Abstract
Development of Mooreonuphis stigmatis, one of the dominant polychaete species in the intertidal soft-bottom community in False Bay (San Juan Island, Washington, USA) has been studied from the 5-chaetiger stage to adult utilizing SEM techniques. Clearly lecithotrophic larvae develop inside the parental tubes until at least the 17-chaetiger stage. The earliest observed stage demonstrated reduced trochal ciliation. Description of development of various ciliated sensory organs is provided. Development of prostomial and peristomial appendages is similar to that found in other onuphid larvae. The chaetal progression pattern of larvae is described in detail and a unified terminology for larval and adult chaetae is suggested. Three major chaetal progression patterns in onuphids can be recognized. The phylogenetic status of some character states such as pseudocompound falciger dentition, origin of branchiae and subacicular hooks is discussed.
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
Introduction
Mooreonuphis stigmatis (Treadwell, 1922) was originally described from the muddy intertidal zone in False Bay, San Juan Island, Washington USA and has since been commonly reported from this area (Hartman Citation1944, Citation1968; Banse & Hobson Citation1974). This species has a wide distribution on the Pacific coast of the USA and Mexico from San Juan Island, Washington to Thurloe Bay, Baja California, and inhabits muddy or sandy environments from intertidal to a depth of 60 m (Hartman Citation1944, Citation1968). Although M. stigmatis is very abundant in some soft-bottom communities (Brenchley Citation1982), there have been no published observations on reproduction or larval development of this species.
All onuphids with known development have lecithotrophic larvae and demonstrate several reproductive strategies classified by Paxton (Citation1993) and divided into four types according to the extent of brood care (). Most species of onuphids belong to type I and have larvae developing in the parental tube until the 20–30-chaetiger stage; in contrast, only a few species have free-swimming lecithotrophic larvae, Paxton's (Citation1993) type IV. Type III sensu Paxton (Citation1993) – development in mucus or jelly masses free or attached to the parental tube – has been reported for four species; type II – development in a mud cocoon – has been described by Day (Citation1960) for one species of Diopatra; however, this observation was considered to be doubtful by Paxton (Citation1993). Paronuphis abyssorum Averincev, Citation1972, described from the Antarctic, has later been considered to be a junior synonym of Leptoecia vivipara by Orensanz (Citation1990). Both authors found small juveniles developing in the body cavity of adult specimens, so viviparity is one additional type of reproductive strategy present among the onuphids (Paxton Citation1986, Citation2005). One more case of viviparity among onuphids has been described by Hartman (1965) for Hyalinoecia bermudensis (Hartman, Citation1965).
Table I. Summary of different developmental patterns in onuphids (pattern types sensu Paxton (Citation1993) with modifications: I – brooding in the parental tube with direct development; II – viviparity; III – brooding in masses or sacs attached to parental tube; IV – free-spawning with planktonic larvae).
The apparent dominance of onuphid species with non-pelagic development inside the parental tube may be skewed by the fact that most of the larvae were found in adult tubes as a by-product of studies for other purposes. Nevertheless, this type of development is very widely distributed within the family and has been reported for both onuphid subfamilies: Hyalinoeciinae Paxton, Citation1986 and Onuphinae Kinberg, 1865.
The earliest significant descriptions of larval development in onuphids were of free-spawning species such as Diopatra cuprea (Bosc, 1802) from Woods Hole, Massachusetts (Allen Citation1959), D. sugokai Izuka, 1912 as D. neapolitana Delle Chaije, 1841 from Japan (Choe Citation1960), and Onuphis elegans (Johnson, 1901) as Nothria elegans from the coast of California (Blake Citation1975). Following artificial fertilization, development from early embryos to different larval stages was studied in the laboratory. At roughly the same time, fragmentary data about direct development of larvae inside parental tubes of various onuphids were reported. The most complete studies on direct development of onuphids were carried out for D. variabilis Southern, 1921 from India (Krishnan Citation1936), Mooreonuphis jonesi Fauchald, Citation1982b from Bermuda (Fauchald Citation1982b), Hyalinoecia auraucana Carrasco, Citation1983 from Central Chile (Carrasco Citation1983), Kinbergonuphis simoni (Santos et al. 1982) from Florida (Hsieh & Simon Citation1987) and D. marocensis Paxton et al., 1995 from the coast of Morocco (Fadlaoui et al. Citation1995). All authors reported the presence of provisional larval chaetae that later in development were replaced by definitive adult chaetae as a main feature of onuphid development. While some of these chaetae are similar in several species, the detailed distributional pattern appears to be species-specific and may be useful in larval taxonomy (Hsieh & Simon Citation1987). Investigations of the jaw apparatus of onuphid larvae have demonstrated that the replacement of juvenile jaws by adult jaws, at least in onuphids with direct development, may be related to the time when larvae have consumed almost all of the yolk deposited in the egg and are ready to leave the parental tube and start to feed (Fauchald Citation1982b; Hsieh & Simon Citation1987).
Chaetal replacement in the development of onuphids was described in detail by Blake (Citation1975), Hsieh & Simon (Citation1987), Orensanz (Citation1990) and Paxton (Citation1996). These authors discussed homology of different types of chaetae in onuphids and eunicids, and a general pattern of chaetal progression in larvae and juveniles of onuphids was suggested.
In the present study we describe the larval development of M. stigmatis and compare our data with known developmental patterns of other onuphids. The terminology that was used by different authors to describe provisional larval chaetae appears to be confusing, and comparison of chaetal distribution in larvae of different species is difficult. We have attempted to unify the terminology used to differentiate the various chaetae in both larval and adult onuphids, with a discussion of the general ontogenetic pattern of chaetal replacement in onuphids. Also based on the current knowledge of onuphid development we discuss the apomorphic and plesiomorphic status of selected characters used in studies of onuphid phylogeny.
Material and methods
Mooreonuphis stigmatis was collected in the intertidal zone in False Bay, San Juan Island in July 2007. Adults with tubes were brought to the laboratory and maintained in flowing seawater for a few days. Larvae of different stages were removed from the tubes under a dissecting microscope and relaxed in 3.5% MgCl2. For light microscopy analysis larvae were preserved in 4% filtered formalin in seawater with subsequent transfer to 70% ethanol. For scanning electron microscopy (SEM) study we followed the protocol of Zograf & Yushin (Citation2004) with modifications. Larvae relaxed in 3.5% MgCl2 were fixed in 25% glutaraldehyde in 0.1 M cacodilate buffer (twice per 1 h without rinsing, on ice in the dark). Then the specimens were rinsed 3 times for 20 min each with filtered seawater at room temperature and postfixed in 4% osmium tetroxide in 0.1 M cacodilate buffer (2 h on ice in the dark). After fixation, the larvae were run through an alcohol series, with 30%, 50% and 70% ethanol for 30 min in each step and then in 75%, 95%, 100%, 100% ethanol for 10 min in each step. Specimens were critically point-dried with CO2 in a Balzers CPD-030 critical point-drier using ethanol as the transition fluid. After the larvae were mounted they were sputter coated with 20–30 nm of gold:palladium alloy 60:40 wt% in a Cressington Scientific 108 auto sputter coater. Samples were imaged on a Leica Stereoscan 440 SEM with lanthanum hexaboride electron source. For SEM study of adults and juveniles of M. stigmatis, worms were fixed in 4% formalin and then transferred to 70% ethanol and dehydrated and coated as described above. Examination of larval jaws was not undertaken due to the low number of specimens.
We followed Hsieh & Simon (Citation1987) in defining young individuals found inside parental tubes as larvae and individuals that have left the parental tube and started to build their own tube but remain immature as juveniles. A total of 28 larvae and juveniles represented five different developmental stages, and 10 adults were used for the morphological study. To study early developmental stages, artificial fertilization was attempted in the laboratory following Blake's (Citation1975) procedure; however, no successfully fertilized eggs were obtained.
Results
Mooreonuphis stigmatis is a dioecious species. Females contain spherical, yellow, yolky eggs up to 300–350 µm in diameter. Segments in the posterior one-third of males contain whitish sperm. Preliminary light microscopy analysis of spermatozoa showed that they have elongated heads with a relatively short flagellum.
Larvae were found in the posterior parts of the tubes (5–16 per tube) in very thin-walled chambers isolated from each other. All larvae from one tube represented the same developmental stage and had the same number of chaetigers.
5-chaetiger larvae
The larvae are about 500 µm long, oblong with indistinct anterior and posterior ends and are full of yolk (A, B). The anterior part has a fragmented akrotroch, a transverse row of ciliary patches (C, D); a prototroch consisting of a number of ciliary fields surrounding the future prostomium and a metatroch of which only two remnant rows of cilia remained ventrally. Simple red eyes are present on the dorsal side of the future prostomium. No segmentation is visible on the dorsal side of the larva. Six pairs of parapodia have started to develop on the ventral side. The two first pairs have rudiments of both notopodial and neuropodial cirri; subsequent parapodia have only ventral cirral rudiments. Five parapodia have aciculae and the first four parapodia bear one early compound bidentate hooded falciger each. Two anal cirri appear in the posterior region of the larva as small rounded buds.
Figure 1. Early development of Mooreonuphis stigmatis. (A–D) 5-chaetiger larva: (A) lateral view; (B) frontal view; (C,D) fragment of akrotroch. (E,F) 7-chaetiger larva: (E) lateral view, (F) ventral view. (G,H) 11-chaetiger larva: (G) lateral view; (H) ventral view. ak, akrotroch; dac, dorsal anal cirrus; fl, frontal lip; la, lateral antenna; ma, median antenna; me, metatroch; p, palp; pg, pigidium; pr, prototroch; pro, prostomium; vac, ventral anal cirrus.

7-chaetiger larvae
The larvae are about 620 µm long with a distinct anterior end, a thick oval body divided ventrally into ten segments, and a pygidium with distinct conical dorsal anal cirri and tiny rudimental ventral anal cirri (E, F). The prostomium bears five rounded rudiments of palps, lateral and median antennae. All larval trochal ciliation has disappeared. Elongated patches of cilia behind the lateral antennae presumably are the beginnings of the nuchal organs. Eyes are present. A transverse fold appears on the border between the prostomium and future peristomium on the ventral side. Aciculae are present in the first seven parapodia. The parapodia are conical and the first four bear dorsal and ventral cirri. The first parapodium has 2–4 provisional pseudocompound bidentate falcigers; these are replaced over chaetigers 2–6 by provisional transitional compound bidentate falcigers, 2–3 per parapodium. The three anteriormost pairs of parapodia have capillary notochaetae, one per parapodium.
10–11-chaetiger larvae
Larvae are about 700–800 µm long, have all major morphological characters of adults: distinct prostomium and peristomium, segmented body and pygidium (G, H). The prostomium has five appendages; both palps and antennae are elongated and have developed single ringed ceratophores at their bases. The frontal part of the prostomium develops into two hemispherical frontal lips. The mouth can open and the muscular proboscis with juvenile jaws can be everted ventrally. A single peristomial ring is well developed and separated from the prostomium; however, peristomial cirri are absent. Palpo- and ceratostyles, rudiments of the frontal lips and anal cirri are covered by scattered patches of long sensory cilia. Nuchal organs are short, developing on the prostomium behind the lateral antennae. Eyes are present. Segmentation is prominent on the ventral side, but still not visible dorsally. The larvae are very yolky and presumably do not feed. All parapodia bear provisional chaetae. The first pair of parapodia has cirriform dorsal and ventral cirri and bears two pseudocompound bidentate falcigers and three pseudocompound bidentate hooded falcigers. Parapodia from the second to sixth–seventh chaetigers have 2–3 transitional compound bidentate falcigers that are replaced by 1–2 compound bidentate hooded hooks in subsequent parapodia. Four to five anterior pairs of parapodia bear capillary notochaetae.
16–18-chaetiger larvae
Larvae are about 1.8 mm long and have the shape of young juveniles (A, C). The body is elongate; segmentation is well developed on the ventral side and becoming visible dorsally, especially in anterior and posterior regions of the larva. The prostomium is rounded and bears paired elongate palps and three antennae with short 1–2-ringed ceratophores (B, D). Frontal lips are oval and are increasingly separated from the prostomium by a groove. Upper and lower lips are developing ventrally. Nuchal organs are becoming wider and longer. Peristomial cirri are now present. The parapodia resemble the adult shape closely, and have cirriform dorsal cirri, and rounded prechaetal and elongated postchaetal lobes. The first two pairs have cirriform ventral cirri which are replaced by glandular pads in subsequent parapodia. All parapodia have long cilia near the ventral bases of dorsal cirri (E, F). These cilia appear in the 10-chaetiger larva, but become increasingly prominent in 16-chaetiger larvae and later stages. Five anterior parapodia have notopodial capillary chaetae. At this stage, adult permanent chaetae start to appear. The first pair of parapodia bears two bidentate provisional pseudocompound falcigers and three permanent tridentate pseudocompound hooded falcigers. From the second to seventh parapodia 2–4 transitional compound bidentate falcigers and 1–2 limbate chaetae are present. Starting from the eighth parapodium, compound falcigers are replaced by 1–3 compound larval bidentate hooded hooks; the first single permanent adult bidentate subacicular hook (SAH) is developed on the ninth parapodium; limbate chaetae are present up to chaetigers 13–14. Branchiae are absent. The upper anal cirri become elongate, covered by patches of cilia, ventral anal cirri remain very short and conical.
Figure 2. 16-chaetiger larva of Mooreonuphis stigmatis: (A) dorsal view; (B) prostomium, dorsal view; (C) lateral view; (D) median antenna; (E) parapodium of the third chaetiger; (F) dorsal cirrus of the third parapodium. cil, ciliae; dac, dorsal anal cirrus; dc, dorsal cirrus; la, lateral antenna; ma, median antenna; n, notochaeta, ng, nuchal groove, p, palp; pc, peristomial cirrus; pso, parapodial sensory organ; vac, ventral anal cirrus; vc, ventral cirrus.

22–24-chaetiger juveniles
Juveniles about 2.5 mm long leave the parental tube and start to build their own tubes. This process was observed in the laboratory. Young individuals have a well-developed prostomium and peristomium with all appendages and sensory organs. The yolk is almost consumed and dorsal segmentation is visible along the whole length of the worm except in a few median segments. Ventral cirri are cirriform in the first two pairs of parapodia and are replaced by glandular pads posteriorly. A digitiform postchaetal lobe is prominent in the first 3–4 parapodia. Provisional capillary notochaetae are present only on the first three chaetigers. The first pair of parapodia bears four larval bidentate pseudocompound hooded falcigers, the second pair has four larval bidentate hooded falcigers and four adult tridentate hooded falcigers. Parapodia 3–8 have three provisional transitional compound bidentate falcigers and 2–3 simple limbate chaetae. Starting from the ninth parapodium, 1–2 bidentate larval subacicular compound bidentate hooded hooks appear in each parapodium. At the same time, one adult subacicular simple bidentate hook per parapodium is present on chaetigers 9–17. Pectinate chaetae are present on chaetigers 12–17, one per parapodium. Branchiae are absent.
118-chaetiger juveniles
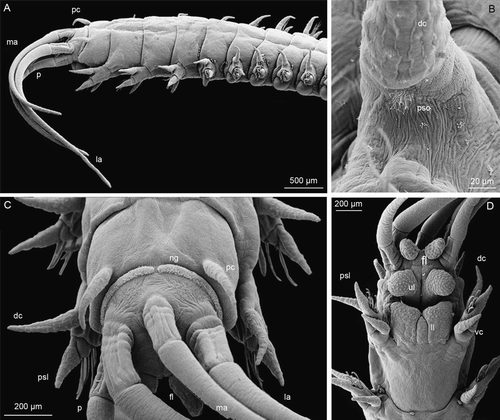
The juvenile is 32 mm long and 0.9 mm wide (without parapodia) and has all characters of adult individuals but remains sexually immature (A). The prostomium is rounded, the two palps reach chaetiger 2, the two lateral antennae reach chaetiger 5 and the median antenna reaches chaetiger 4. The ceratophores have up to four rings (C). The frontal lips are ovate and directed forward, the lower lip has a median section (D). Eyes are present near the base of lateral antennae. Nuchal grooves have become straight, and very long, narrowly separated from each other. No provisional larval chaetae are present, the chaetal distribution is the same as in adults. The first three pairs of parapodia bear only neurochaetae including 1–2 simple capillary chaetae and 4–5 tridentate pseudocompound falcigers with short blunt hoods. Notochaetae are present inside the dorsal cirri. The fourth parapodium has one tridentate pseudocompound hooded falciger, one large median simple tridentate hooded hook, 10–12 simple limbate chaetae and two compound spinigers. Only limbate chaetae and compound spinigers are present on chaetigers 5–16. The compound spinigers are replaced by simple bidentate subacicular hooded hooks starting from chaetiger 16. Ventral cirri are cirriform on the first four parapodia; the postchaetal lobe remains digitiform on the first 15 chaetigers. Branchiae are simple and strap-like, starting from chaetiger 17 and continuing almost to the end of the worm.
Figure 3. 118-chaetiger juvenile of Mooreonuphis stigmatis: (A) lateral view; (B) parapodial sensory organ of the fifth parapodium; (C) anterior end, dorsal view; (D) anterior end, ventral view. dc, dorsal cirus; fl, frontal lip; la, lateral antenna; ll, lower lip; ma, median antenna; ng, nuchal groove; p, palp; pc, peristomial cirrus; psl, postchaetal lobe; pso, parapodial sensory organ; ul, upper lip; vc, ventral cirrus.
Discussion
Mooreonuphis stigmatis broods embryos and larvae in the parental tube until the 17-chaetiger stage and thus belongs to type I sensu Paxton (Citation1993). All larvae in a single tube are in the same developmental stage and probably are the result of a single spawning event. Presumably several such events can occur during the reproductive cycle.
The mode of fertilization remains unclear. Sperm transfer system involving mushroom-shaped spermatophores containing elongate spermatozoa has been described for Kinbergonuphis simoni (Hsieh & Simon Citation1990). Although the spermatophores were not observed in M. stigmatis, the elongate shape of spermatozoids suggests that internal insemination may occur in this species; this could in part explain our lack of success with artificial fertilization.
Distribution of cilia in early embryos
Distribution of cilia in early developmental stages has been described for only two onuphid larvae. Pelagic larvae of Onuphis elegans demonstrate complex ciliation that includes an apical tuft, akrotroch, prototroch, telotroch and transverse ciliary bands on all segments at the 5-chaetiger stage. Although ventral ciliation at the posterior margin of the peristomium was described, the term metatroch was not used (Blake Citation1975). Larvae of Kinbergonuphis simoni have direct development in the parental tube and show less well-developed ciliation. Rudiments of all ciliary bands except the segmental transverse bands are still present, but have patchy structure and never form complete bands. These larvae are unable to swim and stay within the parental tube (Hsieh & Simon Citation1987). In Mooreonuphis stigmatis, distribution of cilia was also patchy. Although we did not observe early embryos and thus could not trace a reduction of the apical tuft and certain patches of the prototroch, the anterior ciliation of 5-chaetiger larvae of M. stigmatis is almost identical to that of 3–4-chaetiger larvae of K. simoni (A–D). No ciliation was observed in the study of 3–13-chaetiger larvae of H. araucana; while cilia may occur at earlier stages of development, it appears probable that the embryos and larvae of this species have completely lost ciliation (Carrasco Citation1983).
Development of prostomium and peristomium
The first rudiments of prostomial appendages appear at the 5-chaetiger stage as five very small, almost indistinguishable bulges between the rudiments of the akrotroch and prototroch (A, B). Later, at the 7-chaetiger stage, all appendages become more prominent. They go from being knob-shaped, through being conical to elongated, approximating the shape of the adult anterior appendages (E, F). The first ring of ceratophores appears at the 11-chaetiger stage, the number of rings increases to two in larvae and later to 4–5 in juveniles and adults. Frontal lips first appear at the 10-chaetiger stage as hemispherical buds, becoming oval and finally tapering during later development (G, H). Upper and lower lips form later becoming well separated in the 16–17-chaetiger larvae. Peristomial cirri appear very late in development after all prostomial appendages have formed (A, B).
Generally, this pattern of development of the anterior region is quite similar in the majority of onuphid species investigated. Kinbergonuphis simoni has the same sequence of formation in all structures, but they form slightly earlier in development: the first signs of prostomial appendages appear at the 3-chaetiger stage; and the late-appearing peristomial cirri become visible at the 7-chaetiger stage. The development of Hyalinoecia araucana is noticeably different. In this species the first three prostomial antennae appear at the 8-chaetiger stage. Palps develop much later, after at least 13-chaetiger larvae. Peristomial cirri are never present even in adults of Hyalinoecia, and do not appear during larval development (Carrasco Citation1983). Absence of peristomial cirri in adults of some onuphid genera such as Hyalinoecia Malmgren, 1866, Epidiopatra Augener, 1918 and Notonuphis Kucheruk, 1978 is here considered a paedomorphic character.
Development of ciliated sensory organs
The body of all onuphids is covered by scattered small patches of short cilia with the highest density being present on the prostomial appendages, frontal, upper and ventral lips, ventral and anal cirri. According to Pflugfelder (Citation1929) and Paxton (Citation1986), these patches combine sensory and secretory structures. Purschke (Citation2005) defined them as multiciliate penetrative sensory cells. In the larval development of Mooreonuphis stigmatis, the first ciliated structures of this kind appear at the 10–11-chaetiger stage and are represented by small patches of cilia randomly distributed on the body surface, with higher concentration on the prostomial appendages and on dorsal, ventral and anal cirri. Later in development the number of the patches increases, they become denser especially on the anterior part of the body. Kinbergonuphis simoni displays a similar pattern (Hsieh & Simon Citation1987).
Two patches of relatively long cilia in front of the lateral antennae and four patches behind the lateral antennae are present at the 7-chaetiger stage of M. stigmatis. One pair of these patches is in the same position and has the same shape as the nuchal organs of the next stage. Later in development all long cilia disappear except in these two patches, which become wider and longer and in adults have the shape of very long transverse grooves narrowly separated from each other. Åkesson (Citation1967) stated that in eunicids the trochoblasts do not contribute to the nuchal organs which are developed exclusively from epishere cells. Purschke (Citation2005) agreed with Åkesson's observation; however, he pointed out that no ultrastructure studies on nuchal organs development in polychaetes have been undertaken.
Another sensory organ located at the bases of the dorsal cirri was described by Hayashi & Yamane (Citation1994) for 14 species from different eunicean families including the onuphid, Kinbergonuphis fragilis (Kinberg, 1865). Similar lateral organs have been found in various polychaete families and considered to be homologous (Rouse & Pleijel Citation2001; Purschke Citation2005). We found lateral organs in larvae, juveniles and adults of M. stigmatis (E, F and 3B). Starting from the 10–11-chaetiger stage, each parapodium has a few long cilia emerging at the base of the knob-like dorsal cirrus. Later in development, cilia increase in number and in adults these sensory organs are of ovoid shape and consist of numerous long cilia.
Development of chaetae
The chaetal progression in onuphids is characterized by the presence of provisional chaetae which occur only in larvae and early juveniles and which are always replaced by the permanent adult chaetae. The number, morphology and distribution of provisional chaetae have been described in detail by several authors (). Description and figures of juvenile chaetae are reported for five species of onuphids (Blake Citation1975; Carrasco Citation1983; Hsieh & Simon Citation1987; Fadlaoui et al. Citation1995; Paxton Citation1996) and one eunicid (Åkesson Citation1967). Orensanz (Citation1990) discussed the homology of different chaetae and provided a comparison of the various chaetal types described by different authors, but did not suggest a uniform descriptive terminology and coding. Paxton (Citation1996) agreed with the homology of provisional and adult chaetae in onuphids as proposed by Orensanz in all but one case. She provided the pattern of chaetal progression in juveniles of Hirsutonuphis Paxton, Citation1986 and for onuphids in general.
Table II. Different types of provisional larval (lowercase) and permanent adult (uppercase) chaetae in comparison with terminology proposed by other authors.
We found the coding of different types of chaetae using letters to be very convenient, especially in comparing chaetal distribution of different species. Here we compare different ways of determination and coding of juvenile and adult chaetae of five species of onuphids () and propose a unified terminology for the different chaetae. This unified terminology is modified from Hsieh & Simon (Citation1987) who provided the most detailed classification of the juvenile chaetae so far published. Some chaetal types are uniform for different genera, others appear to be unique. We use the term ‘falciger’ to cover both provisional and permanent chaetae that occur on modified anterior parapodia of larvae, juveniles and adults and on transitional parapodia of larvae and juveniles. The term ‘hook’ is used for both larvae and adults but only for the subacicular (in Onuphinae) or intrafascicular (in Hyalinoeciinae) chaetae that emerge in posterior parapodia in a ventral position in the fascicle.
Following Hsieh & Simon (Citation1987), we used uppercase letters to code permanent adult chaetae and lowercase letters to code provisional larval chaetae. We used a(b) with a superscript to code various anterior compound or pseudocompound provisional falcigers without (with) hoods. B was chosen to code all permanent adult pseudocompound hooded falcigers. To code transitional falcigers, we used c. For subacicular hooks coding d (for provisional chaetae) and D (for permanent chaetae) were used. All other permanent chaetae were coded with subsequent uppercase letters. n was chosen to code provisional notopodial chaetae and A to code aciculae.
Chaetal composition in Mooreonuphis stigmatis
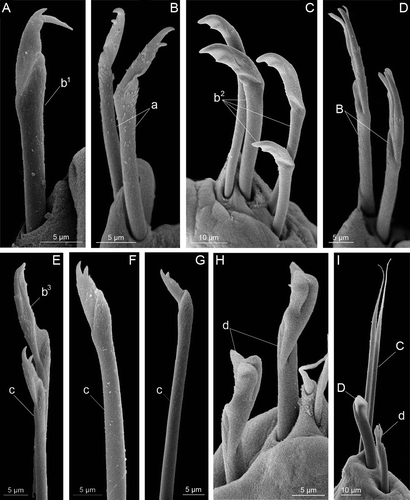
Larval (provisional) chaetae (): notopodial capillary chaeta (n), anterior pseudocompound bidentate falciger (a), anterior early compound bidentate hooded falciger (b 1 ), anterior pseudocompound bidentate hooded falciger (b 2 ), anterior compound bidentate hooded falciger (b 3 ), transitional compound bidentate falciger (c), posterior compound bidentate subacicular hooded hook (d).
Figure 4. Chaetae found on different larvae of Mooreonuphis stigmatis: (A) anterior early compound bidentate hooded falciger – b 1 ; (B) anterior pseudocompound bidentate falciger – a; (C) anterior pseudocompound bidentate hooded falciger – b 2 ; (D) anterior pseudocompound tridentate hooded falciger – B; (E) anterior compound bidentate hooded falciger – b 3 and transitional compound bidentate falciger – c; (F,G) transitional compound bidentate falciger – c; (H) posterior compound bidentate subacicular hooded hook – d; (I) posterior compound bidentate subacicular hooded hook – d, simple limbate chaeta – C and posterior simple subacicular bidentate hooded hook – D. Lowercase letters represent provisional larval chaetae, uppercase letters represent permanent adult chaetae.
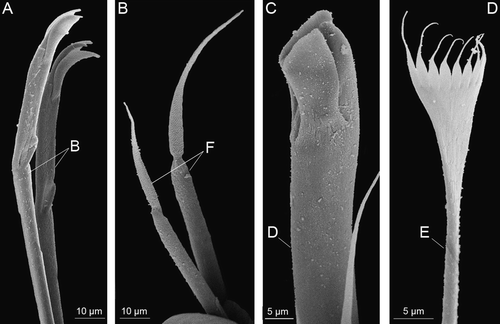
Adult (permanent) chaetae (): acicula (A), anterior pseudocompound tridentate hooded falciger (B), simple limbate chaeta (C), subacicular simple bidentate hooded hook (D), pectinate chaeta (E), compound spiniger (F), large median tridentate hooded hook (G).
Figure 5. Chaetae present on adults of Mooreonuphis stigmatis: (A) pseudocompound tridentate hooded falciger – B; (B) compound spiniger – F; (C) large median tridentate hooded hook – D; (D) pectinate chaeta – E.
Chaetal composition and distribution in M. stigmatis () appears to be very similar to the distribution in Onuphis elegans (Blake Citation1975), Kinbergonuphis simoni (Hsieh & Simon Citation1987), and three species of Hirsutonuphis (Paxton Citation1996). This pattern was described by Orensanz (Citation1990) and Paxton (Citation1996). Provisional falcigers appear on anterior modified parapodia starting from chaetiger 1 appearing consecutively and are later gradually replaced by permanent falcigers. As each anterior parapodium becomes modified during development, the first event is the appearance of provisional pseudocompound falciger as reported in K. simoni (Hsieh & Simon Citation1987), H. armillata Paxton, Citation1996, H. macrocerata Paxton, Citation1996, H. intermedia Paxton, Citation1996 , Rhamphobrachium diversosetosum Monro, 1937 (Paxton Citation1996) and M. stigmatis. These chaetae were considered by Paxton to be homologues to eunicid falcigers, although Orensanz (Citation1990) considered them to be homologous to subacicular hooks. Paxton suggested that the bidentate condition of pseudocompound falcigers was ancestral because such falcigers always appeared first and were later replaced by tridentate in some species. Our observations supported this hypothesis. All anterior provisional falcigers in M. stigmatis were bidentate, while in adults only tridentate pseudocompound falcigers were present.
Table III. The distribution of different chaetae during the development of Mooreonuphis stigmatis. Lowercase letters represent provisional larval chaetae (a – anterior pseudocompound bidentate falciger, b 1 – anterior early compound bidentate hooded falciger, b 2 – anterior pseudocompound bidentate hooded falciger, b 3 – anterior compound bidentate hooded falciger, c – transitional compound bidentate falciger, d – posterior compound bidentate subacicular hooded hook, n – notopodial capillary chaeta). Uppercase letters represent permanent adult chaetae (A – acicula, B – anterior pseudocompound tridentate hooded falciger, C – simple limbate chaeta, D – posterior simple subacicular bidentate hooded hook, E – pectinate chaeta). Numbers represent the number of each chaeta on the parapodium of corresponding chaetiger.
Although in general the chaetal progression in M. stigmatis was very similar to that in some other onuphids, one remarkable exception was observed. Usually the first precursors of pseudocompound anterior falcigers appear on the first chaetiger and form successively as development progresses. In M. stigmatis, the four first chaetigers had a single early bidentate compound hooded falciger (b 1 ) at the 5-chaetiger stage (A). At the 7-chaetiger stage, these falcigers had completely disappeared and the usual chaetal progression occurred. Hsieh & Simon (Citation1987) demonstrated that although different onuphid species have similar types of provisional chaetae, the larval chaetal pattern is species-specific. The presence of anterior early bidentate compound hooded falcigers, a unique type of chaeta, may serve as a diagnostic character for M. stigmatis and possibly for genus Mooreonuphis.
We agree with Orensanz (Citation1990) and Paxton (Citation1996) that the provisional compound subacicular hooded hooks are the precursors of permanent simple bidentate subacicular hooks (SAH). Generally, their distribution and location in the parapodia corresponds to the position of permanent hooks in all studied species, but in all cases permanent hooks start to appear a few chaetigers posterior to the first origin of provisional compound hooks. Distribution of permanent hooks shows great variability in adults of different species of onuphids, but in larval development all species demonstrate remarkable similarity in the place of first origin of permanent SAH ().
Table IV. A comparison of the subacicular hooks (SAH) development among ten species of onuphids.
Paxton (Citation1986) considered that the early origin (chaetiger 8–19) of the SAH in onuphids was a derived neotenic character compared to the late origin of these chaetae (after chaetiger 20) in eunicids. Comparison of eight species of onuphids demonstrates that all of them have the first permanent SAH appearing on chaetigers 7–11. Later in development some species, such as O. elegans and H. armillata, retain the same condition in the adults, whereas in other species the SAH starts more posterior. In development of Eunice valens (Chamberlin, 1919) the first permanent SAH appears very early, on the chaetiger 6 at the 6-chaetiger stage; however, in adults it may start as late as at chaetiger 43. Here we consider the early origin (before chaetiger 11) of the SAH to be ancestral and late origin (after chaetiger 11) to be derived.
The chaetal progression mode appears to have a high phylogenetic value. Phylogeny of onuphids at generic level proposed by Paxton (Citation1986) had reveled two monophyletic subfamilies: Hyalinoeciinae and Onuphinae. Both subfamilies contain two groups of genera: the Nothria group and the Hyalinoecia group are included into Hyalinoeciinae, and the Diopatra group and the Onuphis group form Onuphinae. The relations between the two subfamilies remain unresolved and the most ancestral genus is unknown (Paxton Citation1986). Three different modes of chaetal progression are found to be characteristic for three monophyletic groups of genera. Nevertheless, details of provisional chaetal morphology and replacement have been reported only for few species and all phylogenetic interpretations are preliminary.
The chaetal progression of only one species Hyalinoecia araucana has been studied within Hyalinoeciinae (Carrasco Citation1983). The major character of Hyalinoecia chaetal progression is the absence of provisional anterior pseudocompound falcigers in early developmental stages. Chaetae resembling transitional compound falcigers appear first. Anterior compound falcigers, an autapomorphy of onuphids (Paxton Citation1986), appear on the first chaetiger only at the 13-chaetiger stage. This mode is similar to the chaetal progression in eunicids. In Eunice valens, transitional compound falcigers form first at the 1-chaetiger stage and together with falcate simple chaetae are the only chaetae present up to the 4-chaetiger stage (Åkesson Citation1967).
Two modes reported within Onuphinae have been summarized by Paxton (Citation1996, Citation2005). The members of the Onuphis group sensu Paxton (Citation1986) (e.g. Hirsutonuphis, Kinbergonuphis, Mooreonuphis, Onuphis) demonstrate a very similar pattern of chaetal progression that we consider to be derived. Provisional anterior falcigers are the first chaetae that appear during development. They are replaced by permanent falcigers when the full number of anterior parapodia characteristic for adults of each species has developed. Provisional compound subacicular hooks are the precursors of the permanent simple subacicular hooks. These appear a few segments earlier than the first appearance of the permanent SAH and are replaced gradually by the latter. In fact, the provisional compound subacicular hooks are retained in the posteriormost parapodia even in some adults (Paxton Citation1996). Transitional compound falcigers are present between anterior modified parapodia and parapodia with subacicular hooks. They are present only in larvae and juveniles and completely disappear in adults.
Orensanz (Citation1990) and Paxton (Citation1996) stated that transitional compound falcigers (anterior provisional subacicular hooks sensu Paxton Citation1996) are the precursors and homologues of subacicular hooks of adults. We consider them to be the precursors of adult eunicid compound falcigers and have no homologues among the onuphid permanent chaetae, appearing only in larvae and juveniles. This is supported by the fact that these falcigers resemble the compound falcigers of adult eunicids and always appear before provisional compound subacicular hooks and permanent simple bidentate hooks in larvae and juveniles of onuphids and eunicids.
A third mode of development in onuphids was described for members of the Diopatra group (Paxton Citation2005). Species of Diopatra Audouin & Milne-Edwards, 1833 (Krishnan Citation1936; Allen Citation1959; Fadlaoui et al. Citation1995) are characterized by the presence of two types of anterior provisional chaetae: curved chaetae and wing-tipped capillary chaetae. These chaetae appear first in larval development in anterior parapodia and are later replaced by anterior permanent pseudocompound falcigers. Provisional simple subacicular hooks appear a few segments before the start of the permanent subacicular hooks (Allen Citation1959). Although provisional anterior curved and wing-tipped capillary chaetae have not been reported for Brevibrachium (Estcourt, 1966) (Paxton Citation1986) and Fauchaldonuphis Paxton, Citation2005 (Paxton Citation2005), the presence of simple provisional subacicular hooks links these species to Diopatra and supports the monophyly of the Diopatra group (Paxton Citation2005).
Development of branchiae
The first appearance of branchiae in M. stigmatis was not observed. Juveniles that leave the parental tube are abranchiate and only develop branchiae later. A comparison of branchial development in other onuphid species demonstrates a similar pattern. In all cases branchiae first appear in the median segments (e.g. chaetigers 5–10 in a 20-chaetiger specimen) subsequently, depending on the adult branchial distribution, disperse anteriorly and posteriorly as in Onuphis elegans (Blake Citation1975), Kinbergonuphis simoni (Hsieh & Simon Citation1987) and Diopatra marocensis (Fadlaoui et al. Citation1995) or disappear late in larval development and appear secondary in juveniles as in Mooreonuphis jonesi (Fauchald Citation1982b).
Fauchald (Citation1982a) stated that the beginning of branchiae from chaetigers 5–10 was a plesiomorphic condition while origin of branchiae on or before chaetiger 4 or after chaetiger 10 as apomorphic conditions. Paxton (Citation1986) rejected this hypothesis, and, based on the late origin of branchiae in Hyalinoecia, considered late origin of branchiae (after chaetiger 10) to be a plesiomorphic condition and the early origin (from chaetigers 1–9) to be apomorphic. Comparison of branchial origin during development in onuphids () supports the hypothesis of Fauchald (Citation1982a), but apparently the transformation from plesiomorphic to apomorphic conditions must have occurred several times independently since most of the onuphid genera include species both with primitive and derived branchial distribution, so the identification of apomorphic and plesiomorphic conditions for the family as a whole becomes a disputable question.
Table V. A comparison of the development of branchiae among five species of onuphids.
Editorial responsibility: Kenneth Halanych
Acknowledgements
We are grateful to Svetlana Maslakova and George von Dassow and the staff of the Friday Harbor Laboratories, University of Washington, for help with collecting and preserving specimens for the SEM study. We would like to thank Elena Vortcepneva from Moscow State University for providing the fixation protocol. Also, we are grateful to Scott Whittaker from the National Museum of Natural History, Smithsonian Institution, for assistance with SEM. This work was supported by the Smithsonian Institution fellowship program, the Russian Foundation for Basic Research, grant 06-04-48764 and the tuition and course-related expenses award, Comparative Embryology Course, Friday Harbor Laboratories, University of Washington.
Notes
Published in collaboration with the University of Bergen and the Institute of Marine Research, Norway, and the Marine Biological Laboratory, University of Copenhagen, Denmark
References
- Åkesson , B . 1967 . The embryology of the polychaete Eunice kobiensis . Acta Zoologica , 48 : 141 – 92 .
- Allen , MJ . 1959 . Embryological development of the polychaetous annelid, Diopatra cuprea (Bosc) . Biological Bulletin , 116 : 339 – 61 .
- Averincev VG. 1972 . Benthic polychaetes Errantia from the Antarctic and Subantarctic collected by the Soviet Antarctic Expedition . Issledovaniya fauny morei, Zoologicheskii Institut Akademii Nauk SSSR 11 : 88 292 in Russian
- Banse , K and Hobson , KD. 1974 . Benthic errantiate polychaetes of British Columbia and Washington . Bulletin of the Fisheries Research Board of Canada , 185 : 1 – 111 .
- Blake , JA . 1975 . The larval development of Polychaeta from the northern California coast. II. Nothria elegans (Family Onuphidae) . Ophelia , 13 : 43 – 61 .
- Brenchley , GA . 1982 . Mechanisms of spatial competition in marine soft-bottom communities . Journal of Experimental Marine Biology and Ecology , 60 : 17 – 33 .
- Budaeva , N and Fauchald , K . 2008 . Diopatra tuberculantennata, a new species of Onuphidae (Polychaeta) from Belize with a key to onuphids from the Caribbean Sea . Zootaxa , 1795 : 29 – 45 .
- Carrasco , FD . 1983 . Description of adults and larvae of a new deep water species of Hyalinoecia (Polychaeta, Onuphidae) from the southeastern Pacific Ocean . Journal of Natural History , 17 : 87 – 93 .
- Cazaux , C . 1972 . Développment larvaire d'annélides polychètes (Bassin d’ Arcachon) . Archives de Zoologie Expérimentale et Générale , 113 : 71 – 108 .
- Choe , S . 1960 . On the life history of the polychaete worm Diopatra neapolitana Delle Chiaje . Bulletin of the Japanese Society for Scientific Fisheries , 26 : 430 – 37 .
- Day , JH . 1960 . The polychaete fauna of South Africa. Part 5. Errant species dredged off Cape coasts . Annals of the South African Museum , 45 : 261 – 373 .
- Fadlaoui , S , Lechapt , J-P and Retiere , C . 1995 . Larval development of the onuphid Diopatra marocensis (Annelida: Polychaeta) from the Atlantic coast of Morocco . Journal of the Marine Biological Association of the United Kingdom , 75 : 957 – 66 .
- Fauchald , K . 1982a . Revision of Onuphis, Nothria, and Paradiopatra (Polychaeta: Onuphidae) based upon type material . Smithsonian Contributions to Zoology , 356 : 1 – 109 .
- Fauchald , K . 1982b . Description of Mooreonuphis jonesi, a new species of onuphid polychaete from shallow water in Bermuda, with comments on variability and population ecology . Proceedings of the Biological Society of Washington , 95 : 807 – 25 .
- Hartman , O . 1944 . Polychaetous Annelids. Part V. Eunicea . Allan Hancock Pacific Expeditions , 10 : 1 – 37 .
- Hartman , O . 1945 . The marine annelids of North Carolina . Duke University Marine Station Bulletin , 2 : 1 – 4 .
- Hartman , O. 1965 . Deep-water benthic polychaetous annelids off New England to Bermuda and other North Atlantic areas . Occasional papers of the Allan Hancock Foundation , 28 : 1 – 378 .
- Hartman , O . 1967a . Larval development of benthic invertebrates in Antarctic Seas: Early development of Nothria notialis (Monro) and Paronuphis antarctica (Monro) in Bransfield Strait, Antarctic Peninsula . Jare Scientific Reports Special Issue Proceedings of the Symposium on Pacific–Antarctic Sciences , 1 : 205 – 08 .
- Hartman , O . 1967b . Polychaetous annelids collected by the USNS Eltanin and Staten Island cruises, chiefly from Antarctic Seas . Allan Hancock Monographs in Marine Biology , 2 : 1 – 87 .
- Hartman O. 1968 . Atlas of the Errantiate Polychaetous Annelids from California . Los Angeles : Allan Hancock Foundation, University of Southern California 828 pages
- Hayashi , I and Yamane , S . 1994 . On a probable sense organ newly found in some eunicid polychaetes . Journal of the Marine Biological Association of the United Kingdom , 74 : 765 – 70 .
- Hsieh , H-L and Simon , JL . 1987 . Larval development of Kinbergonuphis simoni, with a summary of development patterns in the family Onuphidae (Polychaeta) . Bulletin of the Biological Society of Washington , 7 : 194 – 210 .
- Hsieh , H-L and Simon , JL . 1990 . The sperm transfer system in Kinbergonuphis simoni (Polychaeta: Onuphidae) . The Biological Bulletin , 178 : 85 – 93 .
- Krishnamoorthi , B . 1963 . Volume regulation in eggs, larvae and adults of a brackish-water polychaete, Diopatra variabilis (Southern) . Proceedings of the Indian Academy of Science , 57 : 275 – 89 .
- Krishnan , G . 1936 . The development of Diopatra variabilis (Southern) . Zeitschrift für wissenschaftliche Zoologie , 147 : 513 – 25 .
- Lieber , A . 1931 . Zur Oogenese einiger Diopatraarten . Zeitschrift für wissenschaftliche Zoologie , 138 : 580 – 649 .
- Lo Bianco , S . 1898 . Notizie biologiche riguardanti specialmente il periodo di maturita sessuale degli animali del golfo di Napoli . Mittheilungen aus der zoologischen Station zu Neapel , 13 : 448 – 573 .
- Monro , CCA . 1924 . On the post-larval stage in Diopatra cuprea Bosc, a polychaetous annelid of the family Eunicidae . Annals and Magazine of Natural History , 14 : 193 – 99 .
- Orensanz , JM . 1990 . The Eunicemorph polychaete annelids from Antarctic and Subantarctic Seas. With addenda to the Eunicemorpha of Argentina, Chile, New Zealand, Australia, and the Southern Indian Ocean . Antarctic Research Series , 52 : 1 – 183 .
- Paxton , H . 1986 . Generic revision and relationships of the family Onuphidae (Annelida: Polychaeta) . Records of the Australian Museum , 38 : 1 – 74 .
- Paxton , H . 1993 . Diopatra Audouin and Milne Edwards (Polychaeta, Onuphidae) from Australia, with a discussion of developmental patterns in the genus. The Beagle . Records of the Northern Territory Museum of Arts and Sciences , 10 : 115 – 54 .
- Paxton , H . 1996 . Hirsutonuphis (Polychaeta: Onuphidae) from Australia, with a discussion of chaetal progression in juveniles . Invertebrate Taxonomy , 10 : 77 – 96 .
- Paxton , H . 2005 . Fauchaldonuphis, a new genus for the enigmatic Polychaete Diopatra paradoxa Quatrefages, 1866 . Marine Ecology , 26 : 209 – 15 .
- Pflugfelder , O . 1929 . Histogenetische und organogenetische Prozesse bei der Regeneration Polychaeter Anneliden. I. Regeneration des Vorderendes von Diopatra amboinensis Aud. et M. Edw . Zeitschrift für wissenschaftliche Zoologie , 133 : 121 – 210 .
- Purschke , G . 2005 . Sense organs in polychaetes (Annelida) . Hydrobiologia , 535 : 53 – 78 .
- Rouse GW , Pleijel F. 2001 . Polychaetes . Oxford : Oxford University Press 354 pages
- Smith , BJ and Jensz , RL . 1968 . Unusual mode of reproduction in a new species of polychaete . Nature , 218 : 777
- Zograf YK , Yushin VV. 2004 . Electron microscopy study of spermatogenesis of free-living marine nematode Paracyatholaimus pugettensis Wieser et Hopper, 1967 (Chromadorida: Cyatholaimidae) . Biologia Morya 30 : 455 61 in Russian