Abstract
Whether the red tide Mesodinium rubrum contains a permanent cryptophyte symbiont or whether it only sequesters chloroplasts from cryptophyte prey was addressed using electron microscopy and the dynamics of photosynthesis, chloroplasts and nuclei. Mesodinium rubrum contains a branched cryptophyte symbiont consisting of many chloroplasts, mitochondria, nucleomorphs, an endoplasmic reticulum and one nucleus. The volume of the symbiont constitutes 36% of the consortium and it is separated from its host by a single-cell membrane. The chloroplasts of Mesodium are larger and morphologically different from two Teleaulax species that served as prey. The symbiont nucleus is also much larger than Teleaulax nuclei. Although M. rubrum is functionally a phototroph, sustained growth beyond two to four generations requires ingestion of prey, but less than one prey cell per generation suffices for maximum growth. This suggests that either the ciliate or its symbiont needs an essential growth factor for continuous growth.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Mesodinium rubrum (Lohmann Citation1908) (=Myrionecta rubra) is a ubiquitous photosynthetic plankton ciliate that often forms conspicuous red tides in fjords, coastal embayments and regions of ocean upwelling (Taylor 1971). It was first described by Lohmann in 1908, but was probably observed as early as 1676 by Leeuwenhook and in 1832 by Charles Darwin on board the “Beagle” (Taylor 1977). The conspicuous brownish-red inclusions have over time been interpreted as symbiotic cyanobacteria (pigments, apparent absence of nuclei) or as symbiotic cryptophytes (pigment composition). Later, transmission electron microscopy indicated that M. rubrum contains a single, branched symbiotic organism delimited from the ciliate cytoplasm by a single membrane (Hibberd Citation1977; Oakley & Taylor Citation1978). The symbiont includes many phycoerythrin-containing chloroplasts arranged in two tenuously linked groups, nucleomorphs, mitochondria with flat cristae, endoplasmic reticulum, and a large non-ciliate nucleus. These properties suggest a cryptophyte origin of the endosymbiont.
Other heterotrophic species of the genus Mesodinium have retractable tentacles at the anterior end of the cell, which are involved in prey capture (e.g. Lindholm et al. Citation1988). Such tentacles were not mentioned for M. rubrum in the original description by Lohmann (Citation1908), nor were they observed in more recent studies (Taylor et al. Citation1971; Hibberd Citation1977; Oakley & Taylor Citation1978). This suggested that the ciliate is incapable of phagocytosis and that the symbiosis is permanent rather than deriving from the sequestered chloroplasts and other organelles from prey organisms. Moreover, high photosynthetic rates of M. rubrum, as well as uptake of inorganic nutrients, indicated that M. rubrum is functionally a phototrophic ciliate (Packard et al. Citation1978; Smith & Barber Citation1979; Wilkerson & Grunseich Citation1990; Stoecher et al. 1991). Tentacles were later observed in living cells by Lindholm et al. (Citation1988), and their role in prey capture was recently described in detail by Yih et al. (Citation2004).
Gustafson et al. (Citation2000) managed, for the first time, to grow M. rubrum in laboratory cultures, feeding it with the cryptophyte Teleaulax sp. They showed that the ciliate has an absolute requirement for prey ingestion, thus explaining past failures in culturing the ciliate. This, and the fact that cryptophyte prey nuclei increased in number inside M. rubrum cells when fed cryptophyte prey, led the authors to suggest that M. rubrum sequesters chloroplasts that are maintained in a functional state for a limited time inside the ciliate, rather than harbouring a permanent symbiont.
Recently, Yih et al. (Citation2004) provided data on ingestion and growth rates of M. rubrum. The ciliates obtained a growth rate of >0.5 day−1 even at low prey concentrations, when the ingestion rate was <1.5 cryptophytes ciliate−1 day−1. This revealed a mismatch between the rate of chloroplast uptake, growth rate and the number of chloroplasts per ciliate. It would mean that replication of chloroplasts does take place inside the ciliate. Also, Johnson & Stoecker (Citation2005) found transient chlorophyll a synthesis in M. rubrum. Their interpretation of the data was that the loss or digestion of prey nuclei resulted in a loss of capability of plastid biosynthesis, but the authors still maintained that the M. rubrum chloroplasts derive from ingested prey. However, neither Gustafson et al. (2002) nor Johnson & Stoecker (Citation2005) mentioned the existence of the large symbiont nucleus described in earlier work (e.g. Hibberd Citation1977; Oakley & Taylor Citation1978; Lindholm Citation1985). The question whether M. rubrum contains a cryptophyte endosymbiont or whether it sequesters chloroplasts from cryptophyte prey is therefore still controversial.
Here we present evidence showing that M. rubrum does harbour a permanent cryptophyte endosymbiont. This evidence is based on fine structure and on the dynamics of photosynthesis, ingestion and growth rates in laboratory cultures.
Material and methods
Isolation and culture of organisms
The food organism Teleaulax sp. was isolated from the northern part of the Sound, Denmark in December 2002. It was grown as non-axenic cultures in f/2 medium (Guillard Citation1983) based on seawater (30 ppt) at 15±1°C. Illumination was provided by cool white fluorescent lamps and cultures were maintained at an irradiance of 100 µmol photons m−2 s−1 and a light–dark cycle of 16:8 h. Irradiance was measured using a LI-COR LI-1000 radiation sensor equipped with a spherical probe.
Mesodinium rubrum was isolated from the Isefjord, Denmark during a bloom event in May 2004. Single cells were collected with a micropipette and transferred three times to sterile-filtered water from the station (salinity 23 ppt). Single cells were then transferred to multidish wells each containing 2 ml of dilute Teleaulax sp. suspension in f/2 medium. After 1 week the wells contained a dense suspension of Teleaulax sp. and some M. rubrum cells. In order to avoid problems associated with dense prey populations in terms of a high pH, all the ciliates were transferred to a new well containing a dilute suspension of Teleaulax cells. This procedure was repeated until dense suspensions of M. rubrum cells were obtained. Thereafter the ciliates were cultured in tissue culture flasks initiated with 500 and 2000 cells ml−1 of M. rubrum and Teleaulax sp., respectively.
Microscopy
The numbers and dimensions of nuclei and chloroplasts were estimated with fluorescence microscopy on formalin- or glutaraldehyde-fixed cells. Cells were filtered on to black Nuclepore® filters with a pore size of 1 µm and the chloroplasts inside each cell were counted in the fluorescence microscope with green excitation light. At least 30 cells were observed on each occasion. For nuclei, cells were stained with 4′-6-Diamidino-2-phenylindole prior to filtration and observed with ultraviolet excitation. Mesodinium cell volumes were based on formalin-fixed cells and calculated as the sum of a hemisphere and a half prolate ellipsoid. The volume of Teleaulax sp. was calculated as the sum of a cone and a hemisphere.
For transmission electron microscopy, cells were fixed in a solution of glutaraldehyde (5%) and OsO4 (1%) in sterile seawater, dehydrated, and embedded in epon. Serial sections (0.1 µm) were stained in uranyl acetate and observed with a Zeiss EM 900 electron microscope. The volume fraction of the symbiont was estimated as an area fraction on median sections (Aherne & Dunnill Citation1982).
Measurements of growth and photosynthesis
All experiments were carried out in 65 ml tissue culture flasks at an irradiance of 100 µmol photons m−2 s−1 with a light–dark cycle of 16:8 h and a temperature of 15±1°C. Preliminary experiments showed that M. rubrum does not grow well on a plankton wheel (often used to ensure uniform distribution of phytoplankton in incubation bottles). The cultures were therefore placed on a glass table without mixing and with illumination supplied from below. The pH of the culture medium was measured regularly as some ciliates are sensitive to the high pH that develops in dense algal cultures (Pedersen & Hansen Citation2003). Our strain of Teleaulax sp. could drive pH up to 9.2. The growth and ingestion of our strain of M. rubrum is affected when pH exceeds 8.5, and it dies out when pH exceeds 8.7. Therefore, cultures were transferred to fresh medium before the pH exceeded 8.5.
For the enumeration of cells, samples (2–3 ml) were withdrawn every 2–3 days, fixed in acidic Lugol's (final concentration 2%), and counted in a Sedgewick-Rafter cell with an inverted microscope. All experiments were carried out in triplicate.
Growth rate constants were estimated as µ=[ln (Nt /N0)] t−1, where N0 is the initial concentration of cells and Nt is the concentration of cells after t days. The ingestion rate of M. rubrum was determined from the reduction in prey concentrations over 48–72 h periods as compared with prey control cultures according to Jakobsen & Hansen (Citation1997). Briefly, ingestion rates, U, were estimated using the two relationships: dx/dt = µxx − Uy, and dy/dt = µyy, where prey (x) is ingested by grazer (y). It was assumed that the grazer (y) grows exponentially with the rate constant µy (determined from prey control cultures) and that the prey (x) grows with the rate constant of µx. Mortality due to grazing is U, the per capita ingestion rate. The solution for U was found numerically by iteration with time steps of 1 h.
Photosynthetic rates of M. rubrum cells were measured by a modification of the “single-cell method” (Stoecker et al. Citation1988; Skovgaard et al. Citation2000). A 2 ml cell suspension of M. rubrum was added to scintillation vials. A NaH14CO3 − stock solution (specific activity 100 µCi ml−1) was then added to each vial, resulting in a specific activity of ∼1.0 µCi ml−1. The vials were incubated on a glass shelf with light coming from beneath for 2–4 h starting 1–3 h after the onset of the light period. This incubation time was selected based on experiments showing linear 14C uptake by M. rubrum for the first 5 h. All measurements were carried out in triplicates. The vials were always accompanied by parallel dark vials, which were treated similarly, except that they were wrapped in aluminium foil during incubation.
After incubation, the specific activity of the medium was measured by transferring 100 µl from each vial to new vials containing 200 µl of phenylethylamine. The remaining suspension received 2.0 ml of 10% acetic acid in methanol to remove all inorganic C. The vials were dried overnight at 60°C and the residue was then re-dissolved in 1.5 ml of distilled water. Finally, 10 ml of Packard Insta-Gel Plus scintillation fluor was added to all vials (including those for specific activity) and activity was measured using a Packard 1500 Tri-Carb liquid scintillation counter. Calculations of photosynthetic rates were based on Parsons et al. (Citation1984). The total dissolved inorganic carbon content was measured with a 225-Mk3 infrared gas analyser (Analytic Development, Hoddesdon, UK).
Results
Morphological evidence
The morphology and fine structure have previously been studied in considerable detail [see Lindholm (Citation1985) and references therein]. The external morphology (with special reference to the present strain of Mesodinium) is also described in Fenchel & Hansen (Citation2006).
With respect to the fine structure of M. rubrum and its endosymbiont, our results confirm those of Hibberd (Citation1977). The branched symbiont is covered by a single-cell membrane. The symbiont consists of two parts residing in the anterior and posterior parts of the ciliate, respectively, and connected by a narrow strand of cytoplasm in one side in the equatorial part of the ciliate. The symbiont constitutes approximately 36% of the volume of the symbiotic consortium. The cytoplasm of the symbiont can be recognized by the mitochondria; these are small, cylindrical or flattened and with flat cristae. In contrast, the ciliate mitochondria are larger, ovoid and with tubular cristae. The chloroplasts are concentrated in the anterior and posterior ends of the ciliates and are absent in its middle part (Figures ). Chloroplast numbers range from six to 36 per cell. They are flattened and somewhat curved and measure 10–15 µm in length, approximately 10 µm in width, and are 0.3–0.4 µm thick. They are situated in the periphery of the ciliate, always facing their convex side outwards (). The thylakoids show a characteristic arrangement (A). The pyrenoid is stalked and the chloroplasts also include a nucleomorph, and vacuoles containing a storage product; these appear as empty vacuoles in and B.
Figure 1. Epifluorescence microscopy of 4′-6-Diamidino-2-phenylindole-stained Mesodinium rubrum and Teleaulax sp. (insert) showing the distribution of the chloroplasts (red) and nuclei (blue) in both species. Note the differences in the size of nuclei and chloroplasts between the ciliate and Teleaulax sp. sn, symbiont nucleus; mi, micronucleus; ma, macronucleus; chl, symbiont chloroplast; n, Teleaulax nucleus; tchl, Teleaulax chloroplast. Scale bar (for both photographs) =10 µm.
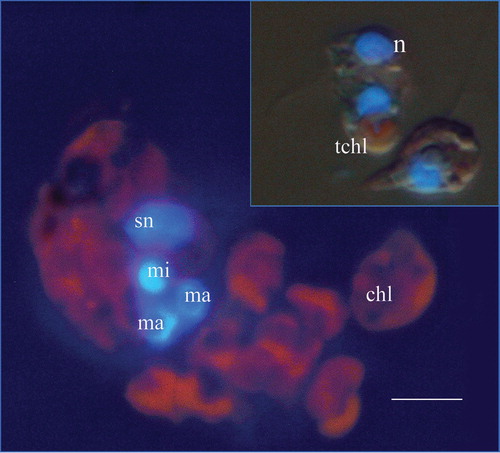
Figure 2. Transmission electron micrograph of a Mesodinium rubrum cell (longitudinal section) showing the ciliate micronucleus and the two macronuclei. ma, macronucleus; mi, micronucleus. Scale bar = 5 µm.
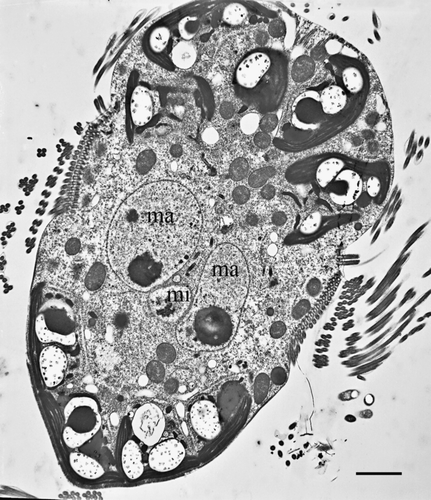
Figure 3. Transmission electron micrograph of a Mesodinium rubrum cell showing two macronuclei and the symbiont nucleus. Also shown is a schematic drawing of the section, indicating the delimitation of the symbiont. ma, macronucleus; sn, symbiont nucleus. Scale bar = 5 µm.
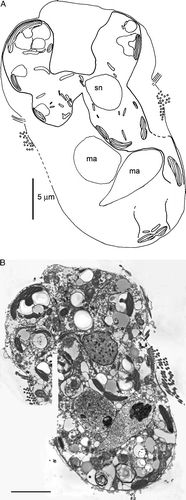
Figure 4. (A) Endosymbiont chloroplast with pyrenoid, starch granules, mitochondria with flat cristae, nucleomorph, and cryptophyte cytoplasm. (B) Transversal section of Teleaulax sp. showing the chloroplast, mitochondria and nucleus. (C) The symbiont nucleus. Unlike the ciliate nuclei it does not have condensed chromatin. mit, symbiont mitochondrion; py, pyrenoid; chl, chloroplast; n, Teleaulax nucleus. Scale bar (for all photographs) = 1 µm.
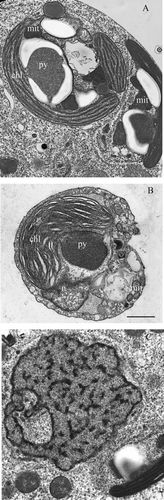
There is usually one symbiont nucleus, but sometimes two are observed. It is possible that this indicates the initiation of division of the symbiotic consortium and that the symbiont nucleus is the first to divide. There are always two symbiont nuclei in dividing Mesodinium cells. The symbiont nucleus is situated anterior to the ciliate nuclei. It is ovoid and its long axis measures 4.5–5.7 µm ( and and C). The nuclei of the ciliate (one micronucleus and two macronuclei) are situated in the middle or slightly behind the middle at the level of the ciliary membranelles ( and ). The mean cell volume of the studied Mesodinium strain was approximately 4570 µm3 (range 2120–7000 µm3).
The morphological features of the prey organism Teleaulax sp. differ in important aspects from those of the symbiont. The single chloroplast measures only 5 − 6×3 µm, the thylakoids are arranged otherwise, and the pyrenoid is not stalked. The Teleaulax sp. nucleus measures only about 3×2 µm ( and B). The cell volume of Teleaulax sp. is approximately 165 µm3.
Growth kinetics, photosynthesis, ingestion rate and maintenance of chloroplasts in the absence of prey uptake
The proliferation of the ciliate and of its prey was studied in mixed cultures initiated with 250 and 2000 cells ml−1 of Mesodinium and Teleaulax, respectively. A monoculture of Teleaulax served as the control. Teleaulax sp. grew exponentially with a rate of 0.9 day−1 for the first 7 days in monoculture (A). In the mixed culture, Teleaulax was ingested by the ciliates, resulting in the depletion of Teleaulax after 7 days. Calculated ingestion rates ranged from an average of 3.5 cells Mesodinium −1 day−1 during the first 2 days to ∼ 0.5 cells Mesodinium −1 day−1 during the period from day 4 to day 7. The ciliates grew exponentially for the first 7 days with a rate constant of 0.4 day−1.On day 7, the mixed culture was sub-cultured to give an initial M. rubrum cell concentration of 200 cells ml−1 and a Teleaulax concentration below detection level (1–2 cells ml−1), see A. The diluted ciliate culture went through an additional three to four cell doublings before cell division stopped. The number of chloroplasts inside the ciliates decreased from an average of 20 to 15 per cell from day 7 to 18 in the diluted culture in the absence of prey (A). This shows that the proliferation of chloroplasts must have taken place even though the ciliates were not feeding.
Figure 5. The first set of experiments. (A) Cell concentrations of Mesodinium rubrum and Teleaulax sp. in a mixed culture and of Teleaulax sp. in monoculture as a function of incubation time. On day 7 (arrows) the mixed culture was diluted and allowed to grow in the absence of prey; the number of chloroplasts per M. rubrum cell was monitored until day 18. (B) On day 18, Teleaulax sp. was added to a subculture of M. rubrum and allowed to proliferate. Teleaulax sp. in monoculture served as a control without predation. All values are treatment means±standard error. (C) Ingestion rates (cells M. rubrum −1day−1) as function of incubation time.
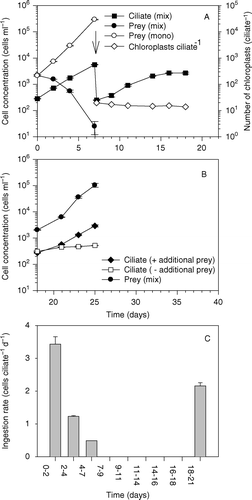
The culture was used to initiate a new experiment on day 18 (B). One set of flasks contained only M. rubrum, whereas the other set contained both M. rubrum and Teleaulax. Despite that initial cell concentrations of both the cryptophyte and the ciliate being the same as at the start of the experiment (day 0), Teleaulax increased in cell concentration to reach 100,000 cell ml−1 at the termination of the experiment on day 25 (B). The M. rubrum population without added prey increased only slightly in cell concentration, whereas the ciliate population to which Teleaulax sp. was added increased. After a period of reduced division rates (days 18–21), growth became exponential from day 21 to day 25. Ingestion rates could only be calculated for the first couple of days, yielding 2.2 cells ciliate−1 day−1 (C), which was significantly lower than previously found at the start of this set of experiments (t-test, P < 0.01).
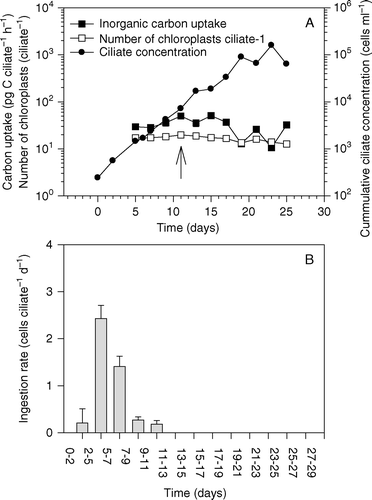
In a second experiment, M. rubrum and Teleaulax were mixed at initial cell concentrations of 250 and 600 cells ml−1, respectively (A). Mesodinium rubrum ingestion rates were low during the first 2 days and Teleaulax cell concentrations increased. However, during the following days, ingestion rates increased and the Teleaulax sp. population was depleted on day 11 (A, B). Photosynthetic rates of the ciliates increased from approximately 30 pg C cell−1 h−1 during days 5–7 to approximately 40 pg C cell−1 h−1 during days 11–17 (A). This increase in photosynthetic rate took place simultaneously with the depletion of prey cells (A). One week later, the photosynthetic rate decreased to an average of approximately 20 pg C cell−1 h−1. Although the Mesodinium cells had tripled in numbers in the absence of prey, the average number of chloroplasts per ciliate had only decreased slightly (from approximately 20 to 13; A).
Figure 6. The second set of experiments. (A) Inorganic carbon uptake by Mesodinium rubrum, cumulative ciliate concentration and number of chloroplasts inside ciliate cells. The arrow indicates when prey cells were depleted. (B) Ingestion rate (cells ciliate−1). Data are presented as a function of incubation time and values represent treatment means±standard error.
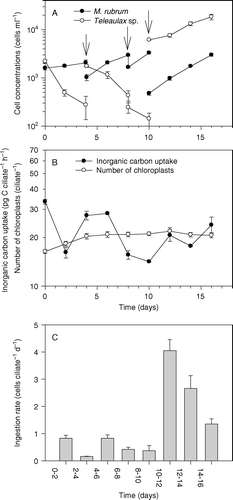
A third experiment was based on Mesodinium cells that had been starved for 2 weeks. They were supplied with a low concentration of Teleaulax cells at the start of the experiment. The ciliates grew slowly for the first 4 days, and the Teleaulax sp. population became almost depleted (A). The cultures were then diluted and additional Teleaulax cells added. This time, M. rubrum grew fast and the prey population became almost depleted on day 10. Dilution and the addition of high concentrations of Teleaulax did not further increase the growth rate of M. rubrum, but this time the Teleaulax population increased during the remaining incubation period. The number of chloroplasts inside M. rubrum cells increased from on average ∼13 to ∼20 per ciliate after 4 days of incubation, whereas the inorganic carbon uptake was approximately 22 pg C cell−1 h−1 during the entire incubation period (B). Ingestion rates were quite low during the first 10 days of the incubation (0.1–1 cells ciliate−1 day−1). Ingestion rates increased considerably after the addition of high concentrations of Teleaulax and ranged from 1.3 to 4 cells ciliate−1 day−1 (C).
Figure 7. The third set of experiments. (A) Proliferation of Mesodinium rubrum and Teleaulax sp. The three arrows indicate times at which the culture was diluted and in the first and the last case additional prey was added. (B) Inorganic carbon uptake and number of chloroplasts inside M. rubrum cells. (C) Ingestion rate. The data are presented as a function of incubation time and values represent treatment means±standard error.
Maintenance of the symbiont nucleus during starvation
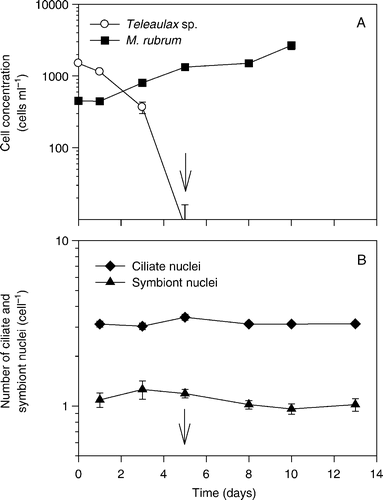
In a mixed culture of M. rubrum and Teleaulax, prey was depleted after 5 days of incubation, whereas the cell concentration of M. rubrum increased from an initial 450 cells ml−1 to 2700 cells ml−1 on day 10 (). During the entire period of starvation, M. rubrum contained three ciliate nuclei and one symbiont nucleus. There were slight deviations in mean values due to dividing cells (that contained two symbiont and six ciliate nuclei, and sometimes two symbiont nuclei and the normal three ciliate nuclei). Also, in a few cases, not all four nuclei could be seen. This may be real or it could be that a nucleus was hidden below other nuclei in the preparations. Under all circumstances, as the ciliates doubled after prey depletion, the symbiont nucleus must also have divided during this period.
Figure 8. The fourth set of experiments. (A) Proliferation of Mesodinium rubrum and Teleaulax sp. in a mixed culture. (B) Numbers of symbiont and ciliate nuclei per ciliate. The arrows indicate when prey cells were depleted. The data are presented a function of incubation time and values represent treatment means±standard error.
Discussion
The cryptophyte chloroplasts of M. rubrum differ in several respects from those of the cryptophyte Teleaulax sp. used as prey. The single chloroplast of Teleaulax is smaller and the thylakoids of this chloroplast are arranged in a different way. Also, the pyrenoids of Teleaulax sp. are not stalked as in the chloroplasts of M. rubrum ( and A, B). Thus, morphological data suggest that the chloroplasts do not derive from ingested Teleaulax.
The generation time of our strain of M. rubrum was on average 1.7 days in cultures supplied with Teleaulax sp. as prey. In such cultures, M. rubrum cells contained approximately 20 chloroplasts. If the ciliate should maintain its chloroplast number by sequestering chloroplasts from its prey, it would have to ingest 11–12 Teleaulax day−1. The highest ingestion rate measured was 3–4 Teleaulax cells day−1, and ingestion rates of <1 cryptophyte cell was, in fact, sufficient to sustain maximum growth. Also, M. rubrum was able to divide three to four times in the absence of prey, with only a minor reduction in the chloroplast number and photosynthetic rates. So, all morphological and experimental evidence suggests that M. rubrum contains permanent endosymbiotic chloroplasts and that the ciliate controls the division of these chloroplasts.
Mesodinium rubrum always contains one symbiont nucleus, which is much larger than the nucleus of the prey species. In the absence of prey, we found that M. rubrum divides and thus replicates the symbiont nucleus several times. This suggests that the ciliate also controls the replication of the symbiont nucleus and that this symbiont nucleus is not derived from the ingestion of cryptophyte nuclei.
Thus, our data do not support the conclusion of Johnson & Stoecker (Citation2005) that M. rubrum sequesters cell organelles from its prey. They based their interpretation on the photosynthetic rates and prey nuclei disappearance upon starvation. The data presented by them on the effect of starvation on the production of chlorophyll a and rates of photosynthesis are not in conflict with our data. They did, however, observe a decline in “prey nuclei” upon starvation of M. rubrum, which we did not see. However, it is unclear what they actually mean by “prey nuclei” in their study. Are these actually prey nuclei or are they in fact the “symbiont nuclei”. Judging from the fact that they report a maximum of one “prey nuclei” per cell in well-fed M. rubrum cells, it may indeed be the “symbiont nucleus” they refer to.
Johnson & Stoecker (Citation2005) reported a decline of “prey nuclei” from 1 M. rubrum −1 to approximately 0.5 M. rubrum −1 after 2 weeks of starvation. We only observed the number of “symbiont nuclei” in M. rubrum cells for about 8 days, but taking into consideration that our experiments were carried out at 15°C, whereas their experiments were carried out at 0–2°C, the data should be comparable. In fact, in both cases, the ciliates divided about four times when subjected to starvation.
It is possible that we may be looking at two different strains with different properties. Our isolate originates from an area quite close to Kiel Bight where Lohmann (Citation1908) collected cells used for the original description of M. rubrum, so we have reason to believe that our isolate is M. rubrum. Further studies are, however, required to sort out the cause of the apparent differences among isolates.
If M. rubrum hosts a single, large permanent endosymbiont, then this is unique among ciliates. However, similar permanent symbiotic consortia occur among certain dinoflagellates. Durinskia baltica (=Peridinium balticum) and Kryptoperidinium foliaceum (=P. foliaceum) are classic examples of dinoflagellates with a single photosynthetic eukaryote endosymbiont (Tomas & Cox Citation1973; Tomas et al. Citation1973; Jeffrey & Vesk Citation1976). The endosymbionts, which in these cases seem to be of diatom origin, are also delimited from the host by a single membrane, as in M. rubrum. Similar relationships between a diatom endosymbiont and a dinoflagellate have been found in other dinoflagellates (Galeidinium rugatum, Peridinium quinquecorne and Gymnodinium quadrilobatum; Horiguchi & Pienaar Citation1994; Tamura et al. Citation2005). Dinoflagellates, which have cryptophyte endosymbionts, have been found in many species of the genus Dinophysis. However, Takashita et al. (Citation2002) and Janson (Citation2004) found evidence that suggests that Dinophysis is an example of chloroplast retention rather than symbiotic chloroplasts.
It is noteworthy that all dinoflagellates with diatom endosymbionts can be grown in inorganic algal growth media with the addition of vitamins, whereas M. rubrum cannot. Perhaps the free-living cryptophyte ancestor of the symbiont to some extent depended on phagotrophy. Not much is known about the need for phagotrophy in marine cryptophytes. It has, however, been shown that freshwater phototrophic cryptophytes (e.g. Cryptomonas spp.) ingest bacteria (Tranvik et al. Citation1989; Marshal & Laybourn-Parry Citation2002; Pålsson & Graneli Citation2003). Measured uptake rates of bacteria are low (<0.5 cell h−1) so that ingestion of bacteria in well-illuminated waters only plays a role for the acquisition of nutrients or growth factors. A closer study of the possible role of phagotrophy in marine phototrophic cryptophytes may illuminate why M. rubrum requires particulate food.
Editorial responsibility: Diane Stoecker
Acknowledgments
We are grateful to Marianne Saiz for technical assistance and to Birgit Thorell for photographical work. The study was supported by grants from the Danish Natural Science Research Council to TF and to PJH and from the Carlsberg Foundation to TF.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Aherne , WA and Dunnill , MS . 1982 . Morphometry , London : Edward Arnold .
- Fenchel , T and Hansen , PJ . 2006 . Motile behaviour of the bloom forming ciliate Mesodinium rubrum . Marine Biology Research , 2 : 33 – 40 .
- Guillard , RRL . 1983 . “ Culture of phytoplankton for feeding invertebrate animals ” . In Culture of Marine Invertebrates , Edited by: Berg , CJ . 123 – 8 . Stroudsburg, PA : Hutchinson Ross .
- Gustafson , DE Jr , Stoecker , DK , Johnson , MD , Van Heukelem , WF and Sneider , K . 2000 . Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum . Nature , 405 : 1049 – 52 .
- Hibberd , DJ . 1977 . Observations on the ultrastructure of the cryptomonad endosymbiont of the red-water ciliate Mesodinium rubrum . Journal of the Marine Biology Association of the United Kingdom , 57 : 45 – 61 .
- Horiguchi , T and Pienaar , RN . 1994 . Ultrastructure of a new sand-dwelling dinoflagellate, Gymnodinium quadrilobatum sp. nov. (Dinophyceae) with special reference to its endosymbiotic alga . Journal of Phycology , 29 : 237 – 45 .
- Jakobsen , HH and Hansen , PJ . 1997 . Prey size selection, growth and grazing responses of a small heterotrophic dinoflagellate Gymnodinium sp. and a ciliate Balanion comatum: a comparative study . Marine Ecology Progress Series , 158 : 75 – 86 .
- Janson , S . 2004 . Molecular evidence that plastids in the toxin-producing dinoflagellate genus Dinophysis originate from the free-living cryptophyte Teleaulax amphioxeia . Environmental Microbiology , 6 : 1102 – 6 .
- Jeffrey , S and Vesk , M . 1976 . Further evidence for a membrane-bound endosymbiont within the dinoflagellate Peridinium foliaceum . Journal of Phycology , 12 : 450 – 455 .
- Johnson , MD and Stoecker , DK . 2005 . Role of feeding in the growth and photophysiology of Myrionecta rubra . Aquatic Microbial Ecology , 39 : 303 – 12 .
- Lindholm , T . 1985 . Mesodinium rubrum – a unique photosynthetic ciliate . Advances in Aquatic Microbiology , 8 : 1 – 48 .
- Lindholm , T , Lindroos , P and Mörk , A-N . 1988 . Ultrastructure of the photosynthetic ciliate Mesodinium rubrum . BioSystems , 21 : 141 – 9 .
- Lohmann , H . 1908 . Untersuchungen zur feststellung des vollständigen gehaltes des meeres and plankton. Wissenschaftliche Meeresuntersuchungen, Kiel . Neue Folge , 10 : 129 – 370 .
- Marshall , W and Laybourn-Parry , J . 2002 . The balance between photosynthesis and grazing in Antarctic mixotrophic cryptophytes during summer . Freshwater Biology , 47 : 2060 – 70 .
- Oakley , BR and Taylor , FJR . 1978 . Evidence for a new type of endosymbiotic organization in a population of the ciliate Mesodinium rubrum from British Columbia . BioSystems , 10 : 361 – 9 .
- Pålsson , C and Graneli , W . 2003 . Diurnal and seasonal variations in grazing by bacterivorous mixotrophs in an ologitrophic clear-water lake . Archiv für Hydrobiologie , 157 : 289 – 307 .
- Packard , TT , Blasco , D and Barber , RT . 1978 . “ Mesodinium rubrum in the Baja California upwelling system ” . In Upwelling ecosystems , Edited by: Boje , R and Tomczak , M . 73 – 89 . Berlin : Springer Verlag .
- Parsons , TR , Maita , Y and Lalli , CM . 1984 . A manual of chemical and biological methods for seawater analysis , Oxford : Pergamon .
- Pedersen , MF and Hansen , PJ . 2003 . Effects of high pH on the growth and survival of six marine heterotrophic protists . Marine Ecology Progress Series , 260 : 33 – 41 .
- Skovgaard , A , Hansen , PJ and Stoecker , DK . 2000 . Physiology of the mixotrophic dinoflagellate Fragilidium subglobosum. I. Effects of phagotrophy and irradiance on photosynthesis and carbon content . Marine Ecology Progress Series , 201 : 129 – 136 .
- Smith , WO and Barber , RT . 1979 . A carbon budget for the autotrophic ciliate Mesodinium rubrum . Journal of Phycology , 15 : 27 – 33 .
- Stoecker , DK , Putt , M , Davis , LH and Michaels , AE . 1991 . Photosynthesis in Mesodinium rubrum: species-specific measurements and comparison to community rates . Marine Ecology Progress Series , 73 : 245 – 52 .
- Stoecker , DK , Silver , MW , Michaels , AE and Davis , LH . 1988 . Obligate mixotrophy in Laboea strobila, a ciliate which retains chloroplasts . Marine Biology , 99 : 415 – 423 .
- Takashita , K , Koike , K , Tadashi , M and Ogata , T . 2002 . Molecular evidence for plastid robbery (kleptoplastidy) in Dinophysis, a dinoflagellate causing diarrhetic shellfish poisoning . Protist , 153 : 293 – 302 .
- Tamura , M , Shimada , S and Horiguchi , T . 2005 . Galeidinium rugatum gen et sp nov (Dinophyceae), a new coccoid dinoflagellate with a diatom endosymbiont . Journal of Phycology , 41 : 658 – 71 .
- Taylor , FJR , Blackbourn , DJ and Blackbourn , J . 1971 . The red-water ciliate Mesodinium rubrum and its “incomplete symbionts”: a review including new ultrastructural observations . Journal of Fisheries Research Board of Canada , 28 : 391 – 407 .
- Tomas , RN and Cox , ER . 1973 . Observations on the symbiosis of Peridnium balticum and its intracellular alga. I. Ultrastructure . Journal of Phycology , 9 : 304 – 23 .
- Tomas , RN , Cox , ER and Steidinger , KA . 1973 . Peridinium balticum (Levander) Lemmermann, an unusual dinoflagellate with a mesocaryotic and eukaryotic nucleus . Journal of Phycology , 9 : 91 – 8 .
- Tranvik , LJ , Porter , KG and Sieburth , JM . 1989 . Occurrence of bacterivory in Cryptomonas, a common fresh-water phytoplankter . Oecologia , 78 : 473 – 6 .
- Wilkerson , FP and Grunseich , G . 1990 . Formation of blooms by the symbiotic ciliate Mesodinium rubrum: the significance of nitrogen uptake . Journal of Plankton Research , 12 : 973 – 89 .
- Yih , W , Kim , HS , Jeong , HJ , Myung , G and Kim , YG . 2004 . Ingestion of cryptophyte cells by the marine photosynthetic ciliate Mesodinium rubrum . Aquatic Microbial Ecology , 36 : 165 – 70 .