Abstract
Due to increased fishing activity, catches of larger species and individuals decrease. Behaviour, reproductive biology and life history data are fundamental to determine vulnerability to fishing. Groupers (Serranidae: Epinephelinae) are valuable targets for fisheries in many parts of the world but are highly susceptible to heavy and unregulated fishing. For many regions, biological information on groupers necessary for management purposes is still scarce. The North Andhra region, central east coast of India, has been the centre for a variety of fishing activities for a long time. Many species of groupers are landed by a variety of gears at fishing harbours and nearby fishing villages of this region. The present paper incorporates data on species diversity and length groups represented in the catches, results of length-weight relationship studies, length at first maturity, and fecundity of grouper species belonging to two genera, Cephalopholis and Epinephelus, that are represented in the commercial catches of this region. The consideration of length at first maturity and fecundity estimations is a very important prerequisite to prevent exploitation and detrimental effects for these fisheries resources.
Introduction
Groupers (Serranidae: Epinephelinae) are major targets of artisanal, recreational and commercial fisheries (Heemstra & Randall Citation1993; Craig et al. Citation2011). Groupers have white, tender and tasty meat that makes them a highly priced marine food fish. They are the preferred species in the live reef fish trade in Southeast Asia and demand the highest price. The high demand for these fishes in the international markets has led to indiscriminate fishing, resulting in the depletion of natural stocks at an alarming level. Many smaller species are also highly valued in the aquarium trade. Groupers are being subjected to increasing fishing pressure for food, ornamental display and medical purposes globally (Pauly et al. Citation2002; Vincent Citation2006).
Groupers are top predators, sedentary in character; most of them are strongly territorial, typically long-lived and slow-growing, and many assemble in large numbers to spawn. These characteristics contribute to their over-exploitation and consequently they have become endangered by recent fishing operations. The loss of groupers can have a serious effect on local ecosystems, since these fish play an important role in the structure of rocky-bottom and coral-reef communities (Aburto-Oropeza et al. Citation2008).
According to the Food and Agricultural Organization of the United Nations (FAO), groupers contributed more than 275,000 tons to global capture fisheries production in 2009 (Sadovy de Mitcheson et al. Citation2011). Of the total grouper production in 2008, more than 80% was reported from Asia (Sadovy de Mitcheson et al. Citation2011). The potential yield estimates of demersal resources along the Indian coast reveals that groupers constitute 1.40%, 1.47% and 0.97% of the total potential yields of 1.93, 0.2 and 0.1 million tons in the depth zones 0–100 m, 100–200 m and 200–500 m, respectively (Fishery Survey of India Citation2010).
The central eastern coast of India (north Andhra region; 17°01′N–19°22′N; 83°23′E–85°14′E) is known for its rocky habitats and associated fish species and has been the basis of traditional fishing for a long time. There has been a massive development of fisheries in this region over the past three decades. Vessels now extend their trawling operations into deeper waters, catching more varieties of demersal fishes which include groupers. While in earlier studies the occurrence of large-sized groupers along the northeast coast of India was considered to be only sporadic and rare (Sujatha Citation2004), there has been a recent increase in landings of grouper species by artisanal fishermen using hook and line and trawl catches in the north Andhra region. Because of the increase in catches and the export value of groupers, there is a need for proper information on these resources to ensure sustainable development and management.
Valuable information on the exploitation of perch fisheries, which includes groupers, from the rocky grounds off Kerala, Tamil Nadu, Gulf of Mannar, Gulf of Kutch, Paradeep and in the Andaman Sea was provided by Mathew (Citation2003). Based on a trawl survey in the Gulf of Mannar, Joseph et al. (Citation1987) and Sudarsan et al. (Citation1989) reported that groupers are more abundant at 20–50 m and 100–150 m depths and their abundance decreases beyond 150 m depth. The first reference to species of epinephelines represented in Indian waters is that of Russell (Citation1803), who described four serranid species: Perca bontoo = Epinephelus malabaricus (Bloch & Schneider, 1801); Perca madinawa bontoo = Epinephelus coioides (Hamilton, 1822); Perca rahtee bontoo = Cephalopholis formosa (Shaw, 1812); Perca suggalathoo bontoo = Epinephelus lanceolatus (Bloch, 1790). James et al. (Citation1996) produced a review of the known status of groupers and snappers of India. They reported 38 species of groupers from the seas around India. For that area several studies on grouper diversity and fisheries biology have been carried out, e.g. by Sujatha (Citation1986), Pokapunt et al. (Citation1993), Rajan (Citation2002); Sluka & Lazarus (Citation2004) and Sujatha et al. (Citation2008).
While regional reviews of groupers have been carried out along the west coast of India (James et al. Citation1996), our knowledge on the distribution and occurrence of these species along the east coast of India is far from complete. The biology of groupers is a major factor in their susceptibility to heavy and unregulated fishing and biological information is essential for management purposes. Hence, our knowledge of the biological parameters of a stock, including the Length-Weight Relationship (LWR), is of great importance in fishery biology studies. LWR studies of different species of groupers have been carried out by some workers from different regions of India (e.g. Premalatha Citation1989; Ameer Hamsa & Mohamad Kasim Citation1992; Chakraborty Citation1994; Rangaswamy et al. Citation1999; Manojkumar Citation2005; Sivakami & Seetha Citation2006; Jayasankar et al. Citation2007; Sujatha et al. Citation2010).
Studies of reproductive and biological aspects such as length at first maturity and fecundity facilitate the development of management strategies such as minimum size limit and assessing fishery resilience (Armswoth Citation2001). This information is particularly important for groupers, given that sex-changing species can be more susceptible to heavy fishing (Levin & Grimes Citation2002). Studies on various aspects of the reproductive biology of groupers in Indian waters were carried out by Chakraborty & Vidyasagar (Citation1996), Chandrasekhara Rao & Krishnan (Citation2009) and Mathew & Mathew (Citation2010).
In the present study we pay special attention to the fact that grouper species are rich in diversity but poor in abundance. Hence, a commercial fishery wholly based on natural stocks will soon face the threat of over-exploitation if the fishery is not properly managed (Shakeel & Ahmad Citation1996). No detailed species-specific catch data for grouper species from Indian waters are currently available. Without quantitative data there is little possibility of assessing the impact of commercial fishing on these species, which are currently listed as Data Deficient on the IUCN Red List (Sadovy Citation2007).
Two priority research areas were identified by the Workshop Group for Global Red List Assessments of Groupers (Sadovy Citation2007, Sadovy de Mitcheson et al. Citation2011):
All larger Data Deficient and Least Concerned species should be the immediate focus of more data-gathering, especially in Southeast Asia.
For threatened species, fishery exploitation is the major threat and needs to be the focus of future research and management activities, given that exploitation is expected to grow.
Materials and methods
The present study is based on grouper specimens collected from the traditional fish landing centres at the fishing harbours of Visakhapatnam () (where the catches from nearby fishing villages are also brought for sale) during the period September 2009 to October 2011. Groupers were caught using traditional fishing gear along with sharks (e.g. Carcharhinidae), Scombridae, Ariidae and Carangidae by handline, longline and trammel nets in shallow waters with non-motorized catamarans and in deeper waters using motorized boats. Hook and line fishing is the method most widely used to catch groupers in this region. They are also caught in trawls, but trawlers are not operated in this region for a 45 day period from 16 April to 1 June, which the Government of Andhra Pradesh has declared as a closed fishing season (trawl ban period). For fishing on rocky and coral areas an additional rope with rollers or bobbins is fastened to the ground rope of the trawl net to protect the net. Locally in Telugu it is called ‘Bamboo vala’. Species of groupers were identified based on previous literature (Heemstra & Randall Citation1993; Carpenter & Niem Citation1999; Sujatha Citation2004; Craig et al. Citation2011).
Length frequency distribution and Length-Weight Relationship (LWR) studies were based on a total of 870 specimens of nine grouper species. Total length (TL) was obtained from the fish measured to the nearest millimetre from the tip of mouth to the tip of caudal fin ray and weight measured to nearest gram. The data from samples of each month were pooled and percentage length frequency and LWR were calculated. The determination of the relationship between length and weight of nine species of groupers was based on the combined data regardless of time of capture, sex and stage of maturity. Sexes were not considered separately for species, as males were rarely represented in the catches. The LWR was calculated by the method of least square employing the equation of Le Cren (Citation1951)
where W = body weight (g); L = TL (mm); ‘a’ is a coefficient related to body form and ‘b’ is an exponent indicating isometric growth when equal to 3 (Edwards Citation1976). The same can be written in the logarithmic form as
.
The methodology for estimation of length at first maturity and fecundity follows standard procedures (Prabhu Citation1956; Shapiro Citation1987; Murua & Saborido-Rey Citation2003; Erisman Citation2008). Based on maximum recorded size and length at first maturity, groupers recorded from the north Andhra region were categorized into small-, medium- and large-size groups. For each group the average maturity length was calculated so that it would be proposed as the minimum allowed capture size. Three species belonging to two genera, Cephalopholis and Epinephelus, were placed into the small-size group (–) with an average maturity length of 230 mm TL: Cephalopholis formosa (Shaw & Nodder, 1812), E. areolatus (Forsskål, 1775) (as E. angularis (Valenciennes in Cuvier & Valenciennes, 1828) in Sujatha 1993), and E. longispinis (Kner, 1864). Seven species, Cephalopholis sonnerati (Valenciennes, 1828), E. bleekeri (Vaillant, 1877), E. chlorostigma (Valenciennes, 1828), E. epistictus (Temminck & Schlegel, 1842), E. radiatus (Day, 1867), E. tauvina (Forsskål, 1775) and E. undulosus (Quoy & Gaimard, 1824) were placed in the medium-size group (–) with average maturity length of 420 mm TL. Four species, E. coioides (Hamilton, 1822), E. magniscuttis (Postel, Fourmanoir & Gueze, 1963), E. malabaricus (Bloch & Schneider, 1801) and E. latifasciatus (Temminck & Schlegel, 1842) were grouped into the large-size group (–) with average maturity length of 550 mm TL. Length at first maturity was estimated as TL (mm) at which 50% of the specimens were in a mature condition, mainly in the samples collected for the large-sized groupers. The maximum total lengths of groupers as recorded in the present study and as reported by Heemstra & Randall (Citation1993) and Froese & Pauly (Citation2013) were used for grouping these species ().
Table I. Grouper species represented in the catches of the north Andhra region.

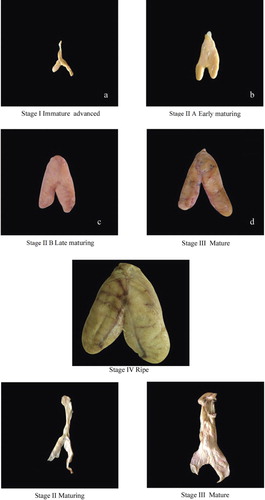
For fecundity estimation, after measuring the total lengths of the fish, gonads were extracted and weighed to the nearest 0.1 g and preserved in 5% neutral formalin for further analyses. For fecundity studies a total of 158 specimens of groupers belonging to two species of the genus Cephalopholis and 10 species of the genus Epinephelus were analysed using macro- and microscopic inspection methods. Total number of mature oocytes obtained from mature active or ripe females (maturity stages III or IV, see also below) was determined under consideration of methods described in earlier studies (e.g. Hunter & Macewicz Citation1985; Shapiro Citation1987; Erisman Citation2008). Gonad maturity stage III was defined as large ovaries occupying three-quarters to almost filling body cavity, yellowish-orange in colour, and oocytes in vitellogenesis stage; maturity stage IV was defined as translucent oocytes mostly larger than in stage III, in migratory nucleus stage or hydration stage, with ovaries often flowing when applying pressure.
Fecundity was estimated by multiplying the number of mature oocytes in the samples taken from the anterior, middle and posterior region of ovaries by the ratio of ovary weight to sample weight. The relationships between fecundity and the three variables, total length (TL), total weight of fish (TW) and weight of gonads (GW), were also calculated using the following equation for the species E. areolatus, E. bleekeri, E. coioides, E. magniscuttis, E. malabaricus and E. radiatus:
where L = total length/fish weight/ovary weight; a and b are constants.
Results
Several patterns were evident when groupers were categorized by their typical depth ranges and maximum reported body sizes (Heemstra & Randall Citation1993; Craig et al. Citation2011; Sadovy de Mitcheson et al. Citation2011). In general, species red-listed as Threatened and Near Threatened tended to have larger maximum sizes, whereas smaller species were rarely placed in a threat category. Data Deficient species occurred in all three size categories. In addition, Threatened and Near Threatened groupers were more commonly species whose general depth range is deep (typically >30 m) or broad (occurring across a wide depth range). Shallow-water species (typically <30 m depth) had the largest proportion of Least Concern classifications. Sadovy de Mitcheson et al. (Citation2011) recognized 42 species (25% of all grouper species) as facing or nearly facing imminent threat of extinction based on IUCN criteria of threat trends, mainly if uncontrolled fishing continues.
Species composition of groupers off north Andhra
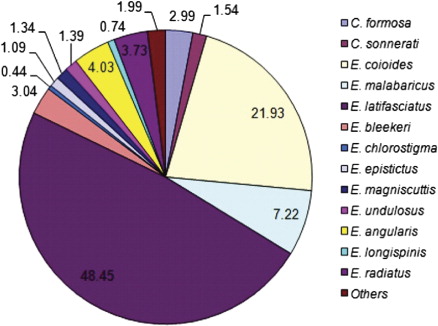
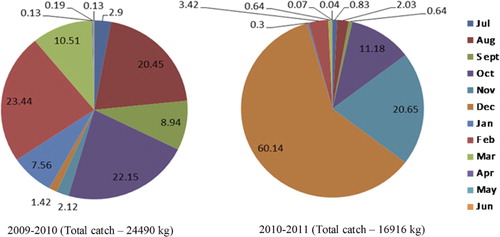
For several species of groupers there is a consistent pattern of depth distribution when the adult individuals of medium- and large-sized species move to deeper waters as they grow. The smaller species remain closer to the coast. From discussions with the fishermen, it was understood that larger individuals are found in deeper waters at around 120 m and weight per individual is usually between 4 and 8 kg, sometimes even more. During the spawning season the same medium and larger species form large aggregations and migrate offshore. It is confirmed that specimens of these larger species are found in the trawl catches from deeper waters. Occurrence information of exploited grouper species off the north Andhra region is given in . Species of groupers were caught from three different depths along the north Andhra coast: in shallow waters (up to 20 m depth) using non-motorized boats, in deeper waters (80–120 m) using motorized boats and from 20–100 m depth using trawlers. The total annual landings of groupers were estimated at 24,490 kg during the period 2009 to 2010 and 16,916 kg during 2010 to 2011. The monthwise catch (as weight in kg) of exploited grouper species is given in .
Length frequency distribution and length at first maturity of exploited grouper species
Length at first maturity, relative abundance in the fished area and the distribution in space and time of the individual species constitute important information for fishery management. In the present study, three different size groups were distinguished, allowing to some extent referral to different fishing areas and methods. For instance, all small-size groupers except Epinephelus areolatus were represented in the catches of traditional gear in near-shore waters.
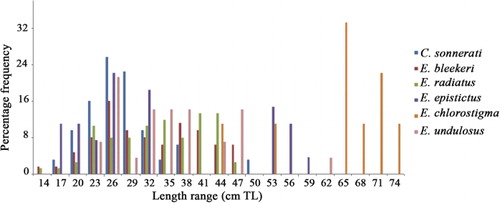
Small-size exploited groupers
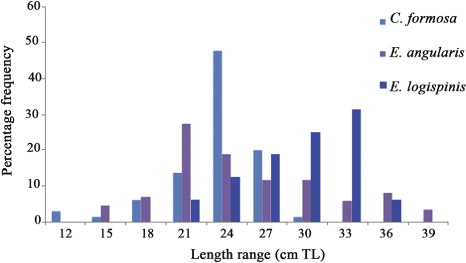
The small-size groupers are represented in handline and trawl catches. The annual pooled length frequency distribution of these commonly represented grouper species is given in . All size groups of Cephalopholis formosa are represented in the catches throughout the year. The common sizes in commercial catches for C. formosa, Epinephelus areolatus and E. longispinis were 21–24 cm TL, 19–21 cm TL and 25–35 cm TL, respectively. The estimated lengths at first maturity for C. formosa, E. areolatus and E. longispinis were 17 cm TL, 20 cm TL and 23 cm TL, respectively. All size groups of E. areolatus were represented in the catches and the mature specimens were collected in the months of March, April and October to December. Only one mature specimen of E. longispinis was collected in the month of January.
Medium-size exploited groupers
The pooled annual length frequency distribution of Cephalopholis sonnerati, Epinephelus bleekeri, E. chlorostigma, E. epistictus, E. radiatus and E. undulosus is shown in . The common sizes in commercial catches for these six medium-size grouper species were 20–28 cm TL, 26–37 cm TL, 65–75 cm TL, 15–40 cm TL, 35–45 cm TL and 23–47 cm TL, respectively. The present study reveals that among medium-size groupers only one species, E. bleekeri, is represented in the catches throughout the year. The length at first maturity for these six medium-size groupers ranges from 28 cm TL (for E. chlorostigma) to 36 cm TL (for E. bleekeri).
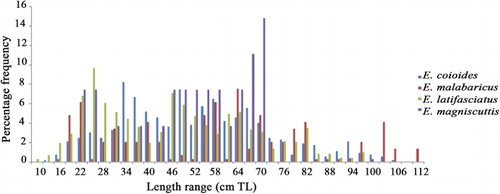
Large-size exploited groupers
Of the large-size grouper species, Epinephelus coioides and E. malabaricus were represented both in traditional gear and trawl catches of the north Andhra region; large-size specimens of these species were also caught with hook and line from deeper waters. Epinephelus latifasciatus and E. magniscuttis were represented only in the trawl catches. The pooled annual length frequency distribution of large-size groupers is given in .
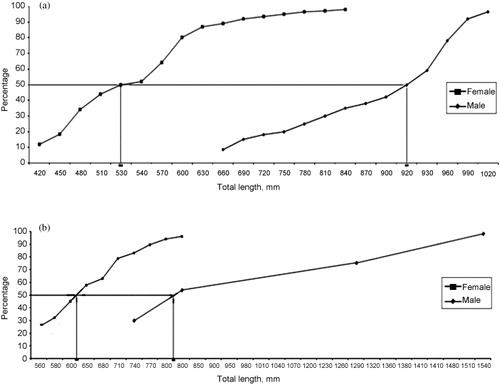
The length at first maturity for E. coioides was estimated at 53 cm TL for females and 92 cm TL for males (). Percentage occurrence of females of E. coioides off Visakhapatnam is given in . In E. malabaricus the pooled annual length frequency distribution indicates that juvenile and mature specimens were captured during most months. The length at first maturity was estimated at 64 cm TL for females and 82 cm TL for males (). The pooled annual length frequency distribution of E. latifasciatus indicates that juveniles and mature specimens are captured during most months in this region (Sujatha et al. Citation2013).
Table II. Percentage occurrence of females of Epinephelus coioides in different stages of maturity in various months represented in the catches at Visakhapatnam during the period October 2009 to September 2011.
Epinephelus magniscuttis was represented in the catches throughout the year except in the months April to June and November. Mature specimens of this species occur in September, October and January to March.
Common sizes in commercial catches of the species E. coioides, E. malabaricus, E. latifasciatus and E. magniscuttis range from 30–65, 21–64, 22–52 and 45–70 cm TL, respectively.
Specimens of small- and medium-size groupers with mature gonads are encountered in the catches during the months from October to March and large-size groupers with mature gonads are encountered in the catches throughout the year except in the months of April to June.
The length at first maturity was suggested as the minimum size that should be allowed to be caught in commercial fishing, as it will allow the fish to gain considerable biomass and spawn at least once in its lifespan.
Fecundity studies of exploited grouper species
An appraisal of the reproductive strategy and fecundity is necessary to evaluate the reproductive potential of individual fish species (Murua et al. Citation2003). Length range, body weight, gonad weight and fecundity for 12 grouper species is given in . Studies on various aspects of reproduction and fecundity of commercially important species like groupers are scarce, especially along the east coast of India. Information pertaining to fecundity estimates of six species of groupers of the genus Epinephelus are given in Tables S1–S6 (Supplementary online material) for a better understanding of how fecundity varies with body size, weight and gonad weight. In small-size exploited groupers fecundity is in the range of 22,128–93,607, with an average of 49,368. In small-size groupers minimum and maximum fecundity was identified from Epinephelus areolatus. In medium-size exploited groupers fecundity is in the range of 42,252–1,599,384, with an average of 422,068. Minimum fecundity was observed in Cephalopholis sonnerati and a maximum in E. bleekeri. For large-size exploited groupers fecundity is in the range of 43,618–2,319,876, with an average of 560,903. Minimum fecundity was observed in E. coioides and maximum in E. malabaricus. Specimens of large-size groupers with mature gonads are encountered in the catches throughout the year except in the months from April to June. This may be because this is the trawl ban period in this region. Different maturity stages of gonads (male and female) of E. coioides are shown in . In the present study fecundity was estimated for the first time for C. formosa, C. sonnerati, E. areolatus, E. longispinis, E. epistictus, E. radiatus, E. magniscuttis, E. malabaricus and E. coioides.
Table III. Fecundity of 12 grouper species (genera Cephalopholis and Epinephelus) off the north Andhra region.
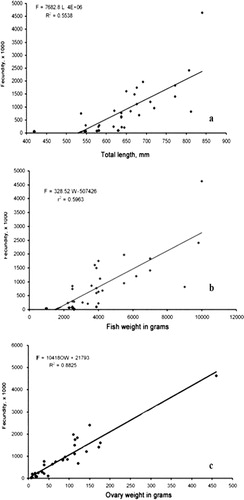
The relationships between fecundity (F) and the three variables total length (L), body weight (W) and ovary weight (O) of the two large-size groupers are shown in – and –.
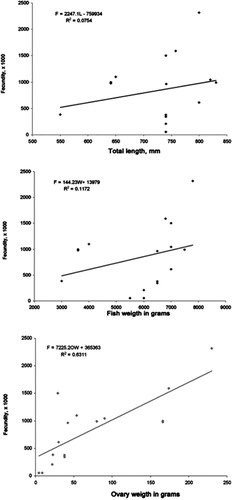
The relationships between fecundity (F) and the three variables total length (L), body weight (W) and ovary weight (O) of six species of the genus Epinephelus are recorded in .
Table IV. Relationship between fecundity and the variables total length, body weight and ovary weight in six species of the genus Epinephelus.
Discussion
Groupers are not only biologically diverse and fascinating in their own right, but are also among the most commercially important and highly regarded of all the fish species associated with reef fisheries globally. Given the importance of grouper fisheries in many parts of the world, it is surprising how little is known about the biology and ecology of all but a few species. Longevity, size and age of sexual maturity, mortality and reproductive parameters, among other life-history characteristics, are of great value for fishery modelling and management, as well as for assessment of conservation status (Craig et al. Citation2011). Groupers form a sizable portion of fishery after the extension of trawling operations to offshore waters off the central eastern coast of India (Sujatha et al. Citation2010). Shakeel & Ahmad (Citation1996) stated that in commercial fishery, the removal of groupers less than the average maturity length of 30.5 cm from the medium-size group and 40.5 cm from the large-size group should be prohibited. The small- and large-size groups contain species which are of no commercial value because they are either too small or extremely rare. Catching a fish larger than 30.5 cm from the small-size group will do no harm. These two size restrictions are expected to result in about 80% of commercial and potentially commercial grouper species being caught after they have spawned at least once. In groupers, males are larger, fewer in number and occur in deeper waters (Mathew et al. Citation2002). In the present study region, length at first maturity for Epinephelus latifasciatus was estimated at 61 cm TL for females and 66 cm TL for males. Above 65 cm TL all specimens are mature. Mature (stages III and IV) specimens occur in the months November to February (Sujatha & Shrikanya Citation2013).
Groupers are among the most fecund demersal and reef fishes. According to Heemstra & Randall (Citation1993), fecundity in E. coioides is estimated as 850,186 for a fish of 35 cm TL and 2,904,912 in one measuring 62 cm TL. Grandcourt et al. (Citation2009) studied the reproductive biology of E. coioides from the southern Arabian Gulf, stating that males were derived from the transition of post-spawning females. Studies on the fecundity of E. latifasciatus from Visakhapatnam waters have been carried out by Sujatha & Shrikanya (Citation2013). This species is gonochoric with fecundity in the range of 153,419–3,772,803. The relationship between fecundity (F) and the three variables total length (L), body weight (W) and ovary weight (O) is F = 5.002L–3.7854 (r2 = 0.1939); F = 12.459W1.0754 (r2 = 0.1492); F = 59.484O1.1163 (r2 = 0.8929).
Obtaining accurate LWR parameters is an important factor in the assessment of fish stocks (Demirhan & Can Citation2007). For small-, medium- and large-size groupers LWR parameter estimates are given in . In the present study ‘b’ values range from 2.7642 (E. longispinis) to 3.2492 (E. undulosus). According to Pauly & Gayanilo (Citation1997), ‘b’ values may range from 2.5 to 3.5, suggesting that the result of this study is valid. The slight variation in the ‘b’ value of a species obtained from different waters may be due to the change in ecological conditions.
Table V. Length range and Length-Weight Relationships of groupers (genera Cephalopholis and Epinephelus).
The once dominant and small groupers in the past have been replaced by large grouper species such as E. coioides, E. malabaricus and E. latifasciatus in the commercial catches. Small species such as C. formosa and E. hexagonatus, that were more abundant in traditional catches (Sujatha Citation1986), now occur in very small numbers in the catches. On the other hand, species that did not appear in traditional catches in the past, such as E. longispinis, are now represented in considerably large numbers. The apparent shift in species composition in the current fishery particularly threatens nearshore grouper species. Formerly abundant small-size species are now rare or uncommon.
Parenti (Citation2001) stated that E. coioides and E. malabaricus, two closely related and similar species in appearance, occupy the same habitats along the continental shelf and shores of large islands of the Indo-Pacific. They also show great adaptability to different water salinities and juveniles of these species have been collected from estuarine river mouths.
It is essential that the status of these two Near Threatened species is monitored closely. Management action is needed to prevent them from becoming listed as Threatened in the future. Grouper stocks, as in other serranids, were typically the first rocky and reef fish stocks to collapse in response to increasing fishing pressure (Johannes Citation1998). Efforts should focus on discovering the aggregation sites of the species C. formosa, E. coioides, E. malabaricus and E. latifasciatus, as mature and juvenile stages of these species are found in the study area.
According to Sadovy (Citation2007): ‘A Data Deficient (DD) listing does not mean that a species is not of concern. Indeed, unless fisheries management improves immediately and we can dramatically enhance our knowledge of these species it will undoubtedly result in more Data Deficient grouper species qualifying for Threatened categories.’ The present study provides preliminary data for the species E. magniscuttis, E. undulosus and E. epistictus, that are categorized as DD.
The size at sexual maturity and the spawning season are critical stages of fish life history. Sex-changing species may be more susceptible to heavy fishing pressure than gonochoristic fishes (Levin & Grimes Citation2002). Fisheries targeting large individuals of grouper stocks or generally heavy fishing pressure that potentially leads to overfishing differentially reduce the number of male fish in protogynous groupers (Alonzo & Mangel Citation2004). This may lead to sperm limitation if fishing pressure on large individuals persists (Coleman et al. Citation1996). Furthermore, management efforts need to consider that reproductive characteristics vary among species but also show regional variations (Sadovy Citation1996).
Conclusions
The current databases which support policies for the management of many grouper fisheries are highly inadequate. Shrimp trawlers continue to be a major problem for grouper and many other species, since they constantly target the same grounds where juveniles and spawning aggregations are located and continue to take a toll in the number of individuals that can reach maturity and are able to reproduce. Thus, the creation of marine protected areas (e.g. of the spawning grounds) is a promising approach to ensure the well-being of grouper species affected by these fisheries. The adequate placement of protective zones depends on a thorough understanding of the life cycle and reproductive biology of target species. Alternatively, the establishing of ‘closed areas’ for fisheries during the brief annual spawning aggregation periods might be an effective approach for protection. The use of No Take Areas (NTAs) for improving fishery yield is an increasing trend both in tropical and temperate waters (Unsworth et al. Citation2007).
To avoid future stock depletion of this important resource along the east coast of India, the identification of spawning aggregation sites is vital for the development of a management plan. A grouper minimum capture size (which should be taken into consideration when delineating marine protected areas or NTAs) is also important. To conserve and manage commercially important small-size groupers, more detailed information on reproductive biology is needed.
Supplementary material
Supplementary material for this article is available via the Supplemental tab of the article’s online page at http://dx.doi.org/10.1080/17451000.2014.949271
Editorial responsibility: Franz Uiblein
Tables SI-SVI
Download MS Word (141.5 KB)Acknowledgements
The authors are grateful to the Ministry of Earth Sciences (MoES), New Delhi for providing financial assistance and to the Head, Department of Marine Living Resources, Andhra University for providing facilities to carry out this research work. We are grateful to the Fishery Survey of India (FSI) for providing us with samples. Our sincere thanks to Dr Franz Uiblein and anonymous referees for constructive criticism of the manuscript.
References
- Aburto-Oropeza O, Erisaman B, Valdez-Ornelasy V, Danemann G. 2008. Commercially important serranid fishes from the Gulf of California – Ecology, fisheries and conservation. Ciencia y Conservacion 2008:1–23.
- Alonzo SH, Mangel M. 2004. The effects of size selective fisheries on the stock dynamics of and sperm limitation in sex-changing fish. Fisheries Bulletin 102:1–13.
- Ameer Hamsa KMS, Mohamad Kasim H. 1992. Growth and production potential of young grouper Epinephelus tauvina (Forskal) reared in fixed net cages. Journal of the Marine Biological Association of India 34:271–76.
- Armswoth PR. 2001. Effects of fishing on a protogynous hermaphrodite. Canadian Journal of Fisheries and Aquatic Sciences 58:568–78.
- Carpenter KE, Niem VH. 1999. FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. Volume 4. Rome: Food and Agriculture Organization, p 2442–47.
- Chakraborty SK. 1994. Age, growth, mortality and stock assessment of Epinephelus diacanthus (Valenciennes) from Bombay waters. Bulletin of the Central Marine Fisheries Research Institute 47:130–33.
- Chakraborty SK, Vidyasagar KD. 1996. Growth, mortality and stock assessment of two perches moontail bullseye Priacanthus hamrur (Perciformes/Priacanthidae) and thorny cheek grouper Epinephelus diacanthus (Perciformes/Serranidae) from Bombay waters. Indian Journal of Marine Sciences 5:313–15.
- Chandrasekhara Rao A, Krishnan L. 2009. Studies on the reproductive biology of the female spiny cheek grouper, Epinephelus diacanthus (Valenciennes, 1828). Indian Journal of Fisheries 56:87–97.
- Coleman FC, Koenig CC, Collins LA. 1996. Reproductive styles of shallow-water groupers (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environmental Biology of Fishes 47:129–141. 10.1007/BF00005035
- Craig MT, Sadovy de Mitcheson Y, Heemstra PC. 2011. Groupers of the World: A Field and Market Guide. Grahamstown, South Africa: CRC Press, NISC. 424 pages.
- Demirhan SA, Can MF. 2007. Length–weight relationships for seven fish species from the southeastern Black Sea. Journal of Applied Ichthyology 23:282–83. 10.1111/j.1439-0426.2007.00835.x
- de Moussac G. 1986. Synthèse des données sur la pêche artisanale aux Seychelles. Biologie – resources – exploitation [Overview of the artisanal fishing data in the Seychelles: Biology – resources – exploitation]. Seychelles: SFA/R & D/006. 64 pages.
- Edwards AL. 1976. An Introduction to Linear Regression and Correlation. San Francisco, CA: W. H. Freeman and Company. 213 pages.
- Erisman BE. 2008. Reproductive Biology and Evolution of Epinephelid and Serranid Fishes (Perciformes, Epinephelidae, Serranidae). Doctoral Thesis. Scripps Institute of Oceanography, University of California, San Diego. 210 pages.
- Fishery Survey of India, 2010. Annual Report. Department of Animal Husbandry, Dairying and Fisheries. Mumbai: Government of India. 50 pages.
- Froese R, Pauly D. 2013. FishBase. World Wide Web electronic publication. www.fishbase.org ( accessed 9 October 2014).
- Grandcourt EM, Al Abdessalaam TZ, Francis F, Al Shamsi AT, Hastmann SA. 2009. Reproductive biology and implications for management of the orange-spotted grouper Epinephelus coioides in the southern Arabian Gulf. Journal of Fish Biology 74:820–41. 10.1111/j.1095-8649.2008.02163.x
- Heemstra PC, Randall JE. 1993. Groupers of the World. (Family Serranidae, Subfamily Epinephelinae). FAO Species Catalogue 16. Rome: Food and Agriculture Organization. 382 pages.
- Hunter JR, Macewicz BJ. 1985. Measurement of spawning frequency in multiple spawning fishes. In: Lasker R, editor. An Egg Production Method for Estimating Spawning Biomass of Pelagic Fish: Application to the Northern Anchovy, Engraulis mordax. US Department of Commerce, NOAA Technical Report. California. NMFS 36, p 79–94.
- James PSBR, Sriramachandra V, Murthy VS, Nammalwar P. 1996. Groupers and snappers of India: Biology and exploitation. In: Sanchez A, Munro JL, Balgos MC, Pauly D, editors. Biology, Fisheries and Culture of Tropical Groupers and Snappers. ICLARM Conference Proceedings, International Center for Living Aquatic Resources Management, Campeche, Mexico, 48:106–136.
- Jayasankar P, Jayasankar J, Anand C, Augustine SK. 2007. Information on Epinephelus diacathus from Indian seas. In: Fennessy S, editor. Newsletter of the Groupers & Wrasses Specialist Group, International Union for the Conservation of Nature, Gland, Switzerland 10:9–11.
- Johannes RE. 1998. The case for data-less marine resource management: Examples from tropical nearshore finfisheries. Trends in Ecology and Evolution 13:243–46. 10.1016/S0169-5347(98)01384-6
- Joseph KM, Sulochanan P, John ME, Somavanshi VS, Nair KNV, Joseph A. 1987. Demersal fishery resources of Wadge Bank. Bulletin Fishery Survey of India 12:1–52.
- Le Cren ED. 1951. Length–weight relationship and seasonal cycle in gonad weight and condition of the perch (Perca fluviatilis). Journal of Animal Ecology 20:201–19. 10.2307/1540
- Levin PS, Grimes CB. 2002. Reef fish ecology and grouper conservation and management. In: Sale PF, editor. Coral Reef Fishes. Dynamics and Diversity in a Complex Ecosystem. San Diego: Academic Press, p 337–89.
- Manojkumar PP. 2005. Fishery of the spinycheek grouper, Epinephelus diacanthus (Valenciennes), off Calicut along the Malabar Coast. Journal of the Marine Biological Association of India 47:63–69.
- Mathew G. 2003. Perches. In: Mohan JM, Jayaprakash AA, editors. Status of Exploited Marine Fishery Resources of India. Kochi: Central Marine Fisheries Research Institute, p 102–09.
- Mathew G, Mathew K. 2010. Anatomical changes during early gonad development in the protogynous greasy grouper Epinephelus tauvina (Forsskal). Indian Journal of Fisheries 57(2):21–24.
- Mathew G, Sanil NK, Sreedhar N, Leela Bhai KS, Kambadkar LR, Palaniswamy N. 2002. Experiment on broodstock development and spawning of Epinephelus tauvina (Forskal). Indian Journal of Fisheries 49:135–39.
- Morgans JC. 1982. Serranid fishes of Kenya and Tanzania. Ichthyological Bulletin of the J.L.B. Smith Institute of Ichthyology 46:1–44.
- Murua H, Saborido-Rey F. 2003. Female reproductive strategies of marine fish species of the North Atlantic. Journal of Northwestern Atlantic Fisheries Science 33:23–31. 10.2960/J.v33.a2
- Murua H, Kraus G, Saborido-Rey F, Witthames PR, Junquera S. 2003. Procedures to estimate fecundity of marine fish species in relation to their reproductive strategy. Journal of Northwestern Atlantic Fisheries Science 33:33–54. 10.2960/J.v33.a3
- Parenti P, Bressi N. 2001. First record of the orange-spotted grouper, Epinephelus coioides (Perciformes: Serranidae) in the northern Adriatic Sea. Cybium 25:281–84.
- Pauly D, Gayanilo Jr FC. 1997. A bee: An alternative approach to estimating the parameters of a length-weight relationship from length-frequency samples and their bulk weight. NAGA ICLARM, Manila, Philippines 22:15–26.
- Pauly D, Christensen V, Guenette S, Pitcher TJ, Sumaila UR, Walters CJ, et al. 2002. Towards sustainability in world fisheries. Nature 418:689–95. 10.1038/nature01017
- Pokapunt W, Sirimontraporn P, Bussarawit S. 1993. Serranid fishes (family Serranidae: subfamily Epinephelinae) from the Andaman Sea. Phuket Marine Biological Center Special Publication 12:97–122.
- Prabhu MS. 1956. Maturation of intra-ovarian eggs and spawning periodicities in some fishes. Indian Journal of Fisheries 3:59–90.
- Premalatha P. 1989. Fishery and biology of rock cods (order Perciformes) from the south-west coast of India. Indian Journal of Fisheries 36:285–91.
- Rajan PT. 2002. A Field Guide to Grouper and Snapper Fishes of Andaman and Nicobar Islands. Port Blair: Zoological Survey of India. 102 pages.
- Rangaswamy VS, Marichamy NR, Rajappackiam S, Sundara Rajam D. 1999. Collection and transportation of groupers for farming. Proceedings of the Fourth Indian Fisheries Forum, Kochi, 24–28 November 1996, p 401–13.
- Russell P. 1803. Description and Figures of Two Hundred Fishes Collected at Visakhapatnam on the Coast of Coromandel. London: W. Bulmer & Co. 85 pages.
- Sadovy Y. 1996. Reproduction in reef fishery species. In: Polunin NVC, Roberts CM, editors. Reef Fisheries. London: Chapman & Hall, p 15–59.
- Sadovy Y. 2007. Workshop for global red list assessments of groupers – Family Serranidae; subfamily Epinephilinae. Chair, Groupers and Wrasses Specialist Group. The University of Hong Kong. 24 pages. http://cmsdata.iucn.org/downloads/final_report_for_gwsg_workshop_feb_2007.pdf ( accessed 16 July 2014).
- Sadovy de Mitcheson Y, Matthew TC, Carpenter KE, Cheung WWL, Choat JH, Cornish AS, et al. 2011. Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish and Fisheries 12:1–18.
- Shakeel H, Ahmad H. 1996. Exploitation of reef resources, grouper and other food fishes in Maldives. SPC Live Reef Fish Information Bulletin 2:14–20.
- Shapiro DY. 1987. Reproduction in groupers. In: Polovina JJ, Ralston S, editors. Tropical Snappers and Groupers: Biology and Fisheries Management. Boulder, CO: Westview Press, p 295–327.
- Sivakami S, Seetha PK. 2006. Indiscriminate destruction of juveniles of spiny cheek grouper Epinephelus diacanthus (Valencinnes) off Quilon, Kerala. Journal of the Marine Biological Association of India 48:128–30.
- Sluka B, Lazarus L. 2004. Groupers and wrasses biodiversity along the west coast of India. IUCN Specialist Group of Groupers and Wrasses, Newsheet 8:6–10.
- Sudarsan D, Sivaprakasam TE, Somavanshi VS, John ME, Nair KNV, Joseph A. 1989. An appraisal of the marine fishery resources of Indian Exclusive Economic Zone. Bulletin of the Fisheries Survey of India 18:81.
- Sujatha K. 1986. List of Epinephelus sp. recorded at Visakhapatnam with a description of Epinephelus hata Katayama (1953). Geobios New Reports 5:65–66.
- Sujatha K. 2004. Groupers off Visakhapatnam, north east coast of India. Journal of the Marine Biological Association of India 46:87–92.
- Sujatha K, Shrikanya KVL. 2013. Reproductive biology of striped grouper Epinephelus latifasciatus (Temminck and Schlegel, 1842) off Visakhapatnam, middle east coast of India. Indian Journal of Geo-Marine Science 42:183–90.
- Sujatha K, Iswarya Deepti VA, Padmavathi P. 2008. Species diversity and exploitation of groupers (Pisces: Serranidae) off Visakhapatnam, east coast of India. In: Natarajan P, Jayachandran KV, Kannaiyan S, Babu Ambat, Arun Augustine, editors. Glimpses of Aquatic Biodiversity. Glimpses of Aquatic Biodiversity. Kochi: Rajiv Gandhi Chair Special Publication 7:205–12.
- Sujatha K. Shrikanya Rao KVL, Padmavathi P. 2010. Length–weight relationship of four species of Epinephelus Bloch, 1793 in the catches of Visakhapatnam, east coast of India. Journal of the Marine Biological Association of India 52:110–13.
- Sujatha K, Shrikanya KVL, Iswarya Deepti VA. 2013. Length–weight relationship of three species of groupers (Pisces: Serranidae) off Visakhapatnam, middle east coast of India. Journal of Fishery Technology 50:277–79.
- Unsworth RKF, Powell A, Hukom F, Smith DJ. 2007. The ecology of Indo-Pacific grouper (Serranidae) species and the effects of a small scale no take area on grouper assemblage, abundance and size frequency distribution. Marine Biology 152:243–54. 10.1007/s00227-007-0675-3
- Vincent ACJ. 2006. Live food and non-food fisheries on coral reefs and their potential management. In: Cote IM, Reynolds JD, editors. Coral Reef Conservation. Cambridge: Cambridge University Press, p 183–236.