trawl
In situ observations and selective targeted sampling using ROV Isis added the following seven species of holothurians that had not been collected by trawling within ECOMAR: Hansenothuria sp., Synallactidae gen. et sp. indet., Kolga nana, Peniagone longipapillata, Enypniastes eximia, Ellipinion alani sp. nov. and Laetmogone billetti sp. nov. Some of these species occur in habitats where trawling is impossible. For example, Hansenothuria sp. and the Synallactidae gen. et sp. indet. were observed only on steep slopes and cliffs. Laetmogone billetti sp. nov. was more common on slopes and cliffs than on flat sedimentary terrains. Other species, such as Enypniastes eximia, were also inaccessible through trawling because they are benthopelagic and rarely descend to the flat terrace. Finally, there is a group of species of gelatinous consistency. Even if sampled in trawls, they usually are badly damaged, rendering identification impossible. Among such species are Kolga nana, Peniagone longipapillata and Ellipinion alani sp. nov.
Equally, seven species found in ECOMAR were not observed in situ by the ROV video survey. Among them were infaunal species (Molpadia musculus, M. aff. blakei, Labidoplax sp. and Gephyrothuria sp.) and rare species (Bathyplotes natans, Molpadiodemas violaceus and Penilpidia midatlantica). Some holothurians, such as species of Mesothuria and Pseudostichopus, are difficult to identify in situ and their identification is only possible in the lab.
Our results demonstrate that significantly more information is obtained on deep-sea holothurians by combining data from trawl catches with observations and sampling from ROV video surveys. Many deep-sea holothurians are gelatinous and features of their external morphology are lost in specimens caught by trawls. Hence, the taxonomy of deep-sea holothurians is based mainly on ossicle morphology; characteristics of external morphology, although distinctive, are difficult to use.
Morphological adaptations
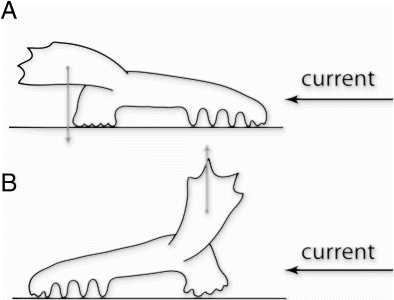
The external morphology in many genera of elpidiids is related to adaptations to a benthopelagic lifestyle, primarily swimming (Hansen Citation1975; Gebruk Citation1990, Citation1994, Citation1995). It has been suggested that swimming, drifting or moving with the current on the sea floor can reduce the energy costs for locomotion in holothurians (Miller & Pawson Citation1990; Gebruk Citation1995; Rogacheva et al. 2012). Swimming elpidiids have an enlarged mouth tube that is formed by a tentacle crown and the anterior part of the body. Expanded tentacles forming the anterior lobe are the most powerful and effective locomotory organ in swimming holothurians. The posterior swimming lobe is formed by fused posterior tube feet, and a flattened posterior part of the body. The role of the velum does not appear to be very important for swimming. The velum in elpidiid holothurians is more important for their positioning at the seafloor: with the aid of the velum and by using the currents holothurians can lift up (B) or press down (A) the tentacle crown relative to the substratum, resulting in optimal positioning for food collection (Gebruk Citation1995). Some species seem to use their velum as a sail – as an extra driving force when ‘walking on legs’. Another common feature in elasipodids are two lateral rows of large tube feet (legs) used for walking (Hansen Citation1975). Free tips of velum papillae, when stretched, can also act as tactile organs exploring the substrate, as suggested by Hansen (Citation1975).
Peniagone is the most species-rich genus in the family Elpidiidae, showing the highest diversity of external morphological features. In the ECOMAR area we observed in situ detailed morphology of the following species: P. diaphana, P. azorica, P. islandica, P. longipapillata and P. coccinea sp. nov. The combination of position and size of their tube feet, velum and tentacle crown is shown in . The enlargement of tentacles (an important adaptation for swimming) correlates with major morphological transformations in the genus. Peniagone azorica and P. islandica (A) have relatively well-developed tube feet in the anterior half of the body and a small velum, and their tentacles are not enlarged. The velum can be relatively larger, as in P. horrifer (B), the species observed in situ at the abyssal plain around the Crozet Islands (Cross et al. Citation2012). In these three species, the anterior part of the body with tentacles used for food collection at the seafloor is balanced through the presence of large anterior tube feet and a velum. These species are more epibenthic, and they actively walk on their large lateral tube feet.
Figure 21. Morphological diversification in Peniagone. Side view (left row) and shape of the velum (right row). (A) Peniagone azorica and Peniagone islandica; (B) Peniagone horrifer; (C) Peniagone coccinea sp. nov.; (D) Peniagone longipapillata; (E) Peniagone diaphana.
Peniagone coccinea (C) and P. longipapillata (D) have enlarged tentacles, which lift the anterior part of the body above the substrate. At this morphological stage anterior tube feet become inefficient, unless their size is much increased. The tube feet get displaced towards the posterior end and are partly fused. A large velum or long velum papillae in these species can be effective in balancing the anterior end when holothurians are on the substrate. Peniagone longipapillata was observed commonly with its papillae stretched frontwards into the current, with the size of its papillae exceeding that of body length. Peniagone coccinea has comparatively the largest velum, which is also commonly observed at the seafloor stretched frontwards into the current. This type of velum increases the buoyancy of the body and can be also effective as a parachuting lobe for drifting in the water column (online supplement 1).
In Peniagone diaphana the tube feet are almost reduced and the velum is weakly developed, forming the anterior skin fold (E). According to in situ observations, P. diaphana spends most of its time drifting above the seabed with its anterior end oriented downward (Barnes et al. Citation1976). Lipps & Hickman (Citation1982) used a term ‘big-bag construction’ for such a morphology and behaviour.
In other genera of Elpidiidae, the velum (if present) is usually rather simple: built of two or three pairs of relatively short papillae, fused to varying extent and forming the anterior dorsal lobe, similar to the morphology of some Peniagone species such as P. azorica and P. islandica. The variability of the velum in Peniagone illustrates one of its adaptations to the deep-sea environment and correlates with the success of this genus in the deep ocean.
Lifestyle and habitat
New observations in situ showed that swimming holothurians often empty their intestines before leaving the seabed. The intestine is long in many deep-sea holothurians (Roberts et al. Citation2000) and acts as ballast when filled with sediment and detrital material. The following phases of activity seem to be common in swimming holothurians: swimming/drifting with the current (i), arriving at a feeding spot at the seabed (ii), actively feeding at the seabed (iii), defecating (iv), and taking off for swimming (v). These activities on the examples of Peniagone coccinea sp. nov. and Benthodytes gosarsi can be seen in the video supplement (online supplements 1 and 2). Many of the holothurian species reported here had not been observed in situ before (Rogacheva et al. 2012).
Holothurians are one of the most successful megafaunal taxa in the ECOMAR area: they are species-rich and abundant in different habitats (Gebruk et al. Citation2010). Most epibenthic deep-sea holothurians are deposit-feeders, usually occurring in flat areas on sedimented surfaces. Sedimented terrains (terraces) are a common feature of the ridge in the studied area and are a suitable habitat for deposit-feeders. Holothurians in the ECOMAR area belong to the following ecological categories: infaunal, epibenthic and benthopelagic. Among the infaunal species, species of Molpadia and Gephyrothuria alcocki were the most abundant. Two small burrowing holothurians, Myriotrochus clarki and Labidoplax sp., were also common, but because of their rather macrofaunal size they were sampled less successfully with OTSB. Several specimens of both species occurred in megacorer samples. Eight out of 11 specimens of Labidoplax sp. were from the upper 2 cm sediment layer, two specimens were from the 2–6 cm layer and 1 specimen was from the 6–10 cm layer. All four specimens of M. clarki were from the 2–6 cm layer. At stations JC048/49, JC048/50 and JC048/52 these two species occurred together: Labidoplax sp. in the 0–2 cm layer, M. clarki in the 2–6 cm layer. Larger specimens of M. clarki are likely to borrow deeper into sediment.
Epibenthic forms were dominated by Pseudostichopus peripatus, Staurocucumis abyssorum and Mesothuria ssp. Among the benthopelagic forms, different species dominated in abundance at different ECOMAR sites. At the southern sites, the dominant species were Benthodytes gosarsi, Psychropotes depressa, Peniagone islandica and Amperima furcata. In the north, the most abundunt species included Paelopatides grisea, Peniagone azorica and Kolga nana. Benthothuria funebris was abundant both in the north and the south. Kolga nana was remarkably numerous at the northeast site: several tens of individuals per square metre occurred in aggregations of this species (Rogacheva Citation2012).
Holothurians were observed even on cliffs, which was an unexpected habitat for this taxon. They were recorded on rocks and in sediment pockets between rocky outcrops. Some species, such as Laetmogone billetti sp. nov., Synallactidae gen. et sp. indet. and Hansenothuria sp., could be specialized cliff forms: they were seen overwhelmingly in habitats only accessible to swimmers (Rogacheva et al. 2012). The data on the abundance and biogeography of the ECOMAR holothurians will be a subject of separate publications.
Editorial responsibility: John Zardus
SMAR_750428_supplemental material.zip
Download Zip (40.6 MB)Acknowledgements
We would like to thank David Billett, Alan Hughes, Pam Talbot, Tammy Horton, Ian Cross, Nataliya Budaeva, Holly Bik and Andrew Cabrinovic for their help during work at sea, NOCS and NHM. Thanks are also due to Alexander Mironov for identification of Myriotrochus clarki, Alexey Smirnov for valuable comments on Labidoplax taxonomy and Mark O'Loughlin and an anonymous reviewer for critical comments to the manuscript. Special thanks to David Shale for remarkable photographs of holothurians made on board RRS James Cook. We also thank Henry Ruhl and Chris Hauton for providing material and in situ images from the JC036 Cruise, Mark Shields and Raimundo Blanco-Perez for providing holothurians from the megafaunal samples, Andy Gooday for identification of Discospirina tenuissima and David Billett for the correction of English usage. We are grateful to the ROV Isis team and the crew of the RRS James Cook for their great assistance and cooperation on board, and Ben Boorman for having led the trawling at the MAR. The BIOICE material was kindly provided by Guðmundur Guðmundsson, Guðmundur Helgason and Olafur Asthorsson. The authors thank Imants Priede for his support of this work from field activities to the manuscript stage. This work was supported by the UK NERC-funded ECOMAR project (NE/C512961/1). AR is grateful for partial financial support from the Norwegian Ministry of Foreign Affairs granted to the Institute of Marine Research, Norway, the MAR-ECO project and RFBR reseach grant 12-05-33049. AR and AG were in part supported by the grant of Minobrnauki of Russian Federation Nr 8664.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Barnes , AT , Quetin , LB , Childress , JI and Pawson , DL . 1976 . Deep-Sea Macroplanktonic Sea Cucumbers: Suspended Sediment Feeders Captured from Deep Submergence Vehicle . Science , 194 : 1083 – 85 . doi: 10.1126/science.194.4269.1083
- Bell FJ. 1892 . Catalogue of the British Echinoderms in the British Museum (Natural History) . London : British Museum . 197 pages
- Bergstad , OA and Godø , OR. 2003 . The pilot project ‘Patterns and progress of the ecosystem of the northern mid-Atlantic’: Aims, strategy and status . Oceanologica Acta , 25 : 219 – 26 . doi: 10.1016/S0399-1784(02)01203-3
- Billett , DSM and Hansen , B. 1982 . Abyssal aggregations of Kolga hyalina Danielssen and Koren (Echinodermata: Holothurioidea) in the north-east Atlantic ocean: A preliminary report . Deep-Sea Research , 29 : 799 – 818 . doi: 10.1016/0198-0149(82)90047-4
- Billett DSM , Hansen B , Huggett QJ. 1985 . Pelagic Holothurioidea (Echinodermata) of the northeast Atlantic . In : Keegan BF , O'Connor BDS , Echinodermata . Proceedings of the 5th International Echinoderm Conference: Galway , 24–29 September 1984 . Rotterdam : A.A. Balkema , p 399 – 411 .
- Bluhm , H and Gebruk , A. 1999 . Holothuroidea (Echinodermata) of the Peru Basin – Ecological and taxonomic remarks based on underwater images . Marine Ecology , 20 : 167 – 95 . doi: 10.1046/j.1439-0485.1999.00072.x
- Brandt JF. 1835 . Prodromus descriptionis animalium ab H. Mertensio in orbis terrarum . Circumnavigatione observatorum . Petropoli . 86 pages .
- Carney , RS and Carey , J. 1976 . Distribution pattern of holothurians of the Northeastern Pacific (Oregon, U.S.A.) continental shelf slope, and abyssal plain . Thalassia Jugoslavica , 12 : 67 – 74 .
- Cherbonnier G. 1941 . Étude anatomique et biogéographique sur deux Cucumaria abyssaux: Cucumaria abyssorum Théel et Cucumaria albatrossi n. sp . Bulletin du Muséum National d'Histoire Naturelle (Paris) 2e sér. 13 : 93 – 103 .
- Cherbonnier , G. 1965 . Expédition Océanographique Belge dans les Eaux Côtiéres Africaines de l'Atlantique Sud (1948–1949). Resultats Scientifiques: Holothurides . Institut Royal des Sciences Naturelles de Belgique , 3 ( 2 ) : 1 – 24 .
- Cherbonnier , G. 1970 . Echinodermes récoltés par la 'Thalassa’ au large des côtes d'Espagne et du Golfe de Gascogne (18–25 octobre, 1968). Bulletin du Muséum National d'Histoire Naturelle . 2e sér. , 45 : 1266 – 77 .
- Clark , HL. 1908 . The apodous holothurians. A monograph of the Synaptidae and Molpadiidae . Smithsonian Contributions to Knowledge , 35 : 1 – 231 .
- Clark , HL. 1913 . Echinoderms from Lower California, with descriptions of new species . Bulletin of the American Museum of Natural History , 32 : 185 – 236 .
- Clark , HL. 1920 . Holothurioidea. Memoirs of the Museum of Comparative Zoology . Report on the scientific results of the expedition to the tropical Pacific, in charge of Alexander Agassiz, by the U.S. Fish Commission Steamer “Albatross”, from August, 1899 to March, 1900 , 39 ( 4 ) : 115 – 54 .
- Clark , HL. 1923a . Echinoderms from Lower California (Supplimentary Report) . Bulletin of the American Museum of Natural History , 48 : 147 – 63 .
- Clark , HL. 1923b . The Echinoderm Fauna of South Africa . Annals of the South African Museum , 13 : 221 – 435 .
- Clark , HL and Deichmann , E. 1936 . On Psolicucumis Heding and its allies . The Annals and Magazine of Natural History , 17 ( 101 ) : 564 – 68 . doi: 10.1080/00222933608655155
- Cross , IA , Gebruk , A , Billett , D and Rogacheva , A. 2012 . Rediscovery of the elpidiid holothurian Peniagone horrifer (Elasipodida, Holothuroidea: Echinodermata) in the southern Indian Ocean . Marine Biodiversity , 42 : 241 – 45 . doi: 10.1007/s12526-012-0111-x
- Deichmann , E. 1930 . The holothurians of the western part of the Atlantic Ocean . Memoirs of the Museum of Comparative Zoology (Harvard) , 71 : 41 – 226 .
- Deichmann , E. 1936 . Notes on Pennatulacea and Holothurioidea collected by the first and second Bingham Oceanographic Expeditions 1925–1926 . Bulletin of the Bingham Oceanographic Collection , 5 ( 3 ) : 1 – 11 .
- Deichmann , E. 1940 . Report on the holothurians, collected by the Harvard–Havana Expeditions 1938 and 1939 with a revision of the Molpadonia of the Atlantic Ocean . Memorias de la Sociedad Cubana de Historia Natural , 14 : 183 – 240 .
- Deichmann E. 1954 . The holothurians of the Gulf of Mexico . In : Galtsoff PS Gulf of Mexico, its Origin, Waters, and Marine Life . Bulletin US Fisheries Commission 55 : 381 – 410 .
- Dilman , AB. 2008 . Asteroid fauna of the northern Mid-Atlantic Ridge with description of a new species Hymenasterides mironovi sp. n . Marine Biology Research , 4 : 131 – 51 . doi: 10.1080/17451000701821736
- Ekman , S. 1926 . Systematisch-phylogenetische Studien über Elasipoden und Aspidochiroten. Zoologische Jahrbücher . Abteilung fur Anatomie und Ontogenie der Tiere , 47 : 429 – 540 .
- Ekman , S. 1927 . Holothurien der deutschen Südpolar-Expedition 1901–1903 aus der Ostantarktis und von den Kerguelen . Deutsche Südpolar-Expedition , 19 ( Zoology 11 ) : 359 – 419 .
- Fisher , WK. 1907 . The Holothurians of the Hawaiian Islands . Proceedings of the United States National Museum , 32 : 637 – 744 . doi: 10.5479/si.00963801.32-1555.637
- Gage , JD. 1985 . New Synaptidae (Holothuroidea: Apoda) from the Rockall Trough . Journal of the Marine Biologocal Association of the United Kingdom , 65 : 255 – 61 . doi: 10.1017/S002531540006094X
- Gage , JD and Billett , DSM. 1986 . The family Myriotrochidae Théel (Echinodermata, Holothurioidea) in the deep northeast Atlantic Ocean . Zoological Journal of the Linnean Society , 88 : 229 – 76 . doi: 10.1111/j.1096-3642.1986.tb01190.x
- Gage , JD , Billett , DSM , Jensen , M and Tyler , PA. 1985 . Echinoderms of the Rockall Trough and adjacent areas. 2. Echinoidea and Holothuroidea. Bulletin of the British Museum (Natural History) . Series Zoology , 48 ( 4 ) : 173 – 213 .
- Gebruk , AV. 1989 . Revision of the family Pelagothuriidae (Holothuroidea, Elasipoda) with a review of Enypniastes eximia and Pelagothuria natatrix. 1. Review of the family Pelagothuriidae (Elasipoda) . Russian Journal of Zoology , 68 ( 12 ) : 57 – 66 .
- Gebruk AV. 1990 . Deep-sea Holothurians of the Elpidiidae Family . Moscow : Nauka . 160 pages .
- Gebruk AV. 1994 . Two main stages in the evolution of the deep-sea fauna of elasipodid holothurians . In : David B , Guille A , Feral J-P , Roux M , Echinoderms Through Time . Proceedings of the Eighth International Echinoderm Conference: Dijon , France , 6–10 September, 1993 . Rotterdam , Brookfield : A.A. Balkema , p 507 – 14 .
- Gebruk AV. 1995 . Locomotory organs in the elasipodid holothurians: Functional–morphological and evolutionary approaches . In : Emson R , Smith A , Campbell A , Echinoderm Research 1995 . Proceedings of the Fourth European Echinoderm Colloquium : London , United Kingdom , 10–13 April 1995 . Rotterdam , Brookfield : A.A. Balkema , p 95 – 102 .
- Gebruk , A. 2008 . Holothurians (Holothuroidea, Echinodermata) of the northern Mid-Atlantic Ridge collected by the ‘G.O. Sars’ MAR-ECO expedition with descriptions of four new species . Marine Biology Research , 4 : 48 – 60 . doi: 10.1080/17451000701842898
- Gebruk , A , Budaeva , N and King , N. 2010 . Bathyal benthic fauna of the Mid-Atlantic Ridge between the Azores and the Reykjanes Ridge . Journal of the Marine Biological Association of the United Kingdom , 90 : 1 – 14 . doi: 10.1017/S0025315409991111
- Gebruk , AV , Solís-Marin , FA , Billett , DSM , Rogacheva , AV and Tyler , PA. 2012 . Review of the genus Zygothuria Perrier, 1898 and the Atlantic group of species of the genus Mesothuria Ludwig, 1894 (Synallactidae: Holothuroidea) with description of the new species Mesothuria milleri sp. nov . Journal of Natural History , 46 : 265 – 348 . doi: 10.1080/00222933.2011.638423
- Gilchrist , JDF. 1920 . Planktothuria diaphana gen. et sp. n . Quarterly Journal of Microscopical Science , 64 : 373 – 82 .
- Grieg J. 1921 (1932) . Echinodermata from the Michael Sars North Atlantic deep-sea expedition 1910 . Report of the scientific results of the ’Michael Sars’ North Atlantic Deep-sea Expedition 1910 3 ( 2 ): 1 – 47 .
- Grube , AE. 1840 . Actinien, Echinodermen und Wurmer des Adriatischen und Mittlemeers , Könisberg : JH. Bon .
- Haeckel , E. 1896 . Systematische Phylogenie der Wirbellosen Thiere (Invertebrata): Zweiter Teil des Entwurfs einer systematischen Stammengeschichte , 720 Berlin : Reimer .
- Hansen , B. 1956 . Holothurioidea from depths exceeding 6000 meters . Galathea Report , 2 : 33 – 54 .
- Hansen , B. 1967 . The taxonomy and zoogeography of the deep-sea holothurians in their evolutionary aspects . Studies in Tropical Oceanography , 5 : 480 – 501 .
- Hansen , B. 1975 . Systematics and biology of the deep-sea holothurians . Galathea Report , 13 : 1 – 262 .
- Hansen B. 1988 . The genus Staurocucumis Ekman and its possible affinity with Echinocucumis Sars (Holothuroidea, Dendrochirota) . In : Burke RD , Mladenov PV , Lambert P , Parsley RL , Echinoderm Biology . Proceedings of the Sixth International Echinoderm Conference , Victoria , 5–9 August 1996 . Rotterdam , Brookfield : A.A. Balkema , p 301 – 08 .
- Hansen , B and Madsen , J. 1956 . On two bathypelagic holothurians from the South China Sea . Galathea Report , 2 : 55 – 59 .
- Harvey , R , Gage , JD , Billett , DSM , Clark , AM and Paterson , GLJ. 1988 . Echinoderms of the Rockall Trough and adjacent areas 3. Additional records. Bulletin of the British Museum (Natural History) . Series Zoology , 54 ( 4 ) : 153 – 98 .
- Heding , S. 1931 . On the classification of the molpadiids . Videnskabelige meddelelser fra dansk Naturhistorisk Forening i Kø´benhavn , 92 : 275 – 84 .
- Heding S. 1935 . Holothurioidea. Part I. Apoda – Molpadioidea – Gephyrothurioidea . The Danish Ingolf Expedition IV ( 9 ): 1 – 84 .
- Heding S. 1940 . Die Holothurien der Tiefsee-Expedition II. Aspidochirote und Elasipode Formen . Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer ‘Valdivia’ 1898–1899 24 : 317 – 95 .
- Heding S. 1942 . Holothurioidea. II. Aspidochirota – Elasipoda – Dendrochirota . The Danish Ingolf Expedition IV ( 13 ): 1 – 39 .
- Heding , S. 1950 . Uber die Planktothuria der Deutschen Tiefsee-Expedition, nebst einigen Bemerkungen uber die Systematik der pelagischen Holothurien . Zoologischer Anzeiger , 145 : 111 – 18 .
- Hérouard , E. 1896 . (Premier) note preliminaire sur les Holothuries provenant des dragegs du yacht ’Princesse Alice’ . Bulletin de la Société Zoologique de France , 21 : 163 – 68 .
- Hérouard , E. 1898 . (Deuxième) note préliminaire sur les Holothuries provenant des dragages du yacht “Princesse-Alice” . Bulletin de la Société Zoologique de France , 23 : 88 – 9 .
- Hérouard , E. 1899 . (Troisième) note preliminaire sur les Holothuries provenant des drageges du yacht ’Princesse Alice’. Revision de la sous–famille Elpidiinae et description de nouvelles especies . Bulletin de la Société Zoologique de France , 24 : 170 – 75 .
- Hérouard , E. 1902 . Holothuries provenant des campagnes de la ‘Princesse-Alice’ (1892–1897) . Résultats des Campagnes Scientifiques accomplies sur son yacht par Albert I, Prince Souverain de Monaco , 21 : 1 – 61 .
- Hérouard , E. 1906 . Holothuries. Résultats du Voyage du S.Y. Belgica en 1897–1898–1899 . Zoologie , 21 : 1 – 16 .
- Hérouard , E. 1923 . Holothuries provenant des campagnes des yachts ‘Princesse-Alice’ et ‘Hirondelle II’ (1898–1915) . Resultats des Campagnes Scientifiques du Prince de Monaco , 66 : 1 – 163 .
- Imaoka , T. 1978 . Three new species of the genus Pseudostichopus from the Japanese waters (Holothurioidea: Gephyrothuriidae) . Publications of the Seto Marine Biological Laboratory , 24 : 377 – 85 .
- Imaoka , T. 1990 . “ Holothuroidea ” . In Echinoderms from Continental Shelf and Slope around Japan , Edited by: Oguro , C , Okutani , T and Horikawa , H . 131 – 54 . Tokyo : Japan Fisheries Resource Conservation Association Tosho .
- Koehler , R. 1896 . Échinodermes. Résultats scientifiques de la campagne du ’Caudan’ dans le Golfe de Gascogne Aôut–Septembre 1895 . Annales de l'Université de Lyon , 26 : 33 – 127 .
- Koehler R , Vaney C. 1905 . Deep-sea Holothuroidea collected by the Royal Indian Marine Survey Ship ’Investigator’ . Calcutta . 123 pages .
- Koehler , R and Vaney , C. 1910 . Description d'Holothuries nouvelles appartenant au Musée Indien . Records of the Indian Museum , 5 : 89 – 103 .
- Lampert , K. 1889 . Die wärhend der Expedition S.M.S. ’Gazelle’, 1872–1874, von Prof. Dr. Th. Studer gesammelten Holothurien . Zoologische Jahrbücher , 4 : 806 – 39 .
- Liao Y. 1997 . Fauna Sinica. Class Holothuroidea . Beijing : Science Press . 334 pages .
- Lipps , JH and Hickman , CS . 1982 . “ Origin, age, and evolution of antarctic and deep-sea faunas ” . In The Environment of the Deep Sea , Edited by: Ernst , WG and Morin , JG . 324 – 56 . Englewood Cliffs , NJ : Prentice-Hall .
- Ludwig HL. 1894 . The Holothurioidea. Reports on an exploration off the west coasts of Mexico, Central and South America, and off the Galapagos Islands, in charge of Alexander Agassiz, by the U.S. Fish Comission Streamer ‘Albatross’, during 1891 . Cambridge : Memoirs of the Museum of Comparative Zoology , p 1 – 183 .
- Ludwig H. 1901 . Arctische und Subarctische Holothurien . In : Römer F , Schaudinn F Fauna Arctica . p 135 – 78 .
- Ludwig , H and Heding , S. 1935 . Die Holothurien der Tiefsee-Expedition I. Fußlose und dendrochirote Formen . Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer ‘Valdivia’ 1898–1899 , 24 ( 2 ) : 123 – 241 .
- Madsen , FJ. 1947 . The echinoderms collected by the ‘Skagerak’ Expedition in the Eastern Atlantic 1946 . Meddelanden från Göteborg Musei Zoologiska Avdelning, Sjätte Följden, Series B , 5 ( 7 ) : 1 – 16 .
- Madsen FJ , Hansen B. 1994 . Echinodermata Holothurioidea . Marine invertebrates of Scandinavia, 9 . Scandinavian University Press : Oslo , 143 pages .
- Marenzeller Ev . 1892 . Note preliminaire sur les Holothuries provenant des campagnes du yacht l’Hirondelle . Bulletin Société Zoologique France 17 : 64 – 66 .
- Marenzeller Ev . 1893 . Contribution a l'étude des Holothuries de l'Atlantique Nord . Resultats des Campagnes Scientifiques accomplies sur son yacht par Albert I, Prince souverain de Monaco 6 : 1 – 22 .
- Miller , JE and Pawson , DL. 1989 . Hansenothuria benti, new genus, new species (Echinodermata: Holothuroidea) from the tropical western Atlantic: A bathyal, epibenthic holothurian with swimming abilities . Proceedings of the Biological Society of Washington , 102 : 977 – 86 .
- Miller , JE and Pawson , DL. 1990 . Swimming sea cucumbers (Echinodermata: Holothuroidea): A survey, with analysis of swimming behaviour in four bathyal species . Smithsonian Contributions to the Marine Sciences , 35 : 1 – 18 . doi: 10.5479/si.01960768.35.1
- Mironov , AN. 2008 . Pourtalesiid sea urchins (Echinodermata: Echinoidea) of the northern Mid-Atlantic Ridge . Marine Biology Research , 4 : 3 – 24 . doi: 10.1080/17451000701847863
- Mitsukuri , K. 1912 . Studies on Actinopodous Holothurioidea . Journal of the College of Science, Tokyo Imperial University , 29 ( 2 ) : 1 – 284 .
- Mortensen T. 1927 . Handbook of the Echinoderms of the British Isles . London : Oxford University Press . 471 pages .
- Müller J. 1850 . Anatomische Studien ueber die Echinodermen Müller's Archiv , Berlin : 225 – 33 .
- Ohshima , H. 1915 . Report on the holothurians collected by the United States Steamer ‘Albatross’ in the northwestern Pacific during the summer of 1906 . Proceedings of the United States National Museum , 48 : 213 – 91 . doi: 10.5479/si.00963801.48-2073.213
- Ohta , S. 1985 . Photographic observations of the swimming behavior of the deep-sea pelagothuriid holothurian Enypniastes (Elasipoda, Holothurioidea) . Journal of the Oceanographical Society of Japan , 41 : 121 – 33 . doi: 10.1007/BF02109182
- O'Loughlin PM. 1998 . A review of the holothurian family Gephyrothuriidae . In : Mooi R , Telford M Echinoderms: Proceedings of the Ninth International Echinoderm Conference , San Francisco , California , USA , 5–9 August 1996 . Rotterdam , Brookfield : A.A. Balkema , p 493 – 98 .
- O'Loughlin , PM. 2002 . Report on selected species of BANZARE and ANARE Holothuroidea, with reviews of Meseres Ludwig and Heterocucumis Panning (Echinodermata) . Memoirs of Museum Victoria , 59 : 297 – 325 .
- O'Loughlin , PM and Ahearn , C. 2005 . A review of pygal-furrowed Synallactidae (Echinodermata: Holothuroidea), with new species from the Antarctic, Atlantic and Pacific Oceans . Memoirs of Museum Victoria , 62 : 147 – 79 .
- Östergren H. 1896 . Zur Kenntnis der Subfamilie Synallactinae unter den Aspidochiroten. Festskrift för Lilljeborg . Uppsala , p 347 – 361 .
- Östergren , H. 1898 . Das system der Synaptiden . Öfversigt af Kongliga Vetenskaps-Akademiens Förhandlingar , 55 : 111 – 20 .
- Östergren , H. 1902 . The Holothurioidea of Northern Norway . Bergens Museum Årbok , 9 : 1 – 34 .
- Östergren , H. 1905 . Zur Kenntnis der skandinavischen und arktischen Synaptiden . Archives de Zoologie expérimentale et générale , 3 ( 4 ) : 133 – 64 .
- Pawson DL. 1963a . The holothurian fauna of Cook Strait, New Zealand . Zoology Publications from Victoria University , Wellington 36 : 1 – 38 .
- Pawson DL. 1963b . Studies on echinoderms of the Southern Pacific Ocean . PhD Thesis . Wellington : Victoria University , 804 pages.
- Pawson , DL. 1965 . Some echinozoans from north of New Zealand . Transactions of the Royal Society of New Zealand Zoology , 5 : 197 – 224 .
- Pawson , DL. 1976 . Some aspects of biology of deep-sea echinoderms . Thalassia Jugoslavica , 12 : 287 – 93 .
- Pawson DL. 1977 . Molpadiid sea cucumbers (Echinodermata:Holothuroidea) of the southern Atlantic, Pacific and Indian Oceans . In : Pawson DL Biology of the Antarctic Seas VI AGU , Washington , DC , p 97 – 123 .
- Pawson , DL. 1982 . Deep-sea echinoderms in the Tongue of the Ocean, Bahama Islands: A survey, using the research submersible Alvin . Australian Museum Memoirs , 16 : 129 – 45 . doi: 10.3853/j.0067-1967.16.1982.362
- Pawson , D , Vance , D and Ahearn , C. 2001 . Western Atlantic sea cucumbers of the Order Molpadiida (Echinodermata: Holothuroidea) . Bulletin of the Biological Society of Washington , 10 : 311 – 27 .
- Perrier , R. 1896 . Sur les Elasipodes recueillis par le ‘Travailleur’ et le ‘Talisman’ . Comptes Rendus Hebdomadaires des Séances de l‘Académie des Sciences Paris , 123 : 900 – 03 .
- Perrier , R. 1898 . Sur les Holothuries recueillies par le Travailleur et le Talisman . Comptes Rendus de l'Academie des Sciences , 126 : 1664 – 66 .
- Perrier , R. 1899 . Diagnose des espèces nouvelles d'Holothuries draguées par le ‘Travailleur’ et le ‘Talisman’ . Bulletin du Muséum National d'Histoire Naturelle, Paris , 5 : 299 – 302 .
- Perrier , R. 1902 . Holothuries . Expeditions scientifiques Travailleur et du Talisman , 5 : 273 – 554 .
- Perrier , R. 1905 . Holothuries antarctiques du Muséum d'Histoire naturelle de Paris. Annales des Sciences Naturelles . Zoologie , 9 : 1 – 146 .
- Risso A. 1826 . Histoire naturelle des principales productions de l'Europe méridionale et particulièrement de celles des environs de Nice et des Alpes Maritimes . Paris : Levrault . 439 pages .
- Roberts , D , Gebruk , A , Levin , V and Manship , BAD. 2000 . Feeding and digestive strategies in deposit-feeding holothurians . Oceanography and Marine Biology: Annual Review , 38 : 257 – 310 .
- Rogacheva A. 2012 . Taxonomy and distribution of the genus Kolga (Elpidiidae: Holothuroidea: Echinodermata) . Journal of the Marine Biological Association of the United Kingdom 92 ( 5 ): 1183 – 93 .
- Rogacheva , A , Cross , IA and Billett , DSM. 2009 . Psychropotid holothurians (Echinodermata: Holothuroidea: Elasipodida) collected at abyssal depths from around the Crozet Plateau in the Southern Indian Ocean . Zootaxa , 2096 : 460 – 78 .
- Rogacheva A , Gebruk A , Alt CHS . 2012 . Swimming deep-sea holothurians (Echinodermata: Holothuroidea) on the northern Mid-Atlantic Ridge . In : Kroh A , Reich M . Echinoderm Research 2010: Proceedings of the Seventh European Conference on Echinoderms , Göttingen , Germany , 2–9 October 2010 . Zoosymposia , 7 : 213 – 24 .
- Rowe , FWE and Gates , J. 1995 . “ Synallactidae ” . In Zoological Catalogue of Australia , Edited by: Wells , A . 261 – 338 . Australia : CSIRO .
- Sars , GO. 1872 . Nye Echinodermer fra den norske Kyst . Videnskabs Selskabet Forhandlinger, Christiania , 1871 : 1 – 31 .
- Sars , M. 1868 . Om nogle Echinodermer og Coelenterater fundne ved Lofoten . Forhandlinger i Videnskabs-Selskabet i Christiania , 1867 : 19 – 23 .
- Sars , M. 1877 . Nye Echinodermer . Fauna Littoralis Norwegiae , 3 : 49 – 75 .
- Sibuet , M. 1977 . Repartition et diversité des Echinodermes (Holothurides – Asterides) en zone profonde dans le Golfe de Gascogne . Deep-Sea Research , 24 : 549 – 63 . doi: 10.1016/0146-6291(77)90527-6
- Sluiter , CP. 1901a . Neue Holothurien aus der Tief-See des Indischen Archipels gesammelt durch die ‘Siboga-Expedition’ . Tijdschrift der Nederlandsche Dierkundige Vereeniging , 7 : 1 – 28 .
- Sluiter , CP. 1901b . Die Holothurien der Siboga-Expedition . Siboga-Expeditie , 44 : 1 – 142 .
- Smirnov , AV. 1997 . New apodid holothurians (Holothurioidea, Apodida) from the New Caledonian continental slope collected during ‘BIOGEOCAL’ expedition 1987 . Zoosystema , 19 : 15 – 26 .
- Smirnov , AV. 1999 . Some remarks on the subgenus Oligotrochus M. Sars, sensu Heding, 1935 (genus Myriotrochus, Myriotrochidae, Holothurioidea) with description of two new species . Zoosystema , 19 : 15 – 26 .
- Solís-Marín FA. 2003 . Systematics and phylogeny of the holothurian family Synallactidae . PhD Thesis . Southampton : University of Southampton . 356 pages .
- Studer , T. 1876 . Über Echinodermen aus dem antarktischen Meere und zwei neue Seeigel von den Papua-Inseln, gesmammelt auf der Reise S.M.S. Gazelle um die Erde . Monatsberichte der Königlich Preussischen Akademie der Wissenschaften zu Berlin , 1876 : 452 – 65 .
- Tabachnick , KR and Collins , AG. 2008 . Glass sponges (Porifera, Hexactinellida) of the northern Mid-Atlantic Ridge . Marine Biology Research , 4 : 25 – 47 . doi: 10.1080/17451000701847848
- Thandar , AS. 1992 . The South African Museum's Meiring Naude Cruises. Part 18. Holothuroidea . Annals of the South African Museum , 101 : 159 – 80 .
- Théel , H. 1877 . Notes sur quelques Holothuries des mers de la Nouvelle Zemble . Nova acta Regiae Societatis Scientiarum Upsaliensis , 17 ( 3 ) : 1 – 18 .
- Théel , H. 1879 . Preliminary report on the Holothuridae of the Exploring voyage of H.M.S. ‘Challenger’. I . Bihang till Kongliga Svenska Vetenskaps-akademiens Handlingar , 5 ( 19 ) : 1 – 20 .
- Théel , H. 1882a . Report on Holothurioidea. Pt. I. Report of the Scientific Results of the Voyage of H.M.S. ‘Challenger’ . Zoology , 4 ( 13 ) : 1 – 176 .
- Théel H. 1882b . Report on the Holothurioidea . In : Tizard TH , Murrey J Exploration of the Faroe Channel, during the summer of 1880, in H.M.'s hired ship ‘Knight Errant’: Proceedings of the Royal Society of Edinburgh , p 694 – 97 .
- Théel , H. 1886a . Report on Holothurioidea dredged by H.M.S. ‘Challenger’ during the Years 1873–76. Part II. Report of the Scientific Results of the Voyage of H.M.S. ‘Challenger’ . Zoology , 14 ( 39 ) : 1 – 290 .
- Théel , H. 1886b . Report on Holothurioidea. Reports on the results of dredging, under the Supervision of Alexander Agassiz, in the Gulf of Mexico (1877–78), in the Caribbean Sea (1879–80), and along the Eastern Coast of the United States during the Summer of 1880, by the U.S. Coast Survey Steamer ‘Blake’ . Bulletin of the Museum of Comparative Zoology , 13 : 1 – 21 .
- Tortonese E. 1965 . Echinodermata. Volume 6. Fauna d'Italia . Bologna : Edizioni Calderini , p 37 – 64 .
- Tyler , PA , Billett , DSM and Gage , JD. 1985 . Life-history biology of Peniagone azorica and P. diaphana . Marine Biology , 89 : 71 – 81 . doi: 10.1007/BF00392879
- Verrill , A. 1884 . Notice of the Remarkable Marine Fauna occupying the Outer Banks off the Southern Coast of New England . American Journal of Science , 3 : 213 – 20 .
- Verrill A. 1885 . Results of the Explorations made by the Steamer ‘Albatross’ off the Northern Coast of the United States in 1883. United States Comission of Fish and Fisheries, Part XI . Report of the Comissioner for 1883 : 503 – 699 .