Abstract
The phyllodocid polychaete Paranaitis wahlbergi occurs in Arctic and northern European boreal waters. Boreal populations are distinct from Arctic ones in having smaller maximal size and larger eggs; in all other respects we find them morphologically inseparable. Phylogenetic analyses and haplotype networks based on the mitochondrial genes 16S rDNA and COI, and the nuclear genes histone H3, ITS1, ITS2, 18S rDNA and 28S rDNA D1–D2 region confirm our suspicion that Arctic and boreal populations belong to different species. We describe the boreal form as a new species, Paranaitis katoi. It is presently known from the Swedish and Norwegian west coasts and from Scotland. Calibrated rates for COI indicate that P. katoi sp. nov. and P. wahlbergi may have been separate for as long as 29–56 million years.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Sibling species in the sea are common, and our awareness hereof has increased considerably during the molecular genetics era (Knowlton Citation2000; Bickford et al. Citation2007). Many of these sibling species are discovered in widely distributed taxa, which when scrutinized are found to consist of several species with more restricted geographical distributions (Knowlton Citation1993). In this study, we take a closer look at the phyllodocid polychaete Paranaitis wahlbergi (Malmgren, 1865), which has a distribution in Arctic and northern European boreal waters.
Following a recent revision by Kato & Pleijel (Citation2003), the phyllodocid polychaete genus Paranaitis comprises 14 species, found mainly on muddy and sandy bottoms in worldwide oceans. Malmgren (Citation1865) described the first member of the genus from Svalbard, Paranaitis wahlbergi (originally as Anaitis wahlbergi), and this species has subsequently been recorded across the eastern Arctic from east Greenland to the Chukchi Sea, as well as from more boreal northern European waters, including the Norwegian Sea, east Iceland, western Scotland, the west coasts of Sweden and Norway, and Gibraltar (Pleijel Citation1993; Kato & Pleijel Citation2003). Kato & Pleijel (Citation2003) carried out a morphology-based phylogenetic analysis of members of Paranaitis, showing that P. wahlbergi is monophyletic based on the absence of a nuchal papilla, a shallow dorsal ligula in segment 1, lateral rows of papilla on the proboscis, and poorly developed dorsal cirrophores. In their redescription of P. wahlbergi, Kato & Pleijel (Citation2003) pointed out that Arctic specimens attain a much larger size than more boreal ones. In addition to this size difference, the boreal form was reported to have fewer chaetae and relatively longer cirri on the anterior-most segments. In the following we will refer to the two forms as ‘Arctic P. wahlbergi’ vs. ‘boreal P. wahlbergi’.
To investigate if the observed size differences between Arctic and boreal populations are due to phenotypic plasticity, or if boreal P. wahlbergi should be referred to as a distinct species, we sequenced the mitochondrial genes COI and 16S rDNA, and the nuclear genes histone H3, ITS1, ITS2, 18S rDNA and 28S rDNA D1–D2 region from a number of specimens from Svalbard, western Norway and the Swedish west coast. As the populations are allopatric, some kind of genetic differences may be expected due to isolation by distance (Slatkin Citation1993), even in the presence of gene exchange. For this reason we compare the genetic structure of the two forms of P. wahlbergi with Phyllodoce groenlandica Ørsted, Citation1842, another member of Phyllodocidae with a similar Arctic–boreal geographic range.
We present two analyses, one phylogenetic analysis in order to assess whether boreal and Arctic populations represent distinct clades or are nested, and a haplotype network analysis to quantify inter-and intrapopulation relationships.
Material and methods
Material from National Museum and Galleries of Wales, Cardiff, UK (NMW) and the Swedish Museum of Natural History, Stockholm (SMNH) was examined. Specimens labelled FP are in the collection of the last author. Newly collected specimens were relaxed with magnesium chloride, preserved in formaldehyde (10%) for a few days, rinsed in fresh water and transferred to 70% alcohol, or for use in DNA sequencing, preserved directly in 95% alcohol. Specimens for scanning electron microscopy (SEM) were fixed in 1% osmium tetroxide in magnesium chloride solution for 1 h, rinsed in fresh water, conserved in 70% alcohol, critical point-dried, sputter-coated and examined in a Philips XL20 microscope. Live specimens were photographed with a Canon EOS 5D connected to a Canon MP-E65/2.8 1–5× macro objective. Origin of specimens, GenBank accession numbers, and deposition of vouchers are detailed in . DNA was extracted using DNAeasy Tissue Kit (Qiagen) following the protocol supplied by the manufacturer. We amplified 658 bp of COI, about 480 bp of 16S rDNA, 337 bp of histone H3, about 1750 bp of 18S rDNA, about 820 bp of 28S rDNA, and about 1200 bp of nuclear ITS 1–5.8S rDNA–ITS 2. We used the same primers as specified in Eklöf et al. (Citation2007) for 16S rDNA, 18S rDNA, 28S rDNA, and we used LCO1490 (Folmer et al. Citation1994) and COI-E (Bely & Wray Citation2004) for COI, H3F and H3R (Brown et al. Citation1999) for histone H3, and ITS18SFPOLY (GAGGAAGTAAAAGTCGTAACA), ITS5.8SFPOLY (GAATTGCAGGACACATTGAAC), ITS5.8SRPOLY (GTTCAATGTGTCCTGCAATTC), and ITS28SRPOLY (ATGCTTAAATTCAGCGGGT) for the ITS 1–5.8S rDNA–ITS2 region. PCR mixtures contained ddH2O, 1 µl of each primer (10 µM), 2 µl template DNA and puReTaq Ready-To-Go PCR Beads (Amersham Biosciences) in a mixture totalling 25 µl. The temperature profile was as follows: 96°C/240 s–(94°C/30 s − 48°C/30 s–72°C/60s)*45 cycles − 72°C/480 s. PCR products were purified with the E.Z.N.A. Cycle-Pure Kit (Omega Bio-tek). Sequencing was performed at Macrogen Inc. facilities (Seoul, Korea). Overlapping sequence fragments were merged into consensus sequences using SeqMan 4.0 (DNAStar).
Table I. Origin of sequenced specimens, specification of vouchers and GenBank numbers. The boreal form of Paranaitis wahlbergi is here referred to as P. katoi sp. nov. as we herein describe this form as a new species. The sequences are registered at GenBank and TreeBase under this name.
In the phylogenetic analysis we used data from mitochondrial COI and 16S rDNA, and data from nuclear histone H3, 28S rDNA and 18S rDNA. Sequences from Arctic and boreal Paranaitis wahlbergi were aligned together with sequences from the five phyllodocid taxa P. kosteriensis (Malmgren, Citation1867), Eteone picta Quatrefages, Citation1866, Chaetoparia nilssoni Malmgren, 1867, Notophyllum foliosum (M. Sars, Citation1835) and Phyllodoce groenlandica using Clustal X (Thompson et al. Citation1997) with default settings (gap/gap length penalties set to 15/6.66). All sites were included in subsequent analyses. Paranaitis kosteriensis lacked data on histone H3 and 16S rDNA, C. nilssoni lacked data on histone H3, one specimen of boreal P. wahlbergi lacked data on histone H3, and additionally we only had 18S rDNA sequences for one boreal and one Arctic specimen of P. wahlbergi. The nuclear and the mitochondrial data sets were analysed separately and combined. We used PAUP*4.0b10 (Swofford Citation2002) to analyse the data with parsimony, using heuristic search option, tree bisection and reconnection (TBR), and 100,000 random additions. All characters were treated as unordered and given equal weight. Clade support was assessed using parsimony jack-knifing (Farris et al. Citation1996). Jack-knife values were calculated from 5000 replicates, heuristic searches, 37.1% character deletion, TBR, and 3 random additions, maxtrees set to 500. We used MrBayes 3.1.2 (Ronquist & Huelsenbeck Citation2003) to conduct Bayesian analyses. For the two sets of protein coding genes, histone H3 and COI, we used a General Time Reversible model (GTR) with site-specific rates, and for the ribosomal genes we used a GTR model with invariant gamma distribution (GTR + I+G). The nuclear ribosomal genes were treated as one unit. The number of generations were set to three million with four parallel chains (three hot, one cold), sample frequency was set to 500, and number of runs were set to two. A quarter of the samples were discarded as burn in. Parameters were altered in the proposal mechanisms to acquire a span within 20–60% acceptance rates for the moves in the cold chain of each run (Roberts et al. Citation1994; Gelman et al. Citation1995). Proposal rates were not changed. The tree files were analysed in AWTY (Are We There Yet) (Wilgenbusch et al. Citation2004; CitationNylander et al. 2008) to visually interpret if the analyses had reached the stationary phase.
For the population genetic analyses, sequences from Arctic and boreal Paranaitis were aligned together with sequences from up to nine specimens of P. groenlandica. The ITS1–5.8S rDNA–ITS2 region was aligned in its entirety, and boundaries for the different regions were determined from Chen et al. (Citation2002). ITS1 and ITS2 were subsequently analysed independently. The ITS2 region was difficult to read for the P. groenlandica specimens, probably due to intraindividual length variation, and was thus excluded before alignment. All sites were included in the population genetic analyses. Statistical parsimony haplotype network was generated with the software program TCS (Clement et al. Citation2000), with a 95% connection limit for the 16S rDNA and histone H3 data sets, and a fix connection limit at 120 steps for the COI, ITS1 and ITS2 data sets. Kimura 2-parameter distances between and within species were calculated in PAUP*4.0b10 (Swofford Citation2002). All data matrices are available at TreeBase, http://www.treebase.org. Divergence time for Arctic and boreal P. wahlbergi were estimated using the substitution rates for hydrothermal polychaetes provided by Chevaldonné et al. (Citation2002), the only calibrated rates for polychaetes published so far (time since divergence = Kimura 2-parameter distance/r where r=0.26–0.50% divergence per million years).
Results
Phylogenetic analysis
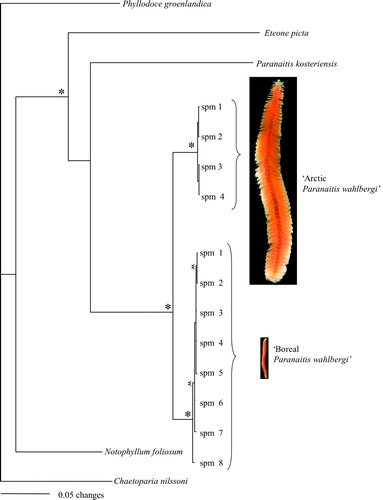
The combined data set of histone H3, 18S rDNA, 28S rDNA, COI and 16S rDNA consisted of 4112 aligned positions, of which 393 were parsimony-informative, and 354 were variable but not parsimony-informative. The parsimony analysis yielded 58 most parsimonious trees (MPTs) with a tree length of 1198, a consistency index of 0.7846, and a retention index of 0.7507. The MPTs differed in the internal relationships within Arctic and boreal Paranaitis wahlbergi. Six of the nodes were well supported with jack-knife values above 80% (). Four of these nodes were supported in the separate mitochondrial and nuclear analyses (indicated with an asterisk in ), while two were only supported by the mitochondrial data (indicated with half an asterisk). These two nodes were not contradicted by the nuclear data, and were thus also supported in the combined analysis. The majority rule consensus trees from the Bayesian analyses were in principal identical to the jack-knife trees in both separate and combined analyses, and the nodes that had jack-knife values > 80% had posterior probabilities >95% ().
Figure 1. Majority rule consensus tree from the Bayesian analysis on the combined mitochondrial and nuclear data set. An asterisk indicates clades supported by posterior probabilities >0.95 and parsimony jack-knife support values >80% in both separate (nuclear and mitochondrial) and combined data sets. Half an asterisk indicates clades with similar support values, but only found in mitochondrial and combined data sets. Photographs show Arctic vs. boreal Paranaitis wahlbergi in scale 1:1.
Population genetic analyses
The COI data set consisted of 658 characters. Including Paranaitis groenlandica, 157 of the characters were parsimony-informative and 2 were variable but not parsimony-informative. Of those, 25 occurred in the first position, 2 in the second position, and 132 in the third position. Excluding P. groenlandica, 90 characters were parsimony-informative and 6 variable but not parsimony-informative. Of those, 9 occurred in the first position and 87 in the third position. Three of the substitutions separating Arctic and boreal P. wahlbergi resulted in amino acid changes. The mean K2P-corrected distance between Arctic and boreal P. wahlbergi was 14.6±0.35%. A total of two haplotypes in Arctic P. wahlbergi, seven haplotypes in boreal P. wahlbergi, and six haplotypes in P. groenlandica were recovered (A). Mean intraspecific variation was 0.1±0.08% in Arctic P. wahlbergi, 0.8±0.8% in boreal P. wahlbergi, and 0.5±0.4% in P. groenlandica.
Figure 2. Haplotype networks for Phyllodoce groenlandica (dark grey circles), Arctic Paranaitis wahlbergi (light grey circles), and boreal P. wahlbergi (white circles). SVA = Svalbard, SE = Sweden, NO = Norway, spm 1, 2, 3, etc. refer to the specimens specified in and . A bar denotes one mutation. A–C: haplotype networks for COI, 16S, and histone H3, respectively.
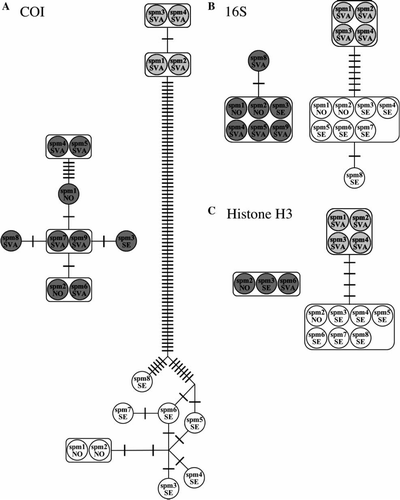
The 16S rDNA data set consisted of 503 characters. Including Paranaitis groenlandica, 72 of the characters were parsimony-informative, and 2 were variable but not parsimony-informative. Excluding P. groenlandica, seven characters were parsimony-informative, and one character variable but not parsimony-informative. The mean K2P-corrected distance between Arctic and boreal P. wahlbergi was 1.5±0.07%. A total of one haplotype in Arctic P. wahlbergi, two haplotypes in boreal P. wahlbergi, and two haplotypes in P. groenlandica were recovered (B). Mean intraspecific variation was 0.06±0.09% in both boreal P. wahlbergi and P. groenlandica.
The histone H3 data set consisted of 337 characters. Including P. groenlandica, 27 of the characters were parsimony-informative. Of those, 4 occurred in the first position and 23 in the third position. Excluding P. groenlandica, four characters were parsimony-informative, all in third positions. None of the substitutions separating Arctic and boreal P. wahlbergi resulted in amino acid changes. The mean K2P-corrected distance between Arctic and boreal P. wahlbergi was 1.2%. Single haplotypes were found in all three taxa (C).
The ITS1 data set consisted of 673 characters. Including P. groenlandica, 388 of the characters were parsimony-informative, and 11 variable but not parsimony-informative. Excluding P. groenlandica, 121 characters were parsimony-informative, and 8 were variable but not parsimony-informative. The mean K2P-corrected distance between Arctic and boreal P. wahlbergi was 22.2±0.4%. A total of three haplotypes in Arctic P. wahlbergi, seven in boreal P. wahlbergi, and three in P. groenlandica were recovered (A). Mean intraspecific variation was 0.85±0.60% in Arctic P. wahlbergi, 0.89±0.31% in boreal P. wahlbergi, and 0.98±0.45% in P. groenlandica.
Figure 3. Haplotype networks for Phyllodoce groenlandica (dark grey circles), Arctic Paranaitis wahlbergi (light grey circles), and boreal P. wahlbergi (white circles). SVA = Svalbard, SE = Sweden, NO = Norway, spm 1, 2, 3, etc. refer to the specimens id in and . A bar denotes one mutation. A, B: haplotype networks for ITS1, and ITS2, respectively.
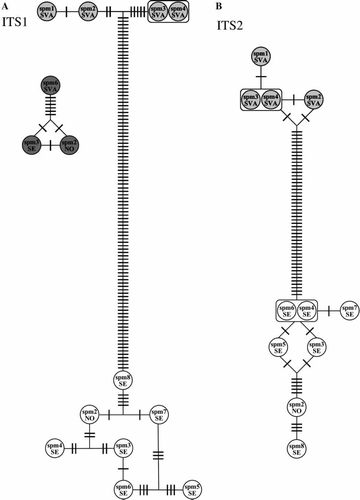
The ITS2 data set consisted of 446 characters. It proved difficult to read this fragment in P. groenlandica and was therefore excluded before alignment. In the alignment with Arctic and boreal P. wahlbergi, 59 characters were parsimony-informative and 11 variable but not parsimony-informative. The mean K2P-corrected distance between Arctic and boreal P. wahlbergi was 16.04±0.33%. A total of three haplotypes in Arctic P. wahlbergi, and six in boreal P. wahlbergi, were recovered (B). Mean intraspecific variation was 0.27±0.17% in Arctic P. wahlbergi, and 1.12±0.95% in boreal P. wahlbergi.
No differences were observed between Arctic and boreal P. wahlbergi in 5.8S rDNA with 158 characters, and 18S rDNA with 1773 characters, but in the 28S rDNA D1–D2 region with 840 characters, there were 6 characters separating the 2 taxa (mean K2P-corrected distance = 0.84%).
Divergence time
Using the rates 0.26–0.50% per million years for the COI data set, the divergence time between boreal and Arctic P. wahlbergi was estimated to be 29–56 million years.
Discussion
Sibling species in the sea are ubiquitous (Knowlton Citation1993, Citation2000). Sibling species may be poorly studied organisms that actually have morphological differences, i.e. pseudo-sibling species, or they may be inseparable to the human eye even after thorough investigations, i.e. true sibling species (e.g. Westheide & Hass-Cordes Citation2001). Sibling species are often detected among widely distributed ‘species’ where several species with different geographical distributions, rather than a single one, are involved (Knowlton Citation1993; Bonse et al. Citation1996). Arctic and boreal Paranaitis wahlbergi are candidates for pseudo-sibling species. Morphologically they may be separated by maximal size, with Arctic P. wahlbergi reaching more than 10 cm in body length (), and boreal ones not exceeding 2 cm. Of course, this would only serve to diagnose large specimens. Additionally, a reproductive character separates them, where Arctic P. wahlbergi have eggs which are c. 100 µm in diameter, whereas in boreal populations they are about 300 µm (see taxonomy section below). Likely, this also indicates different modes of larval development in the two groups, with planktotrophic development in the former and lecithotrophic in the latter.
In our phylogenetic analysis both Arctic and boreal P. wahlbergi are well supported by the mitochondrial as well as the nuclear data (); they are sister taxa and represent two non-nested clades and should be treated as distinct species. Our results from the population genetic analysis based on the mitochondrial COI and 16S rDNA, and the nuclear histone H3, ITS1, ITS2 (A–C, 3A,B), also strongly corroborate this result. The variation within both groups is low compared to the differences between the two, and similar to those found in other studies of polychaetes (). We do not believe that genetic distance per se could be used as a yardstick for delimiting species (as in e.g. Hebert et al. Citation2004). Instead we agree that a combined approach using mitochondrial, nuclear and morphological data is more appropriate for delineating species boundaries and identifying diagnostic characters (Ferguson Citation2002; Rubinoff & Holland Citation2005; Rubinoff Citation2006; Rubinoff et al. Citation2006).
Table II. Mean Kimura two-paramater (K2P) distances and intraspecific variation for a selection of polychaete species pairs or species groups for the mitochondrial COI and 16S, and the nuclear ITS1 and ITS2.
As we have not found Arctic and boreal P. wahlbergi sympatrically, minor genetic differences between populations from Svalbard and the coasts of western Norway and Sweden could result from isolation by distance within a single species. For comparative purposes, and to some extent rule out this explanation, we also sequenced specimens from another phyllodocid, P. groenlandica, sampled at the same locations as P. wahlbergi. We could not detect any geographical separation of haplotypes for P. groenlandica, and the intraspecific variation for the whole geographic area is comparable to that found within Arctic and boreal P. wahlbergi (A–C, 3A). Thus, we conclude that the geographic distance between Svalbard and the Scandinavian localities is unlikely to be the reason for the genetic distance seen between Arctic and boreal P. wahlbergi.
Using the calibrated rate from Chevaldonné et al. (Citation2002), our data from COI suggests a separation of Arctic and boreal P. wahlbergi for as long as 29–56 million years. However, divergence estimates based on mutation rates for mitochondrial genes has been criticized (Rubinoff et al. Citation2006), because the mutation rate of a gene is dependent on the physical location in the genome. The further the gene is located from where the mitochondrion starts its replication, the higher the mutation rate. Since we in most cases are unaware of the gene order, mutation rate will not be the same across the group we study if there has been a gene order rearrangement. In the present case this may not be a problem, because the gene order seems to be very conserved in Annelida (Bleidorn et al. Citation2007). Another more serious objection is that the calibration is based on annelids from hydrothermal vents, and mutation rates in hydrothermal annelids may be very different from those living in more ‘normal’ habitats. It is with these reservations in mind we should look at these estimates. But even if we apply the much faster rate based on transisthmian pairs of Alpheid shrimps where the divergence rate =1.4% per million years, this would mean that Arctic and boreal P. wahlbergi have been separated for about 10 million years. Both estimates are well beyond the suggested minimum time of 3–3.5 million years required for development of strong reproductive isolation under the allopatric model (Knowlton et al. Citation1993).
To conclude, we suggest that we have strong evidence for regarding boreal P. wahlbergi as a distinct and previously unrecognized species, and therefore here describe it as P. katoi sp. nov.
Taxonomy
Family Phyllodocidae Ørsted, Citation1843
Genus Paranaitis Southern, 1914
Paranaitis katoi sp. nov. (–7)
Synonymy
Paranaitis wahlbergi (in part) Pleijel Citation1991: figure 9, 1991: 30–2, figures 17–19, map 10; Pleijel & Dales Citation1991: 96, figure 27A–C; Hartmann-Schröder Citation1996: 91; Kato & Pleijel Citation2003: 385–8, figures 1–3, 37.
Etymology
Named in honour of Dr Tetsuya Kato, for his outstanding contributions to phyllodocid taxonomy.
Material examined
Sweden: Holotype (SMNH Type-7377), northern Bohuslän, Koster area, S Yttre Vattenholmen, 58°52.425’–58°51.905'N, 11°06.053’–11°06.511′E, 130–140 m, mud, detritus sledge, fixed in formaldehyde, coll. FP, 5 October 2007; 3 paratypes, all from type locality, detritus sledge, 2 mounted on SEM stubs (SMNH Type-7380, 7381) and 1 (mature female) fixed in formaldehyde (SMNH Type-7382), coll. FP, 5 October 2007; 2 paratypes (SMNH Type-7387, 7389), northern Bohuslän, Singlefjord, 59°05.030′N, 11°07.394′E, 78 m, mud, detritus sledge, fixed in 95% ethanol, part of both specimens used for DNA extractions, coll. FP, 28 April 2005; 4 specimens (SMNH 97333–97336), northern Bohuslän, Koster area, SV Yttre Vattenholmen, 58°51′N, 11°06′E, 90–110 m, mud, detritus sledge, fixed in 95% ethanol, part of all four specimens used for DNA extractions, coll. AN, 5 May 2005; 1 specimen (FP), northern Bohuslän, Koster area, S Yttre Vattenholmen, 58°52.238’–58°51.905′N, 11°06.546’–11°06.235′E, 135–140 m, mud, detritus sledge, fixed in 95% ethanol, coll. FP, 21 March 2007; 1 specimen (FP), same locality, detritus sledge, fixed in 95 ethanol, coll. FP, 22 May 2007; 3 specimens (FP), northern Bohuslän, Koster area, S Yttre Vattenholmen, 58°51.905'N, 11°06.511′E, 125 m, mud, detritus sledge, fixed in 95 ethanol, coll. FP, 25 June 2007; 1 specimen (FP), northern Bohuslän, Koster area, S Yttre Vattenholmen, 58°52.132′N, 11°06.235′E, 170 m, mud, detritus sledge, fixed in 95 ethanol, coll. FP, 21 March 2007; 1 specimen (SMNH 22501), northern Bohulän, Singlefjord, 80 m, mud, detritus sledge, coll. FP, 1 August 1988; 10 specimens, northern Bohuslän, Säcken, 125 m, mud, coll. FP, 4 April 1988 (mounted for SEM, not kept); 1 specimen, northern Bohuslän, Säcken, fixed in 95% ethanol, coll. marine biology course, 4 April 2005; 3 specimens (SMNH 23277), northern Bohuslän, Koster area, SW Yttre Vattenholmen, 160–180 m, mud, fixed in formaldehyde, 22 July 1988, coll. FP.
Norway: 2 specimens (SMNH 90981, SMNH 97332), Trondheimsfjord, Rødberg, 63°28.36′N, 10°00.04′E, 180-250 m, triangular dredge, Lophelia with mud, fixed in 95% ethanol, part of both specimens used for DNA extraction, coll. FP 29 January 2002.
United Kingdom: 3 specimens (NMW.Z 1985.023.0021), Scotland, Loch Creran, Argyll, 15–22 m.
Description
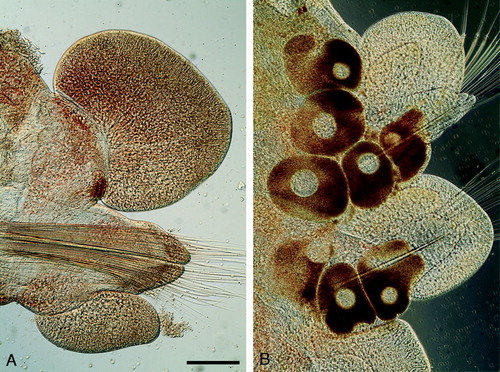
Holotype complete specimen, 12.25 mm long, 1.0 mm wide at mid-body, for 56 segments. For length–segment relationships see . Live specimens yellowish red with denser red pigment on dorsum and inner part of dorsal cirri from segments 5–6, sometimes forming distinct, transverse red bands across posterior part of segments; eyes red (). Preserved specimens whitish, larger specimens iridescent, eyes brownish black. Body dorso-ventrally flattened, fusiform (). Prostomium anteriorly rounded, slightly wider than long, posteriorly covered by segment 1, with shallow, indistinct ligula (A,B). Paired antennae and palps short and narrow, anteriorly to laterally directed. Eyes rounded, with lenses, medium-sized, partly covered by segment 1. Nuchal papilla absent (A,B). Nuchal organs retractile, knoblike, ventro-laterally situated (C). Proboscis with single lateral row of large fleshy papillae each side; dorsal part covered by minute, diffusely distributed papillae. Terminal ring with large number of indistinct papillae, smaller on dorsal part than on ventral and lateral parts. Paired large papillae laterally inside ring. Segments 1 and 2 completely fused dorsally (B). Cirri of segment 1, dorsal and ventral cirri of segment 2, and dorsal cirri of segment 3 cylindrical, basally swollen with long, tapered ends. Cirri of segment 1 reaching segments 4–5. Dorsal cirri of segments 2 and 3 reaching about segment 7. Ventral cirri of segment 2 reaching segment 5. Segment 2 without neuropodial lobes or chaetae. Segment 3 with small neuropodial lobes with c. 5 chaetae and ventral cirri. Dorsal cirri of median segments oval, asymmetrical, slightly wider than long (D, 7A). Dorsal cirrophores short, poorly delineated (A). Neuropodial lobes long, with supra-acicular lobes slightly longer than subacicular lobes, with 20–30 chaetae. Dorsal and ventral chaetae similar within single fascicle. Rostrum of chaetal shaft asymmetrical, with a main tooth and a number of smaller ones (G). Ventral cirri elongated oval, almost as long as neuropodial lobes, with rounded ends but with weak indication of ventrally displaced tip (E, 7A). Pygidial cirri sphaerical, slightly tapered, small (F). Single median pygidial papilla present (F).
Figure 4. Length–segment relationships in Paranaitis katoi sp. nov. from the Swedish west coast. Measurements are based on entire, non-regenerating specimens that were measured live, relaxed.
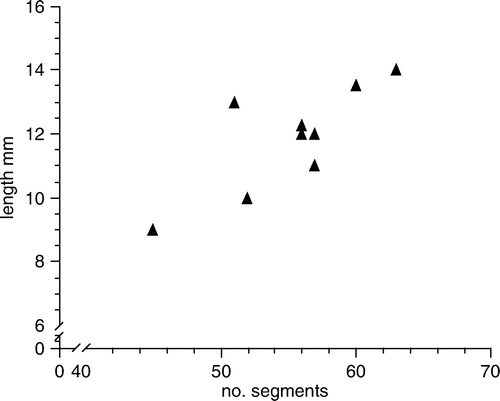
Figure 5. Live, relaxed holotype of Paranaitis katoi sp. nov. (SMNH Type-7377). Specimen is 12.25 mm long.

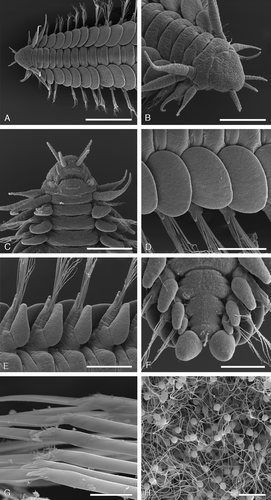
Figure 6. SEM micrographs of paratypes of Paranaitis katoi sp. nov. (A–F SMNH Type-7381; G,H SMNH Type-7380). A. Anterior end, dorsal view. B. Anterior end, antero-lateal view. C. Anterior end, ventral view. D. Median parapodia, left side, dorsal view. E. Median parapodia, right side, ventral view. F. Posterior end, ventral view. G. Articulation of chaetae. H. Sperm. Scale lines: A, 500 µm; B, 200 µm; C, 150 µm; D,E, 250 µm; F, 100 µm; G,H, 10 µm.
Reproduction
A few mature specimens collected in October in the northern part of the Swedish west coast. Males with round-headed sperm (H), females with eggs up to 300 µm in diameter (B).
Habitat
Muddy and silty bottoms, 15–250 m.
Distribution and remarks
Specimens referred to as Paranaitis wahlbergi have been reported from Arctic waters from Labrador and east Greenland to the Chukchi Sea, and from the Norwegian Sea, east Iceland, from the Norwegian, Swedish and Scottish west coasts, western Ireland, and Gibraltar (Southern Citation1914; Pleijel Citation1993; Kato & Pleijel Citation2003). At present, based on the size of examined specimens, we can only conclude that P. wahlbergi is limited to Arctic waters. Paranaitis katoi occurs on the Swedish, Norwegian and Scottish west coasts (specimens from Scotland are referred to P. katoi on their small size). The identities of previous reports of P. wahlbergi from the Norwegian Sea, east Iceland, the Faroes, Ireland, and Gibraltar require confirmation, but in the case of specimens smaller than 20 mm without mature eggs, a correct identification may only be possible to achieve with the help of molecular data.
Editorial responsibility: Christoffer Schander
Acknowledgements
We wish to thank Torkild Bakken and Jon-Arne Sneli for assistance and collaboration during visits to Trondhjem Biological Station, Andy Mackie (NMW) for loan of specimens, and the skippers of R/V Nereus, Anders Billing and John-Ingemar Adolfsson, for skilful dredging. Financial support for AN was obtained from the Swedish Species Information Centre (contract dha/6/03 1.4), for FP from Formas (contract 2004-0085), and for all three from Trondheim Marine Systems Research Infrastructure (European Community ARI-programme).
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Bastrop , R and Blank , M. 2006 . Multiple invasions – a polychaete genus enters the Baltic Sea . Biological Invasions , 8 : 1195 – 200 .
- Bastrop , R , Jürss , K and Sturmbauer , C. 1998 . Cryptic species in a marine polychaete and their independent introduction from North America to Europe . Molecular Biology and Evolution , 15 : 97 – 103 .
- Bely , AE and Wray , GA. 2004 . Molecular phylogeny of naidid worms (Annelida: Clitellata) based on cytochrome oxidase I . Molecular Phylogenetics and Evolution , 30 : 50 – 63 .
- Bickford , D , Lohman , DJ , Sodhi , NS , Ng , PKL , Meier , R and Winker , K. 2007 . Cryptic species as a window on diversity and conservation . Trends in Ecology and Evolution , 22 : 148 – 55 .
- Bleidorn , C , Eeckhaut , I , Podsiadlowski , L , Schult , N , McHugh , D and Halanych , KM . 2007 . Mitochondrial genome and nuclear sequence data support Myzostomida as part of the annelid radiation . Molecular Biology and Evolution , 24 : 1690 – 701 .
- Bonse , S , Schmidt , H , Eibye-Jacobsen , D and Westheide , W. 1996 . Eulalia viridis (Polychaeta: Phyllodocidae) is a complex of two species in northern Europe: results from biochemical and morphological analyses . Cahiers de Biologie Marine , 37 : 33 – 48 .
- Brown , S , Rouse , G , Hutchings , P and Colgan , D. 1999 . Assessing the usefulness of histone H3, U2 snRNA and 28S rDNA in analyses of polychaete relationships . Australian Journal of Zoology , 47 : 499 – 516 .
- Chen , CA , Chen , C-P , Fan , T-Y , Yu , J-K and Hsieh , H-L. 2002 . Nucleotide sequences of ribosomal internal transcribed spacers and their utility in distinguishing closely related Perinereis polychaetes (Annelida; Polychaeta; Nereididiae) . Marine Biotechnology , 4 : 17 – 29 .
- Chevaldonné , P , Jollivet , D , Desbruyères , D , Lutz , RA and Vrijenhoek , RC. 2002 . Sister-species of eastern Pacific hydrothermal vent worms (Ampharetidae, Alvinellidae, Vestimentifera) provide new mitochondrial COI clock calibration . Cahiers de Biologie Marine , 43 : 367 – 70 .
- Clement , M , Posada , D and Crandall , KA. 2000 . TCS: a computer program to estimate gene genealogies . Molecular Ecology , 9 : 1657 – 9 .
- Drake , CA , McCarthy , DA and von Dohlen , CD. 2007 . Molecular relationships and species divergence among Phragmatopoma spp. (Polychaeta: Sabellariidae) in the Americas . Marine Biology , 150 : 345 – 58 .
- Eklöf , J , Pleijel , F and Sundberg , P. 2007 . Phylogeny of benthic Phyllodocidae (Polychaeta) based on morphological and molecular data . Molecular Phylogenetics and Evolution , 45 : 261 – 71 .
- Farris , J , Albert , VA , Källersjö , M , Libscomb , D and Kluge , AG. 1996 . Parsimony jackknifing outperforms neighbor-joining . Cladistics , 12 : 99 – 124 .
- Ferguson , JWH. 2002 . On the use of genetic divergence for identifying species . Biological Journal of the Linnean Society , 75 : 509 – 16 .
- Folmer , O , Black , M , Hoeh , W , Lutz , R and Vrijenhoek , R. 1994 . DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates . Molecular Marine Biology Biotechnology , 3 : 294 – 9 .
- Gelman , A , Roberts , GO and Gilks , WR. 1995 . “ Efficient metropolis jumping rules ” . In Bayesian Statistics 5 , Edited by: Bernardo , JM , Berger , JO , Dawid , AP and Smith , AFM . 599 – 607 . Oxford : Oxford University Press .
- Hartmann-Schröder , G. 1996 . Annelida, Borstenwürmer, Polychaeta. 2., neubearbeitete Auflage . Tierwelt Deutschlands , 58 : 1 – 648 .
- Hebert , PDN , Stoeckle , MY , Zemiak , TS and Francis , CM. 2004 . Identification of birds through DNA barcodes . PLoS Biology , 2 : e312
- Kato , T and Pleijel , F. 2003 . A revision of Paranaitis Southern, 1914 (Polychaeta: Phyllodocidae) . Zoological Journal of the Linnean Society , 138 : 379 – 429 .
- Knowlton , N. 1993 . Sibling species in the sea . Annual Review of Ecology and Systematics , 24 : 189 – 216 .
- Knowlton , N. 2000 . Molecular genetic analyses of species boundaries in the sea . Hydrobiologia , 420 : 73 – 90 .
- Knowlton , N , Weigt , LA , Solórzano , LA , Mills , DK and Bermingham , E. 1993 . Divergence in proteins, mitochondrial DNA, and reproductive compatibility across the Isthmus of Panama . Science , 260 : 1629 – 32 .
- Malmgren , AJ. 1865 . Nordiska Hafs-Annulater. Öfversigt af Kongl . Vetenskapsakademiens förhandlingar , 22 : 181 – 92 .
- Malmgren , AJ. 1867 . Annulata Polychaeta Spetsbergiae, Groenlandiae, Islandiae et Scandinaviae hactenus cognita. Öfversigt af Kongl . Vetenskapsakademiens förhandlingar , 24 : 127 – 235 .
- Nygren , A , Pleijel , F and Sundberg , P. 2005 . Genetic relationships between Nereimyra punctata and N. woodsholea (Hesionidae, Polychaeta) . Journal of Zoological Systematics and Evolutionary Research , 43 : 273 – 6 .
- Nygren , A and Sundberg , P. 2003 . Phylogeny and evolution of reproductive modes in Autolytinae (Syllidae, Annelida) . Molecular Phylogenetics and Evolution , 29 : 235 – 49 .
- Nylander , JAA , Wilgenbusch , JC , Warren , DL and Swofford , DL. 2008 . AWTY (Are WE There Yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics . Bioinformatics , 24 : 581 – 3 .
- Ørsted , AS. 1842 . Udtag af en beskrivelse av Grønlands Annulata dorsibranchiata . Naturhistorisk Tidsskrift , 4 : 109 – 27 .
- Ørsted , AS. 1843 . Annulatorum danicorum conspectus , Copenhagen : Fasc. 1. Maricolæ .
- Pleijel , F. 1991 . Phylogeny and classification of the Phyllodocidae (Polychaeta) . Zoologica Scripta , 20 : 225 – 61 .
- Pleijel , F. 1993 . Polychaeta. Phyllodocidae . Marine Invertebrates of Scandinavia , 8 : 1 – 159 .
- Pleijel , F and Dales , RP. 1991 . Polychaetes: British Phyllodocoideans, Typhloscolecoideans and Tompteroideans . Synopses of the British Fauna (New series) , 45 : 1 – 206 .
- Quatrefages A. 1866 . Histoire naturelle des Annelés marins et d'eau douce. Annélides et Géphyriens . Librarie Encyclopédique de Roret , Paris .
- Rice , SA , Karl , S and Rice , KA. 2008 . The Polydora cornuta complex (Annelida: Polychaeta) contains populations that are reproductively isolated and genetically distinct . Invertebrate Biology , 127 : 45 – 64 .
- Roberts GO , Gelman A , Gilks WR. 1994 . Weak convergence and optimal scaling of random walk Metropolis algorithms . Research Report 94.16, Statistical Laboratory, University of Cambridge .
- Ronquist , F and Huelsenbeck , JP. 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 4 .
- Rubinoff , D. 2006 . Utility of mitochondrial DNA barcodes in species conservation . Conservation Biology , 20 : 1026 – 33 .
- Rubinoff , D , Cameron , S and Will , K. 2006 . A genomic perspective on the shortcomings of mitochondrial DNA for ‘Barcoding’ identification . Journal of Heredity , 97 : 581 – 94 .
- Rubinoff , D and Holland , BS. 2005 . Between two extremes: mitochondrial DNA is neither the panacea nor the nemesis of phylogenetic and taxonomic inference . Systematic Biology , 54 : 952 – 61 .
- Sars M. 1835 . Beskrivelser og iagttagelser over nogle maerkelige eller nye i havet ved den bergenske kyst levende dyr af polypernes, acephalernes, radiaternes, annelidernes og molluskernes classer, med en kort oversigt over de hidtil af forfatteren sammesteds fundne arter og deres forekommen . Bergen : Thorstein Hallegers Forlag hos Chr . Dahl .
- Schulze , SR , Rice , SA , Simon , JL and Karl , SA. 2000 . Evolution of poecilogony and the biogeography of North American populations of the polychaete Streblospio . Evolution , 54 : 1247 – 59 .
- Slatkin , M. 1993 . Isolation by distance in equilibrium and non-equilibrium populations . Evolution , 47 : 264 – 79 .
- Southern , R. 1914 . Clare Island Survey. Archiannelida and Polychaeta . Proceedings of the Royal Irish Academy , 31 : 1 – 160 .
- Swofford DL 2002 . PAUP*. Phylogenetic Analysis Using Parsimony (* and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts .
- Thompson , JD , Gibson , TJ , Plewniak , F , Jeanmougin , F and Higgins , DG. 1997 . The CLUSTAL X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools . Nucleic Acids Research , 25 : 4876 – 82 .
- Westheide , W and Hass-Cordes , E. 2001 . Molecular taxonomy: Description of a cryptic Petitia species (Polychaeta: Syllidae) from the island of Mahé (Seychelles, Indian Ocean) using RAPD markers and ITS2 sequences . Journal of Zoological Systematics and Evolutionary Research , 39 : 103 – 11 .
- Wiklund H , Glover AG , Johannessen PJ , Dahlgren TG . 2009 . Cryptic speciation at organic-rich marine habitats: A new bacteriovore annelid from whale-fall and fish farms in the North East Atlantic . Zoological Journal of the Linnean Society 155 : 774 – 85 .
- Wilgenbusch JC , Warren DL , Swofford DL. 2004 . AWTY: A system for graphical exploration of MCMC convergence in Bayesian phylogenetic inference . http://ceb.csit.fsu.edu/awty .