ABSTRACT
Achieving efficient scaffolds for bone tissue engineering (TE) requires smartly defined parameters for reaching a balance between physical–chemical properties, biocompatibility and complex architectures. Three-dimensional (3D) printing offers precise geometry control of the desired scaffold at micro-scale. However, the performance of 3D printing is highly dependent on the formulation, the challenge being to achieve the suitable ink and establish the most efficient printing parameters. Gelatin methacryloyl (GelMA) emerges as a promising ink due to superior biological properties, photocrosslinking ability and printability. The present review focuses on the evolution of GelMA-based inks and bioinks from the simplest to the most advanced multicomponent formulations capable of bone tissue regeneration. Additionally, a comparative analysis between the different photoinitiators is covered, indicating each one's advantages and disadvantages. Furthermore, the main printing and bioprinting methods that are used in GelMA printing are outlined with the required parameters and their influence on the final product performance.
1. Introduction
Bone tissue engineering (TE) describes an interdisciplinary field that combines knowledge and technologies from different disciplines, including biology, chemistry, engineering, medicine, and materials science, in order to restore or replace damaged tissues [Citation1]. The first successful use of bone grafting can be dated back to 2000 BC in the ancient centre of Ishtkunui, where a skull was found containing a xenograft in a 7 mm bone defect. The success of the procedure was demonstrated by examination of the skull, which showed regeneration around the grafted bone [Citation2]. It was much later, in 1965, that Urist revealed the mechanism of osteoinduction and laid the groundwork for further advancement of artificial substitutes for osseous implantation [Citation3]. The first attempts of bone substitution involved autogenous and allogenous bone grafting, but as they impose limitations in terms of availability, calcium phosphate ceramics have been later employed in clinical applications as they resemble the mineral phase of bone, are biocompatible, bioactive and osteoconductive [Citation4]. Among the most studied calcium phosphates are hydroxyapatite (HAp) and tricalcium phosphate (TCP). Whereas HAp is the main bone component, is osteoconductive and allows the formation of chemical bonds with bone, TCP is a more biodegradable ceramic that is able to support bone regeneration [Citation5,Citation6]. Albeit, these ceramics often present low plasticity, are brittle and only resemble the inorganic bone component. Therefore, polymers such as collagen and gelatin have been introduced for such applications due to their high elasticity and likeness to the organic bone component [Citation7]. Nevertheless, the use of polymeric scaffolds is limited due to their low mechanical stability, and in order to overcome these limitations, composite materials have been developed [Citation8]. By combining the high mechanical properties of the ceramics and the elasticity of the polymers, composite scaffolds simulate both the organic and the inorganic bone components, representing good candidates for bone TE [Citation9].
An ideal scaffold for bone TE has to provide a microenvironment that mimics the properties of the extracellular matrix (ECM) in order to sustain cell attachment, migration and proliferation. The macro- and microstructures, as well as morphology, are key characteristics that influence the cellular responses, and three-dimension (3D) printing paved the way to a precise control of the manufacturing process allowing the achievement of the aforementioned features required for bone regeneration [Citation10]. Furthermore, 3D printing is a versatile technique that uses an input file format to manufacture the desired 3D shape, while 3D bioprinting refers to the fabrication of tissue-mimicking constructs starting from an input file by employing living cells alongside biomaterials with cell-protecting capacities, able to stimulate their growth and proliferation [Citation11,Citation12]. Whereas in 3D printing biomaterials are mostly used as inks, in bioprinting the concept of bioink is introduced, which covers not only the printable cell-supporting material, but also the cell suspension, or even tissue spheroids [Citation13]. The selection of the biomaterial for 3D printing depends on the application and on the printing technology, the main challenge being in designing a material possessing good printability and stability while also maintaining high cell viability [Citation14,Citation15]. Several categories and subcategories of bioprinting techniques are available, although the most frequently used classification is the one established for the standardisation of terminology by the reference document ISO/ASTM 52900:2015-12 Standard Terminology for Additive Manufacturing, which distinguishes the methods in extrusion-based bioprinting, jetting-based bioprinting and vat photopolymerisation, the latter including stereolithography (SLA) and digital light processing (DLP) [Citation16–19]. Arguably, the first bioprinting method introduced was extrusion-based by Landers et al.[Citation20], closely followed by jetting-based bioprinting in 2003, [Citation21,Citation22] and stereolithography bioprinting in 2004, from Boland et al. [Citation23].
In extrusion-based bioprinting, the filament is extruded through a nozzle via piston, screw or pneumatic pressure, and deposited onto the printing surface. The main parameters that influence the printing process are pressure, printing speed, geometry of the nozzle and viscosity of the material, as there is a strong relationship between ink rheology and structure fidelity. The ideal extrusion bioink should present a viscosity low enough to minimise the shear forces exerted on the cells, but high enough to induce good resolution. Modular viscoelastic characteristics are required in extrusion-based bioprinting. In addition to viscosity, yield stress is another important parameter that needs to be considered as it is correlated with shear-thinning, a desirable viscoelastic characteristic in extrusion-based bioprinting that must be properly modulated since even short-term high levels of shear stress could decrease cell viability and lead to alterations in the proliferation or function of the surviving cells [Citation24–26]. Moreover, the viscoelastic features of bioinks are described by the shear storage modulus (G′) and loss modulus (G″). G′ accounts for the elastic property of materials, representing the ability to recover instantaneously after deformation, whereas G″ accounts for the viscous property of materials, corresponding to the ability to remodel irreversibly over time. When the storage modulus is significantly greater than the loss modulus, the material is regarded to be solid, in contrast, when the loss modulus is much greater than the storage modulus, the material is referred to be liquid, therefore, if the storage modulus and the loss modulus are comparable, the material can be considered to be in gel state [Citation27,Citation28]. These parameters describe the printing behaviour of the materials, where the G″/G′ ratio gives the loss tangent, a parameter that indicates the suitability of a material for bioprinting [Citation29]. The advantages of the extrusion-based technique are the rapid print speed and the ability to print high cell densities (108–109 cells·mL−1) [Citation30]. However, limitations arise in terms of resolution as it is dependent on the inner diameter of the nozzle, which needs to be wide enough not to affect the viability of the encapsulated cells. Thus, for high cell densities nozzle diameters larger than 200 µm are required, which can provide limited resolution within the 200–500 µm range [Citation31]. Material jetting is a non-contact printing technique where droplets of the material are ejected by one of three approaches: thermal, piezoelectric and electromagnetic. Owing to the small volume deposited through this method, the resolution that can be obtained is superior to that achieved with extrusion-based methods [Citation32]. Thermal material jetting is considered the most suitable for bioprinting as the frequencies used in the other methods can cause cell lysis by disrupting the cell membrane. Biomaterials used in jetting-based bioprinting should have a lower viscosity than those used in the extrusion-based method in order to reduce shear stress and maintain high cell viability. Therefore, viscosity is recommended to be maintained in the range of 3–30 mPa·s, surface tension between 20 and 70 mJ m−2 and density at around 1000 kg m−3 [Citation33]. The vat photopolymerisation methods employ a light source to irradiate and photocrosslink the bioink contained in a vat, point by point and layer by layer. SLA is the earliest vat photopolymerisation technique, patented in 1986 by Charles Hull [Citation34]. The bioprinting resolution in SLA is also higher than in the extrusion-based technique, allowing modulation by varying the irradiation parameters and reaching values below 100 µm [Citation32,Citation35]. When SLA is performed using a digital photomask, the method is referred to DLP, which allows bioprinting at higher speed and improved resolution owing to its precise manner of patterning [Citation32]. The employed mask can be a liquid crystal display or a digital micromirror device [Citation36]. With the aid of a photoinitiator, the beam polymerises the formulation in a specific pattern, thus obtaining the desired structure. The stereolithographic formulation must present reactive groups such as acrylates, methacrylates, epoxides or vinyl-ethers, required for the photopolymerisation process [Citation30]. This technology allows high accuracy bioprinting with high viscosity bioinks without decreasing the cell viability [Citation37].
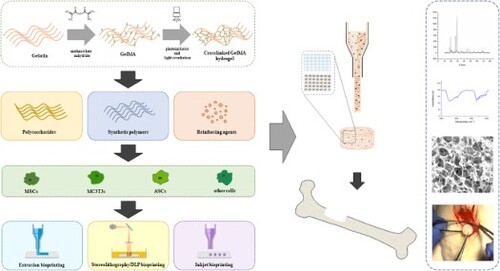
One of the most studied biomaterials for bioprinting is gelatin methacryloyl (GelMA), owing to its biological properties as a polymer obtained by hydrolysis of collagen, as well as to the high stability offered by the photocrosslinking. The primary biological advantage resides in the presence of the arginine-glycine-aspartic acid (RGD) sequences in its chains, which enhances integrin-mediated cell adhesion [Citation38–40]. GelMA hydrogels exhibit higher stability than gelatin as it presents the ability to covalently crosslink under light exposure in the presence of a suitable photoinitiator. The central challenge of implementing GelMA hydrogels as TE scaffolds is balancing accurate printability and biological functionality, which require the use of different formulations [Citation41]. Several review papers have been devoted to the optimisation of bioinks for medical applications [Citation42–44], and we can state that GelMA is one of the most documented inks, attracting a lot of attention over the last years [Citation45–48]. However, to the best of our knowledge, no review paper has been dedicated to 3D bioprinting of GelMA formulations specifically for bone regeneration. Considering the promising results indicated in the literature regarding GelMA and GelMA-based bioinks for both printing technology and bone regeneration applications, it is timely to provide a review paper dedicated to the aforementioned topics. The current review provides an overview of the most recent trends in coupling the appropriate 3D fabrication technique with GelMA-based inks and bioinks for bone TE. Firstly, the most important methods used for GelMA crosslinking are presented. Further on, an exhaustive report is provided on the bioprinting methods and protocols developed for formulations containing solely GelMA, GelMA with polysaccharides and GelMA with synthetic polymers. Lastly, an outline of the main reinforcing agents employed in bioinks alongside GelMA and the future prospects concludes this paper.
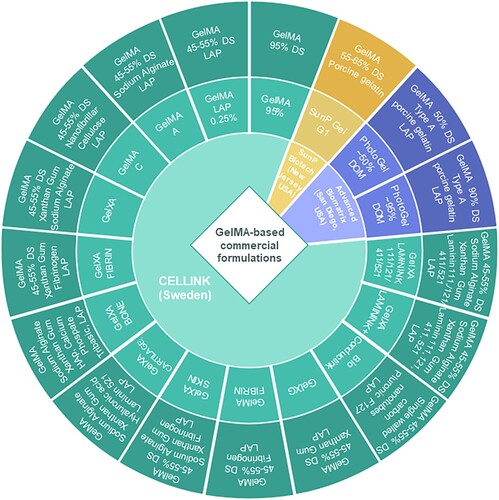
2. Commercial GelMA ink formulations
Gelatin methacrylation using glycidyl methacrylate was patented in 1998 by Koepff et al. [Citation49], and two years later, Van Den Bulcke et al. researched the rheological properties that make this material suitable for different applications [Citation50]. These two studies paved the way for new formulations in TE, and with the development of 3D bioprinting techniques, GelMA-based formulations have become some of the most widely used bioinks. Due to the broad potential to explore the cellular behaviour and several other medical applications, GelMA-based formulations have also been developed into commercial products. Currently, there are commercial formulations on the market both containing GelMA solely as well as GelMA in combination with other polymers, owing to the reproducibility of the products and the increased potential to be approved for clinical trials in the future [Citation51]. In their 2024 publication, Huang et al. indicated the four stages that 3D bioprinting underwent from 1995 to the present, with the last stage representing the use of living cells, proteins and other extracellular matrix components in the bioprinting process [Citation52]. We anticipate that the developed commercial inks could stand as common reference printing formulations with excellent and reproducible performances able to generate results of the highest robustness, significant reduction in variability across different laboratories and approaching the fifth stage i.e. commercial printed medical devices approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) and clinical applications for both printing formulations and printing technique. illustrates the three main providers of commercial GelMA inks for 3D printing. It can be clearly observed the expanded portfolio of products offered by CELLINK®, providing options for printing from skin grafts up to bone or cartilage scaffolds. Apart from GelMA 95%, all commercial inks offered by CELLINK® exhibit a degree of substitution (DS/DOM) with methacrylic groups between 45 and 55% and contain lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as photoinitiator. The predominant polymers used in these formulations along with GelMA are polysaccharides, mainly sodium alginate (ALG) and xanthan gum (XG), and for the reinforcement necessary for higher loading structures, HAp, single-walled carbon nanotubes and tribasic calcium phosphate are the preferred compounds [Citation53]. An extensive range of formulations have been developed by this manufacturer containing laminin 111, 121, 411 or 521 in their GelXA LAMININK series, including GelXA LAMININK + which contains all four types of laminins previously mentioned. Research involving these laminin-based materials commercialised by Cellink indicated an increase in cell proliferation and structural integrity of the printed scaffolds [Citation54,Citation55]. In contrast, SunP Biotech markets only one GelMA-based ink with a substitution degree of 55–65% and 0.25% LAP for photoinitiation. A study led by Yang et al. in 2022 using this formulation demonstrated a compression modulus of up to 22.6 kPa and a cell viability of ∼90% for the bioprinted constructs, thus proving the potential of this ink in biomedical applications [Citation56]. The third mentioned provider, Advanced Biomatrix Solutions, offers two GelMA options, one with 50% substitution degree and one with 95%. These inks were subjected to a comparative study by Bedell et al. and the results indicated that the GelMA formulation exhibited higher biocompatibility than sodium alginate formulations, the GelMA ink presenting similar results to those of collagen methacryloyl and hyaluronic acid inks [Citation57].
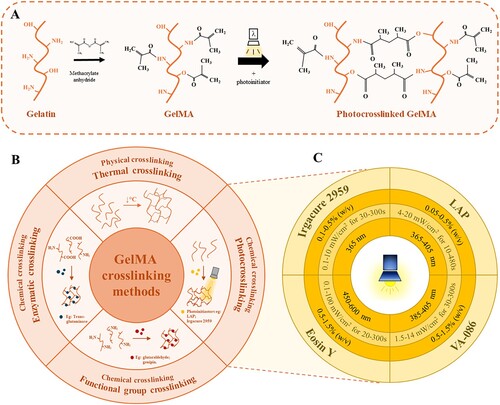
3. GelMA crosslinking strategies
The GelMA crosslinking methods can be classified into two main categories depending on the bonding that occurs, which are physical and chemical methods. As GelMA is gelatin chemically modified with methacrylic groups, it maintains gelatin's capacity to form thermosensitive networks through thermal gelation [Citation58]. However, the transition temperature is influenced by source, degree of substitution and parameters of methacrylation. By using phosphate buffer solution in the methacrylation system, an increase in the degree of substitution is obtained which leads to an increase in the transition temperature, as shown in the study conducted by Chen et al. 2023, indicating a phase transition temperature of ∼30°C for 80% degree of methacrylation. In contrast, when carbonate–bicarbonate buffer solution is used in the system, the transition temperature decreases with an increase in the degree of methacrylation, accordingly for 80% degree of substitution the transition does not occur even at temperatures as low as 4°C [Citation59]. The modulation of the methacrylation parameters towards an appropriate sol–gel transition temperature is critical in order to create intermolecular interactions that result in enhanced printing fidelity. However, these bonds are weak and reversible, thus insufficient for obtaining the superior stability and mechanical properties required in bone TE [Citation58]. Such performance can only be achieved by the formation of covalent bonds by chemical crosslinking. The use of chemical crosslinking agents, such as glutaraldehyde or carbodiimide, notably improves the mechanical stability of GelMA hydrogels, but have a strong cytotoxic effect, limiting their suitability for biomedical applications. A considerably more biocompatible alternative is crosslinking with genipin, a natural agent capable of forming covalent bonds between the amino groups of lysine. However, genipin presents a higher cost than other chemical crosslinking methods, reducing feasibility and increasing the production expenses [Citation60]. A highly biocompatible alternative method is represented by enzymatic crosslinking, i.e. transglutaminases that catalyse the formation of covalent bonds between the γ-carbonyl and ϵ-amino groups of glutamine and lysine. Nevertheless, both genipin and enzymatic crosslinking are dependent on the existence of the lysine groups, which are deprived at higher degrees of substitution, hence the crosslinking capacity of these agents is inefficient for highly methacrylated GelMA [Citation61]. Therefore, a much more cost-effective and powerful method is photocrosslinking. Through gelatin methacrylation, the appropriate methacrylate functional groups are provided, enabling the formation of covalent bonds between the polymer chains in the presence of a photoinitiator. This process ensures the printing fidelity of the filament and improved mechanical strength of the resulted scaffold. However, there are limitations, such as the use of opaque particles, which prevent light from penetrating the entire printed filament, thus reducing the crosslinking efficiency [Citation62].
The most used photoinitiators for GelMA photocrosslinking are presented in (C), namely LAP, 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959), Eosin Y (EY) and 2,2'-azobis[2-methyl-N-(2-hydroxyethyl)propionamide] (VA-086). The photoinitiator concentration used should be high enough to allow efficient crosslinking, yet low enough not to affect cell viability. Xu et al. reported that at concentrations of 0.3% and 0.5% (w/v), both Irgacure 2959 and LAP maintain high cell viabilities regardless of the printing time. Conversely, for concentrations of 0.7% and 0.9% (w/v), Irgacure 2959 resulted in a considerably greater decrease in cell viability than LAP. The photoinitiator type does not only interfere with the biological properties, but also with the structural ones, as GelMA samples treated with Irgacure exhibit larger pore sizes, higher degradation rate and higher swelling rate compared to samples treated with LAP [Citation63]. Additionally, other studies have reported the formation of oxidative stress alteration due to UV irradiation and a decrease in DNA damage at wavelengths above 400 nm (VIS light) [Citation64,Citation65]. In a 2009 research, Fedorovich et al. demonstrated a decrease in cell viability and cell cycle progression with increasing concentration of Irgacure 2959 up to 0.1% (w/v) [Citation66]. Thus, the use of visible light can be considerably safer for GelMA crosslinking in comparison with UV light exposure and the photoinitiator employed for the visible spectrum is Eosin Y. Eosin Y is a photoinitiator excited at wavelengths of 450–600 nm, which has been shown to induce GelMA photocrosslinking resulting in constructs with superior mechanical properties without affecting cell viability [Citation67]. A safer choice for UV photocrosslinking than LAP or Irgacure 2959 can be achieved by using VA-086, a photoinitiator that crosslinks at safer UV wavelengths (385–405 nm), thereby minimising its cytotoxicity. The efficiency of VA-086 in GelMA photocrosslinking was presented by Occhetta et al. in 2014 through their research in which they obtained biocompatible hydrogels with tunable mechanical properties [Citation68].
4. GelMA-solely ink and bioink formulations
4.1. GelMA-solely inks/bioinks for extrusion-based bioprinting
Several studies focused on GelMA extrusion printing and bioprinting as this technology allows high control over the printing parameters and enables the achievement of clinically relevant structures by employing high viscosity bioinks (). However, cell viability during extrusion bioprinting is relatively decreased, 40–80%, compared to other techniques such as inkjet bioprinting where the values are higher than 85% [Citation69]. Therefore, the need for precise calibration of the extrusion bioprinting parameters is to achieve the optimum cell viability values during bioprinting is emphasised. The most important parameters include dispersing pressure, nozzle geometry and diameter, rheological properties of the material and printing speed, all of which are closely interrelated [Citation70–73]. A 2022 computational fluid dynamics study by Chand et al. indicates the influence of all these variables on the extrusion bioprinting process and more specifically on shear stress, a parameter which increase leads to a decrease in cell viability. Their findings indicate that for cylindrical nozzles there is a larger region of shear stress in comparison to conical nozzles, where higher pressure is exerted at the nozzle tip. Furthermore, it was highlighted that the employment of smaller nozzle diameters leads to a decrease in cell survivability due to higher shear forces on cells. However, increasing the outlet nozzle diameter results in a decrease in printing resolution, hence, it is necessary to achieve a balance between optimal printing resolution and high cell viability. Nonetheless, a compromise must also be reached between nozzle properties, printing speed and dispersing pressure. Higher pressure leads to increased shear stress, hence lower cell viability, but decreasing the pressure often results in disruptions of the printing filament. Simultaneously, decreasing the viscosity of the material facilitates deposition, thus requiring lower printing pressures, but can negatively influence printability and post-printing structural stability. In terms of printing speed, it has been shown to have a greater effect on filament continuity and less influence on cell viability, thus lower values for printing speed result in the deposition of uniform but wider diameter filaments [Citation74]. Moreover, when bioprinting requires an additional photocrosslinking step, as in the case of GelMA, parameters such as wavelength, intensity and exposure time will have a major effect on post-printing cell viability [Citation75,Citation76]. As aforementioned, wavelengths in the UV spectrum are more cytotoxic than those in the VIS spectrum [Citation64,Citation65]. Additionally, decreasing the intensity and exposure time reduces the cytotoxic effect, but also lowers the stability and mechanical strength of the final product, thus emphasising the importance of optimisation of such photo-exposure parameters [Citation75,Citation76].
Table 1. Formulations for 3D bioprinting of GelMA.
Another study designed to explore the effect of nozzle geometry on cell viability was described by Billet et al. in 2014 in which they reported the fabrication of GelMA scaffolds with a highly interconnected pore network and high cell viability. The bioprinting was performed with a 5–20% (w/v) GelMA formulations at a 0–500 kPa pressure, through a cylindrical and a conical nozzle with an inner diameter of 150–200 µm, at a temperature of 24–30°C, a printing speed of 0–1000 mm·min−1 and a cooled to ∼5°C platform. The polymer was mixed with hepatocarcinoma cells (HepG2) at a cell density of 1.5 × 106 cells·mL−1 to obtain 1–3 mm thick constructs of 13 × 13 mm. Furthermore, two different photoinitiators were employed, Irgacure 2959 and VA-086. The highest cell viabilities (>97%) were observed for the 10% (w/v) GelMA formulation, at low dispensing pressures (100 kPa) using the conical needle type (Ø 200 µm). Both G′ and G″ values increased with the increase in GelMA concentration. The use of VA-086 resulted in increased porosity as a result of UV-induced radical formation. In addition, cell viability values were higher for VA-086 samples compared to Irgacure 2959. However, VA-086 treated samples required 10-times higher photoinitiator concentrations to obtain the required network properties indicating limitations in its practicality [Citation77]. In the same year, Bertassoni et al. published a study on the extrusion of formulations containing 5–15% GelMA. In this research, GelMA was dissolved in Dulbecco's phosphate buffer saline (DPBS) using 0.5% Irgacure 2959 with HepG2 and embryonic mouse fibroblast cells (NIH3T3) in Dulbecco's modified Eagle medium (DMEM) with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin-streptomycin (P-S). Extrusion was carried out through a straight nozzle with an inner diameter of 500 µm, at a printing speed of 120 mm·min−1, for 10% GelMA concentration and 1, 1.5, 3 and 6 × 106 cells·mL−1 cell densities were bioprinted, followed by UV crosslinking between 10 and 60 s. The elastic modulus increased with higher GelMA concentration, at 60 s UV crosslinking being 60.3 kPa for 15% GelMA and 19.0 kPa for 7% GelMA. They concluded that for successful bioprinting, concentrations higher than 7% GelMA are required [Citation78]. Three years later, in 2017, Liu et al. demonstrated the ability to bioprint quantities of less than 7% GelMA by fabricating physical gels, obtained by cooling GelMA during the extrusion process. The formulations were obtained with 3%, 4% or 5% GelMA and 0.5% (w/v) Irgacure 2959 and 4 × 106 cells· mL−1 cell density of human umbilical vein endothelial cells (HUVECs). Extrusion was performed using both a straight and a conical nozzle with a printing speed of 400 mm·min−1, at 21°C, to obtain constructs of 5 × 5 × 1.6 mm3, which were further UV crosslinked for 30 s. Live/Dead assay of the samples showed higher cell viability for the samples extruded by the conical nozzle, with values for 3%, 4% and 5% GelMA of 88.7% 85.8% and 85.1%, compared with 75.1%, 73.7% and 69.0% for the straight one. Furthermore, it was observed that more shear stress was recorded by employing the straight nozzle (∼26, 115 and 183 Pa for 3%, 4% and 5% respectively), in contrast to the conical nozzle extrusion (∼23, 104 and 174 Pa). Additionally, lower concentrations provided the constructs with larger pores and less stiffness. The pore diameter decreased from 135.6 µm to 66.7 and 49.9 µm with the increase of GelMA concentration, while the Young’s modulus increased from 1.8 kPa to 3.6 and 6.9 kPa respectively as GelMA concentration increased. Moreover, the gelation kinetics were studied by measuring the evolution of G′ and G″. The recorded values of G′ for 3, 4 and 5% GelMA concentration were 47.5, 147.0 and 233.9 Pa, while the ones for G″ were 1.2, 2.4 and 3.3 Pa, respectively [Citation79]. A more recent study, from 2024, also focused on bioprinting with GelMA concentrations in the 3–10% range in a bioink containing 0.25% LAP and dental pulp stem cells (DPSCs) at a cell density of 4 × 106 cells·mL−1. Extrusion pressure varied between 50 kPa for 3% GelMA and 65 kPa for 10% GelMA, and the printing speed remained constant at 300 mm min−1. For photocrosslinking, a UV source was employed at 405 nm for 40 s. Although the results showed the best printability values for the 5% GelMA concentration, the compressive modulus displayed values lower than 50 kPa, while the cell viability after 28 days was lower than for the structures with 3% or 10% GelMA [Citation80]. Overall, these results indicate that when inks and bioinks containing only GelMa are bioprinted by extrusion GelMA concentration values higher than 5% are needed to obtain good shape fidelity during printing and proper post-printing mechanical strength. However, with increasing GelMA concentrations, a decrease in cell viability due to shear stress can be expected.
4.2. GelMA-solely inks/bioinks for vat photopolymerisation-based bioprinting
Compared to extrusion-based bioprinting, the vat-photopolymerisation-based approach offers advantages both in terms of resolution and cell viability, representing a nozzle-free technique that does not exert shear forces on the encapsulated cells [Citation81,Citation82]. However, the use of a UV source and the generation of free radicals specific to the photocrosslinking process may lead to DNA damage and could induce a cytotoxic effect. Consequently, parameters such as type and concentration of photoinitiator, wavelength, duration and intensity of the exposure should be carefully selected to minimise the toxic effect on the encapsulated cells [Citation83]. As discussed in the previous chapter, the use of VIS wavelengths represents the safer choice, whereas when UV photoinitiators are used, a concentration of below 0.5% is recommended [Citation63–65]. Moreover, research conducted by Ruskowitz et al. indicates that even at 365 nm, short-term exposures with an intensity source of less than 20 mW cm−2 do not affect cell function and proliferation. Negative contradictory results were observed for the 254 nm wavelength, which induced a significant decrease in cell proliferation even at 0.3 mW cm−2 for 0.5 min exposure, suggesting cell death or cell-cycle arrest [Citation84].
Both vat photopolymerisation techniques, SLA and DLP, have been used extensively in studies for GelMA-based ink bioprinting due to their ability to achieve improved resolution and higher cell viability compared to extrusion-based techniques [Citation85,Citation86]. SLA is a maskless technique that employs a light beam to selectively treat the bioink layer by layer with high resolution. Meanwhile, the technique derived from SLA, namely DLP uses a digital mirror device to focus the light beam onto the material, which makes the approach faster, encompassing an entire 2D layer [Citation87,Citation88]. In 2016 Wang et al. described a fast photopolymerisation method for GelMA with a laser diode, decreasing the risk of cell DNA damage. The procedure involved the employment of a blue laser diode of a 405 nm wavelength with a maximum intensity of 300 mW, mounted 10 cm above the petri dish, containing 2 mL of solution. The solution consisted of 10% GelMA with 1% VA-086 in phosphate buffered saline (PBS) and NIH3T3 fibroblast cells (8 × 106 cells·mL−1) in DMEM treated with 10% FBS and 1% P-S. Three different light intensities of 100, 200 and 500 mW cm−2 were applied using a circular beam spot of 8 mm diameter and a 180 mm·min−1 platform movement. The data indicated that the minimum crosslinking time was dependent on the laser output power, hence for 50, 100 and 250 mW, the minimum time required was determined to be 30 s, 20 and 10 s respectively. For all three power values, cell viability values greater than 80% were obtained up to 4 days after bioprinting [Citation89]. Another research conducted by Wang deployed VIS SLA for GelMA crosslinking. Eosin Y was chosen as photoinitiator and the GelMA solution with concentrations between 10% and 20% was blended with NIH3T3 cells at a density of 3 × 106 cells·mL−1 prepared in supplemented DMEM. The technique used for bioprinting was DLP, employing an array of digital mirrors to pattern control the light in the projection field for selective crosslinking. The distance between the mirror and the printing plane was 6 cm, at a beam intensity of 48.6 mW cm−2, a reflected beam angle of 12°, each layer being crosslinked for 120 s. It was observed that the 20% GelMA concentration exhibited too high a viscosity for DLP bioprinting, while the 10% samples required a higher amount of photoinitiator, resulting in increased cytotoxicity. Thus, the 15% concentration presented the most suitable characteristics for this technique, as they displayed cell proliferation and intracellular network formation after five days [Citation90]. A 2020 research directed by a member of the previous study, H. Kumar, used the very same experimental method to bioprint GelMA synthesised by reverse osmosis (RO). The formulations included concentrations of 10% GelMA, 0.2% Eosin Y as a photoinitiator and NIH3T3 cells or U118 astrocytes at 8 × 106 cell density for both types of cells. It was determined that GelMA synthesis by reverse osmosis produced structures with higher mechanical stability exhibiting the highest compressive Young's modulus of the samples, ∼13 kPa. Furthermore, RO-GelMA samples also displayed increased cell viability and cell-matrix integration after 5 days [Citation91]. The studies highlight the ability of GelMA inks and bioinks to produce constructs with high cell viability by DLP bioprinting, however limited mechanical properties, even at high GelMA concentrations are obtained.
4.3. GelMA-solely inks/bioinks for jetting-based bioprinting
Jetting-based bioprinting poses limitations in terms of bioink choice, requiring specific viscosities and densities depending on the actuation method (e.g. thermal, piezoelectric or electrostatic). There are several parameters that influence not only printing accuracy but also cell viability during printing, including the rheological properties of the bioink, shear rate in the printing chamber, droplet impact velocity and droplet volume. Increased droplet impact velocity is associated with decreased cell survivability, due to high mechanical forces exerted on the encapsulated cells. Studies have shown that an increase in bioink viscosity not only increases deposition accuracy but also leads to an increase in cell viability, as higher polymer concentrations protect encapsulated cells from deposition impact [Citation92]. Additionally, an increase in the concentration of encapsulated cells has been shown to lead to higher viability by mitigating droplet slashing, and in terms of volume droplet, a minimum of 20 nL per spot has proved necessary to reduce evaporation-induced cell damage [Citation93]. In modulating all these parameters, one must consider their influence on the shear rate in the printing chamber. In material-jetting methods shear stress levels can reach values as high as 500 Pa even if only for a very brief period of ∼ 100 µs, therefore, higher viscosity will lead to increased shear stress, which will result in a decrease of cell viability during bioprinting [Citation94].
For employing GelMA in jetting-based bioprinting, additional treatments are required to reduce its long molecular chains, which cause its high viscosity. A 2022 study by Suntornnond et al. describes a two-step method for modifying GelMA through a saponification process under highly alkaline conditions (pH of 11), followed by thermal treatment through autoclaving at 121°C. For inkjet bioprinting, a frequency of 1 kHz was employed, dispersing a volume of approximately 0.345 nL at a nozzle-to-plate distance of 15 mm. The bioink consisted of 10% GelMA with a cellular density of 2 × 106 cells·mL−1 of L929 murine fibroblasts in supplemented medium. A decrease in the bioink viscosity was observed after each heat treatment. Untreated GelMA exhibited a viscosity of 17.6 mPas, after one treatment cycle it decreased to 4.0 mPa·s, after two cycles to 3.1 mPa·s, and after three cycles to 2.8 mPa·s, at a shear rate of 10 000 s−1. The two-step method generated a shortening of the molecular chain. In order to maintain the crosslinking ability without affecting cell viability, the process with two cycles of heat treatment has proven to be the most efficient [Citation95]. However, the low concentrations required for this technique led to the formation of structures with mechanical strengths insufficient for bone TE. Therefore, the focus was shifter towards ink and bioink formulations of GelMA in combination with different polymers.
5. Ink and bioink formulations based on GelMA and polysaccharides
5.1. GelMA-polysaccharides inks/bioinks for extrusion-based bioprinting
The complexity of the ECM in terms of composition, architecture and mechanical strength as well as the limitations of GelMA have led to the need of integrating other materials in the bioink formulations. Owing to their versatility and ability to increase the stability of GelMA, polysaccharides have been the most widely used in such compositions [Citation96]. One of the most widely used polysaccharides in bioprinting, ALG, has also been incorporated into bioink formulations alongside GelMA, providing dual crosslinking potential, UV for GelMA and ionic for ALG. The inclusion of these polymers in bioinks results in the generation of interpenetrating networks (IPNs), as reported by Krishnamoorthy et al. in their 2019 study. The researchers bioprinted a formulation containing 5–10% GelMA, 0.5–1% ALG, 0.5% Irgacure 2959 and 5 × 106 cells·mL−1 in 10% SBF and 1% antibiotic, by extrusion through a 150 µm inner diameter nozzle a pressure ranging from 13 to 220 kPa and a printing speed varying from 20 to 220 mm·min−1. Following the printing, UV crosslinking was performed for 60 s with a 7 mW cm−2 intensity source and ionic crosslinking in a 2% CaCl2 solution for 60 s. For this approach, it was determined that for uniform and continuous filament formation the required pressure is 27–110 kPa and the printing speed 100–140 mm·min−1. Furthermore, the mechanical properties of the bioinks increased with increasing polymer concentration, the formulation with 10% GelMA and 1% ALG presenting a tensile strength of 75 kPa and a tensile modulus of 39.1 kPa, while those with 5% GelMA or 0.5% ALG exhibited inferior results. However, regarding the degradation kinetics, these networks did not exhibit high stability even for the 10% GelMA and 1% ALG samples, presenting 75% degradation after only 4 days [Citation97]. A more comprehensive study on the mechanical and rheological properties of ALG-GelMA-based bioinks was carried out in 2020 by Aldana et al. using higher ALG concentrations, 5–7%, and lower for GelMA 4–8%. In the formulations 0.5% Irgacure 2959 and a cell density of 1 × 106 cell·mL−1 of sheep adipose derived stem cells (SADSCs) in DMEM with glucose, FBS and P-S were added. The extrusion was carried out through a 150–300 µm nozzle, at pressures between 55 and 240 kPa at a room temperature of 25°C, and after UV crosslinking was carried for 5 min and ionic crosslinking for 10 min in a 100 mM CaCl2 solution. Results indicated the highest compressive modulus for samples containing 8% GelMA and, in addition, samples containing 8% GelMA and 7% ALG exhibited the highest Young's modulus values (98.1 kPa for parallel direction and 65.7 kPa for perpendicular direction). G′ and G″ values also indicated the advantage of the 8% GelMA and 7% ALG samples, which displayed a viscous liquid-like behaviour due to the high modulus. Concerning stability, the lowest degradation after 14 days of incubation was achieved for the sample with 6% GelMA and 7% ALG reaching values of ∼35% and indicating that increasing ALG concentration slows the degradation rate. In terms of biocompatibility, samples with 6% GelMA and 5% ALG demonstrated higher cell viabilities, however formulations with 8% GelMA and 7% ALG also displayed values higher than 75% after 24 h [Citation98].
Another polymer used in bioink formulations with GelMA to improve the rheological properties is gellan gum, a hydrolysable bacterial polysaccharide, classified as a Generally Recognised As Safe (GRAS) substance by FDA in the food industry as gelling agent. A 2014 study headed by Melchels reveals improvements in the pseudo-plastic properties of a bioink containing 0.75–1% gellan gum and 10% GelMA. Extrusion was carried at 37°C, with a 420 µm diameter nozzle, at a 500 mm·min−1 stage speed. The rheological study demonstrated that samples containing GelMA and gellan exhibited 1–3 times higher viscosity than the separate constituents. In addition, the bioink exhibited a cell viability of 81% and it has been proven that the compression modulus increases as the concentration of gellan gum increases [Citation99]. Higher polymer concentrations were tested by Mouser et al. in 2016 for bioprinting of cartilage-like structures. They studied concentations of 3–20% GelMA, 0–1.5% gellan gum, 0.1% Irgacure with 10–20 × 106 cell·mL chondrocytes cell densities. However, the findings indicated that low of polymer concentrations led to the formation of a discontinuous filament, while high concentrations damaged cell viability. Thus, concentrations of 7.5–10% GelMa and 0.5–0.75% gellan gum proved to be the most reliable. Additionally, for bioprinting at 28°C of 10% GelMA and 0.5% gellan gum a Young's modulus of 47 kPa was obtained, while for 1% gellan gum the value increased to 77.8 kPa [Citation100].
A different approach implies the use of xanthan gum (XG), another important bacterial polysaccharide suitable for extrusion bioprinting. Its potential has been documented in several studies [Citation101–103], including that of Iervolino et al. where they tested concentrations of 6–10% GelMA and 0–4% xanthan gum to examine the rheological behaviour and biocompatibility on murine fibroblast cell line L929 (). For photopolymerisation they used 0.5% Irgacure 2959 and a UV source of 25 mW cm−2 at an exposure of 15 min. The extrudability was tested at 25°C, with pressures of 20–30 kPa for a conical nozzle and 60–80 kPa for a cylindrical nozzle, at printing speeds of 180–1800 mm·min−1, the diameter of both nozzles being 250 µm. It was concluded that the addition of XG decreases the degradation rate, and the best shape fidelity and extrudability results were achieved for the formulation containing 3% GelMA and 7% xanthan gum, which also exhibited over 90% cell viability after 7 days. In terms of parameters, the most feasible ones to maintain good resolution at low pressures were set at 25 kPa pressure and 600 mm·min−1 speed for the conical nozzle [Citation104]. A more advanced approach was developed in 2021 by Garcia-Cruz et al. who demonstrated the fabrication of a bioink from GelMA and xanthan gum methacryloyl (XGMA), which exhibits a low viscosity under high shear and a rapid increase in viscosity after shear is removed, thus achieving greater shape fidelity. The studied concentrations were 3–5% GelMA and 3–5% XGMA, and for photocrosslinking LAP and a UV source of 10 mW cm−2 for 4 min were employed. C2C12 muscle cells were encapsulated to a cell density of 3 × 106 cells·mL−1 with supplemented DMEM medium. Moreover, extrusion was performed through a 410 µm conical nozzle at a pressure between 70 and 120 kPa and a printing speed of 120 mm·min−1. Regardless of the polymer concentration, similar stability values were achieved, the degradation amounting to ∼25% after 10 days of incubation. With a higher viscosity, the 5% GelMA and 5% XGMA concentration resulted in a cell viability of only 57% after 7 days, therefore the more appropriate formulation was determined to be the 3% GelMA and 3% XGMA which, despite lower fidelity, exhibited better integrity maintenance of the scaffold in culture and a cell viability of 92% after 7 days [Citation105].
Figure 3. Characterization of the GelMA/Xanthan Gum (XG) scaffolds. (A) Representative picture of a line-printing test performed with the GelMA-XG 30% highlighting the used printing speeds. A representative microscope image of well-defined lines is also provided. (B) Viability (%) of L929 cells determined by alamarBlue® assay. The influence of degradation products of GelMA (blue striped bar) and GelMA-XG (orange bar) hydrogels on cell viability was evaluated and compared wftabith control (green striped bar) after 1, 5, and 7 days. Statistical differences are reported with *p < 0.05, **p < 0.01. (C) Ten-layers scaffold printed with GelMA-XG 30% with larger (left) and smaller (right) distances between lines. Reprinted from [Citation104] under Creative Commons CC-BY Licence.
![Figure 3. Characterization of the GelMA/Xanthan Gum (XG) scaffolds. (A) Representative picture of a line-printing test performed with the GelMA-XG 30% highlighting the used printing speeds. A representative microscope image of well-defined lines is also provided. (B) Viability (%) of L929 cells determined by alamarBlue® assay. The influence of degradation products of GelMA (blue striped bar) and GelMA-XG (orange bar) hydrogels on cell viability was evaluated and compared wftabith control (green striped bar) after 1, 5, and 7 days. Statistical differences are reported with *p < 0.05, **p < 0.01. (C) Ten-layers scaffold printed with GelMA-XG 30% with larger (left) and smaller (right) distances between lines. Reprinted from [Citation104] under Creative Commons CC-BY Licence.](/cms/asset/0eedf1c4-1b68-4920-a863-f8226b995ce1/nvpp_a_2378003_f0003_oc.jpg)
GelMA has also been blended in bioink formulations with methylcellulose, a compound derived from cellulose by partial substitution of their hydroxyl groups with methoxy groups. In 2020 Rastin et al. studied the formation of structures with high shape integrity by dissolving 5–10% GelMA in Na2SO4 at 70°C and adding 4–8% methylcellulose and 0.75% photoinitiator at 50°C. Human primary osteoblasts in alpha-modified minimal essential medium were encapsulated in the bioink. Bioprinting was carried out at a 480 mm·min−1 speed, with pressures between 68 and 137 kPa, through a 210 µm nozzle and was succeeded by UV exposure for 1 min. Shape integrity studies showed the best results for samples containing 8% methylcellulose and 5% GelMA, extruded at 103 kPa, with a printing speed of 480 mm·min−1 and a distance between the nozzle and the platform of 0.2 mm. The recorded printability value (Pr) for this bioink was 0.94, indicating high printability, as a Pr = 1 indicates the formation of regular square shapes during the printing. In addition, the compression modulus for this formulation measured 15 kPa while for a bioink containing only 5% GelMA the modulus was only 4.5 kPa, and the cell viability indicated above 90% values, thus suggesting high biocompatibility. In terms of stability, for samples containing 8% methylcellulose, increasing GelMA concentration resulted in an increase of the remaining mass, with degradation for the 10% GelMA sample lower than 20% after 60 days [Citation106]. The studies indicate that GelMA-polysaccharides formulations allow the formation of biocompatible scaffolds, with high printing fidelity, the most reliable GelMA concentration being the 10% one. However, these formulations require further modulations for obtaining the required compressive modulus values for bone tissue replacement ().
Table 2. Formulations for 3D printing and bioprinting of GelMA and polysaccharides.
5.2. GelMA-polysaccharides inks/bioinks for vat photopolymerisation-based bioprinting
The most encountered polysaccharide alongside GelMA in bioinks for stereolithography is methacrylated hyaluronic acid (HAMA). The 2019 study led by Lam indicates the use of 5% GelMA and 1% HAMA with 0.1% LAP to test the performance of a print with low cell density (5 × 106 cells·mL−1) and high cell density (25 × 106 cells·mL−1) of porcine chondrocytes. The employed technique was DLP, with each layer being light-cured with blue light at 385–405 nm for 30 s. Each model presented 3 layers of 300 µm each, the final model being ∼1 mm high and 8 mm in diameter. Shape and size stability was demonstrated for both formulations after 14 days in cell culture conditions. Furthermore, at low densities the model exhibited a more homogeneous distribution of proteoglycans and type 2 collagen, while at high densities a layered distribution was observed [Citation107]. Three years later, Shopperly et al. published their research on concentrations of 5% GelMA and 2% HAMA, for three distinct volume ratios (G20:80H, G50:50H and G80:20H). The method and parameters previously described were reproduced for this experiment. An increase in Young's modulus was observed with increasing HAMA percentage, thus G20:80H presented a modulus of 5.26 kPa, G50:50H a modulus of 4.95 kPa and G80:20H of 3.09 kPa. All samples displayed a high biocompatibility, with cell viability values greater than 90% after 14 days, and G80:20H samples showed the highest increase in gene expression (553%) for collagen type I alpha 1 chain (COL1A1), collagen type II alpha 1 chain (COL2A1) and aggrecan [Citation108]. Despite these significant biological results, the poor mechanical performance indicates the need for either higher polymer concentrations or the inclusion of other materials with higher mechanical capacities.
5.3. GelMA-polysaccharides inks/bioinks for jetting-based bioprinting
Given GelMA’s incompatibility with the inkjet method, even in formulation with polysaccharides, a pretreatment and process modification are required for successful printing. In 2018, Yoon et al. modified the inkjet technique by combining it with the spray-coating method and obtained a technology that allows high-resolution printing of GelMA. For achieving the bioink, concentrations of 0.7% ALG, 3.5–6% GelMA, 0.05% Irgacure 2959 and Hs68 or HDF fibroblast cells at 0.5 × 106 cells·mL−1 were employed. In order to obtain an adequate degree of viscoelasticity, GelMA was subjected to saponification treatment under high alkaline (pH = 11) and thermal (121°C) conditions. The fabrication system involved inkjet printing of the hydrogel onto the substrate, followed by spray coating with CaCl2 solution for crosslinking. An 80 µm inkjet nozzle was used for bioprinting, at 37 °C, with an input voltage of ±80 V and the airbrush pressure for spray coating was set at 85.4 ± 5.6 µL s−1. Additionally, photocrosslinking was performed by UV exposure for 5 min. By increasing the GelMA concentration, an increase in the compression modulus was observed, the value for 6% GelMA being 22.7 kPa with an increase in cellular activity as well [Citation109]. Limitations of this technique can be observed both in terms of the low cell density that can be bioprinted and low compression modulus, due to the reduced concentration values of GelMA and polysaccharide that can be bioprinted through the inkjet technique.
6. Bioink formulations based on GelMA and synthetic polymers
6.1. GelMA-synthetic polymers inks/bioinks for extrusion-based bioprinting
By shifting the focus towards formulations of GelMA with synthetic polymers, the intention was to improve the structural and mechanical stability of bioprinted scaffolds. Synthetic polymers are known for their processability which enable the achievement of the required mechanical resistance and degradation rate. However, the selection of the right synthetic polymer must be thoroughly considered, as it should not only be biocompatible but also water soluble and suitable to be used alongside GelMA [Citation110]. Therefore, biocompatible synthetic polymers have been studied for bioprinting alongside GelMA () one of them being [...] polycaprolactone (PCL). PCL-based medical devices have been approved by the FDA for internal use in the human body and inks containing PCL are employed in 3D printing for tissue engineering due to their printability, increased biocompatibility and superior mechanical properties [Citation111,Citation112]. A study by Buyuksungur et al. indicates the manufacture of a hybrid scaffold that combines the mechanical properties of PCL and the biological ones of GelMA by bioprinting them side-by-side. Therefore, a 15% GelMA solution was extruded at 22°C, at a pressure of 180 kPa and a nozzle speed of 600 mm·min−1 alongside a 50,000 g mol−1 PCL solution at 160°C, 730 kPa and 120 mm·min−1 nozzle speed through a 400 µm nozzle. Prior to extrusion, dental pulp stem cells (DPSCs) were incorporated into the GelMA solution at a cell density of 1 × 106 cells·mL−1 and 0.5% Irgacure 2959. The photocrosslinking process was carried out at 365 nm for 5 s at an intensity of 15 W cm−2. The PCL/GelMA scaffolds presented enhanced compressive modulus of 106 MPa, compared to the GelMA structure at only 0.36 MPa, and cell viability of 90%, indicating high biocompatibility and appropriate printing fidelity. Stability was also significantly increased, with weight losses at 21 days of 10% for GelMA constructs and only 2% for the PCL/GelMA ones. Additionally, a significant increase in the mineralised area was determined after 3 weeks, reaching values of almost 60% [Citation113].
Table 3. Formulations for 3D printing and bioprinting of GelMA and synthetic polymers.
A more complex bioink was proposed by Jia et al. comprising 5–7% GelMA, 1–3% ALG and 1–3% poly(-ethylene glycol)-tetra-acrylate (PEGTA). The extrusion was performed through a two-channel coaxial nozzle with diameters ranging from 0.16–1.55 mm, a nozzle speed between 120 and 360 mm·min−1, the outer channel being connected to a CaCl2 solution. Two cell types, HUVECs and mesenchymal stem cells (MSCs), were encapsulated in the bioink, both at a cell density of 3 × 106 cells·mL−1. Simultaneously, Irgacure 2959 was used as a photoinitiator and crosslinking was performed at 360–480 nm with an intensity of 6.9 mW cm−2 for 20, 30 and 40 s followed by soaking in EDTA solution for 5 min to remove ALG from the structure. Following printability tests, it was established that the optimal concentrations are 7% GelMA, 3% ALG and 2% PEGTA. In addition, a significant increase in the compressive modulus was observed with increasing PEGTA concentration, with the 1, 2 and 3% samples exhibiting values of 24.2, 34.5 and 50.7 kPa respectively. Moreover, in terms of biological tests, it was found that the 20 and 30 s UV exposure resulted in higher cell viability (>80%) than the 40 s exposed samples after 1, 3 and 7 days. Thus, it was concluded that crosslinking at 30 s provides scaffolds with high structural integrity and the best cell spreading and organisation results [Citation114]. These data indicate an increase in the mechanical strength of the GelMA scaffolds through the addition of synthetic polymers, but also highlight limitations in terms of the types of such polymers that can be used in these applications without affecting cell viability.
6.2. GelMA-synthetic polymers inks/bioinks for vat photopolymerisation-based bioprinting
One material present in formulations with GelMA for DLP is poly(ethylene glycol) diacrylate (PEGDA) due to its mechanical properties. An approach described by Tilton et al. in 2023 involves the fabrication of scaffolds by visible light (VL) DLP containing a formulation of 10% GelMA, 5–15% PEGDA, 0.1% LAP and a cell density of 1 × 107 cells·mL−1 MCT3T3-E1 in supplemented α-DMEM. For bioprinting a 420 nm wavelength was projected at an intensity of 21 mW cm−2 with an exposure of 10 s per layer, except for the first layer which was crosslinked for 40 s. Considering both biological and mechanical properties, the samples containing 10% PEGDA demonstrated the best results, offering cell spreading similar to the control after 3 days, ALP activity comparable to the control after 14 days in osteogenic culture, as well as printability fidelity and a high compression modulus of almost 260 kPa [Citation115]. In 2023 Gao et al. have also studied PEGDA in formulations with GelMA and Pluronic F127 diacrylate (F127DA). Their chosen concentrations were 5% GelMA, 10% PEGDA, 5% F127DA, 0.25% LAP and rabbit bone marrow mesenchymal stem cells (rBMSCs). The DLP parameters involved using UV light at an intensity of 20 mW cm−2, for 4 s per layer, at a layer height of 100 µm and a temperature of 25°C. The obtained scaffolds provided the required properties for bone TE, with the formulation containing GelMA exhibiting high porosity, with a pore size of ∼508 µm, good osteoconductivity, a compressive modulus of 92.3 kPa, a compressive strength of 829.5 kPa and a remaining weight of 57% after 50 days as it can be observed in [Citation116]. A different ink, obtained this time by using 3–6% polymethacrylic acid (PMAA) and 15% GelMA, was developed in a research also coordinated by Gao for obtaining scaffolds with mechanical properties superior to those exclusively made out of GelMA. For DLP printing each 100 µm layer was exposed for 11–15 s to a UV light of 10 mW cm−2 intensity at 29°C. The findings indicated that the formulation containing 3% PMAA exhibited a higher compression modulus than that containing 6% PMAA, and in addition it exhibited better promotion of chondrogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) and higher biocompatibility. However, in stability comparisons, the time until complete degradation of the 6% PMAA constructs, of 55 days, was higher than for 3% PMAA, at 42 days [Citation117]. It can be observed that the DLP method produces structures with better mechanical properties than the extrusion bioprinting technique when synthetic polymers are used in formulations alongside GelMA. However, even if in vitro testing did not indicate cytotoxicity induced by these formulations, it is known that synthetic polymers exhibit a different behaviour once they are implanted, as in vivo tests revealed a higher predisposition of these polymers to induce inflammation and strong immune responses post-implantation [Citation118]. In addition, the biodegradability of synthetic polymers is considerably lower than that of natural polymers, potentially hindering the regeneration of new bone tissue [Citation119].
Figure 4. Characterization of the GelMA/F127DA/PEDGA (GPF) scaffolds compared to the control F127DA/PEDGA (PF). (A) Image of 3D modelling. (B,C) Images of the GPF scaffold; (D) SEM image of the GPF scaffold. (E) Live/dead assay of MC3T3-E1 cells co-cultured with 25% extract for 2 days. Green represents living cells. (F) Reconstructed 3D patterns from micro-CT images of femur defects at 4 and 12 weeks. (G) Pore size of the CAD and scaffolds. (H) Swelling ratio of different scaffolds. (I) In vitro degradation behaviour of the two scaffolds in PBS (37 °C, pH = 7.4). (J) Compressive stress–strain curves of the scaffolds. (K) Compressive modulus of the scaffolds. (L) Compressive strength of the scaffolds. Data were analysed using a paired t test and one-way ANOVA and are shown as the mean ± standard deviation (** p < 0.01, n = 3). Reprinted from [Citation116] under CC by 4.0 Licence.
![Figure 4. Characterization of the GelMA/F127DA/PEDGA (GPF) scaffolds compared to the control F127DA/PEDGA (PF). (A) Image of 3D modelling. (B,C) Images of the GPF scaffold; (D) SEM image of the GPF scaffold. (E) Live/dead assay of MC3T3-E1 cells co-cultured with 25% extract for 2 days. Green represents living cells. (F) Reconstructed 3D patterns from micro-CT images of femur defects at 4 and 12 weeks. (G) Pore size of the CAD and scaffolds. (H) Swelling ratio of different scaffolds. (I) In vitro degradation behaviour of the two scaffolds in PBS (37 °C, pH = 7.4). (J) Compressive stress–strain curves of the scaffolds. (K) Compressive modulus of the scaffolds. (L) Compressive strength of the scaffolds. Data were analysed using a paired t test and one-way ANOVA and are shown as the mean ± standard deviation (** p < 0.01, n = 3). Reprinted from [Citation116] under CC by 4.0 Licence.](/cms/asset/af4bca5f-f3ef-4849-9b7b-3c487c954c57/nvpp_a_2378003_f0004_oc.jpg)
6.3. GelMA-synthetic polymers inks/bioinks for jetting-based bioprinting
The use of poly(ethylene glycol) dimethacrylate (PEGDMA) alongside GelMA is favourable as it represents a polymer compatible with the inkjet technique and it forms structures with a significantly higher compression modulus than natural polymers. Gao et al. described the bioprinting of such a bioink containing 1.5% GelMA, 10% PEGDMA, 0.05% Irgacure 2959 and human MSCs at a cell density of 6 × 106 cells·m L−1. The inkjet bioprinting was performed at 3.6 kHz, with a resolution of 300 dpi and a drop volume of 130 pL. Even though immediately after bioprinting the compressive modulus of the structures was lower than that of samples containing PEGDMA alone, the values increased by 100% after 21 days under osteogenic differentiation, exceeding the values of PEGDMA alone scaffolds. This result also confirms that the proposed bioink promotes hMSCs cell differentiation and extracellular matrix production [Citation120]. However, the mechanical properties of inkjet bioprinted structures do not match those of DLP bioprinted GelMA-synthetic polymer compositions.
7. Nanocomposite and multicomponent bioink formulations based on GelMA
7.1. Composite and multicomponent GelMA inks/bioinks for extrusion-based bioprinting
In the aforementioned that constructs manufactured exclusively of polymeric materials are not sufficient to mimic the complex composition of load-bearing tissue and to provide the necessary mechanical stability, the requirement for reinforcement with various agents is outlined. Formulations of this variety range from the simplest composites, employing one or more reinforcing agents, to complex multicomponent bioinks, containing several other polymers as well as reinforcing agents from micron down to the nm scale (). The most commonly used reinforcing agent in bone TE is HAp, based on its similarity to the mineral constituent of bone, and it was often found in bioink formulations alongside GelMA. One such formulation was described by Leu Alexa et al. in 2022 by reinforcing GelMA with HAp doped with cerium ions. Compositions of 20, 30 and 35% GelMA reinforced with a cerium-doped HAp at 3% concentration were printed through a 260–340 µm nozzle, at pressures between 90 and 310 kPa and printing speeds of 120–600 mm·min−1. The degradation studies revealed an increase in stability with increasing GelMA concentration, with the remaining weight increasing from ∼83%, for 20% GelMA constructions, to ∼85%, for 35% GelMA constructions, after 1 d of incubation in PBS. The 30% GelMA and 3% cerium-doped HAp inks resulted in high structural integrity scaffolds that enhance cell proliferation and support osteogenic differentiation [Citation121]. A multicomponent bioink was explored by Wenz et al. containing 13% GelMA, 1% HAMA and 5% HAp particles and encapsulating a density of 5 × 106 cells·mL−1 human adipose-derived stem cells (hASCs). The used nozzle featured a diameter of 300 µm, and bioprinting was conducted at a temperature of 21.5°C with a speed of 30–60 mm·min−1, followed by photocrosslinking at 365 nm with an intensity of 9.0 mW cm−2 for 120 s for each double layer. They reported that HAp reinforcement increased G′ by 126%, doubled G″ and increased bone matrix production and remodelling after 28 days [Citation122]. Nanohydroxyapatite (nano-HAp) has also been employed for such applications. Osi et al. tested the influence of these nanoparticles on the cytotoxicity and mechanical properties in GelMA and methacrylated chitosan formulations. Employing 10–20% GelMA, 1% chitosan methacryloyl and 0.5% nano-HAp, constructs were printed through a 200 µm nozzle at a temperature of 17–25°C, succeeded by UV cross-linking at 365 nm with an intensity of 1 W cm−2. Similar to the previous study, the addition of nano-HAp resulted in an increase in G′, and the 20% GelMA formulations exhibited the highest compressive modulus values and the greatest ECM production. Furthermore, all samples displayed cell viability values greater than 70%, indicating that nano-HAp reinforcement does not interfere with the biocompatibility of the structures [Citation123].
Table 4. Composite and multicomponent GelMA formulations for 3D printing and bioprinting.
HAp is not the sole ceramic within the calcium phosphate class that has been exploited in GelMA-based bioinks. In 2019, Kosik-Koziol et al. investigated the performance of a multicomponent GelMA and ALG bioink after the addition of different concentrations of β-tricalcium phosphate (β-TCP). Concentrations employed included 6% GelMA, 4% ALG, 0.5–3% β-TCP and 0.05% Irgacure 2959. To achieve a dual crosslinking, a co-axial extrusion system with a 250 µm internal nozzle for the bioink and an external nozzle of 690 µm for CaCl2 extrusion was used. By encapsulating a density of 107 cells·mL−1 bone marrow-derived hMSCs, bioprinting was achieved at a printing speed of 234 mm·min−1, followed by UV crosslinking at 365 nm with a 12 mW cm−2 source for 30 s. Higher concentration of β-TCP prevented GelMA photocrosslinking and thus resulted in lower compressive modulus constructions. Accordingly, the optimal β-TCP concentration determined was 0.5%, and the obtained scaffolds exhibited a high degree of viability for bone marrow-derived hMSCs and formation of calcified osteochondral tissue areas [Citation124].
Another osteoconductive material is silica, which interacts with both calcium and phosphate ions through its silanol groups. In 2021 Choi et al. tested the ability of a nanocomposite bioink based on 15% GelMA and 3–10% silanated silica to enhance mechanical and biological properties. After the encapsulation of 5 × 106 cells·mL−1 hMSCs, a pressure of 950 kPa and a printing speed of 180 mm·min−1 were used to achieve bioprinting through a 300 µm nozzle. Additionally, both a cooling jacketed for the bioink and a cooled platform at 5°C were employed before the UV photocrosslinking of the construct at 360–380 nm with an intensity of 10 mW cm−2 for 180 s. Using this method they successfully bioprinted scaffolds with up to 10% silanated silica, which exhibited a 23% higher Young's modulus than samples containing GelMA alone. Additionally, these samples also displayed the highest cell viability values and an increase in osteopontin expression which promotes osteocyte mineralisation [Citation125]. In a different study, Chimene et al. investigated the potential of nanosilicates (nano-Si) in GelMA bioink reinforcement. More precisely, the researchers employed concentrations of 2–4% nano-Si in multicomponent formulations containing 5–15% GelMA and 0–2% kappa-carrageenan (kCA) to allow the formation of electrostatic noncovalent bonds. As two-dimensional synthetic nanoclays, nano-Si exhibits both a positive charge on the faces and a negative one on the edges, allowing weak bonds to form. The encapsulated cells were hMSCs, and the bioprinting parameters included a printing speed of 900 mm·min−1, a nozzle diameter of 840 µm, and an intensity UV source of 25 mW cm−2 at 365 nm for the photocrosslinking initiated with 0.25 Irgacure 2959. The results indicated that concentrations of 7.5% GelMA, 1% kCA and 2% nano-Si led to structures with adequate mechanical properties and bone reconstruction capacities [Citation126].
In their 2023 study, Liu et al. incorporated 1–4% nano-attapulgite (nano-ATP) into 5–20% GelMA bioinks to assess their ability to improve the mechanical properties and the expression of genes responsible for osteoblast differentiation. A 0.25% LAP concentration was selected for photoinitiation, and bioprinting was performed through a 260 µm nozzle after the encapsulation of BMSCs or MUVECs at cell densities of 2 × 106 cells·mL−1. In addition, photocrosslinking was performed at a wavelength of 405 nm with an intensity of 4 W cm−2 for 15–480 s. The study focused on the ability of the obtained scaffolds to promote both bone regeneration and angiogenesis, and the results indicated that a concentration of 1% nano-ATP produced scaffolds with superior mechanical, angiogenic and osteogenic properties quantified by gene expression [Citation127]. Another osteogenic and angiogenic nanocomposite bioink was developed by Cidonio et al., this time by using 0.5–2% laponite and 5–10% GelMA. Laponite is a smectite nanomaterial capable of improving printability and enhancing biological properties. For this research, a density of 5 × 106 cells·mL−1 hBMSCs were encapsulated in the bioink bioprinted at a speed of 550 mm·min−1, with a 300 µm nozzle, followed by photocrosslinking at 400–450 nm with an intensity of 3 mW cm−2 for 180 s. The study reported that both printability and bicompatibility were best enhanced by the formulation containing 7.5% GelMA and 1% laponite, resulting in a bioink with adequate viscosity, capable of osteogenetic and angiogenetic differentiation [Citation128]. Enhanced bone regeneration was also described by Wang et al. in 2024 by bentonite reinforcement of a bioink containing 5% GelMA. The bioink containing a cell density of 5 × 106 cells·mL−1 BMSCs and 0.25% LAP as photoinitiator was reinforced with three different concentrations of bentonite, specifically 1.25%, 2.5% and 5%. Bioprinting was carried out through a 410 µm nozzle at a 50–200 kPa range of pressure, a printing speed of 240–480 mm·min−1 and a platform temperature of 15, whereas a 3W source was used for UV irradiation at a wavelength of 405 nm for 10 s. Mechanical tests revealed a significant increase in mechanical stability with increasing bentonite concentration, as well as enhanced deformability, demonstrated by the Young's modulus of 2.4 MPa for the scaffold reinforced with 5% bentonite. Furthermore, the 5% bentonite scaffold exhibited superior cell spreading with a pronounced network of living cells, displayed the highest collagen deposition, indicating extracellular matrix formation and the lowest degradation rate. Therefore, it is demonstrated that this concentration is the most adequate for achieving scaffolds with enhanced osteoinduction and promotion of tissue regeneration [Citation129].
A different reinforcement strategy involves the use of carbon-based nanomaterials such as graphene oxide (GO). Mendes et al. studied the behaviour of scaffolds printed with GO concentrations from 0.005 to 1.4 mg mL−1, 5% GelMA and 0.1% LAP. After printing the constructs at 15–20 kPa, 300 mm·min−1 printing speed, through a 250 µm nozzle, a photocrosslinking process at 405 nm with an intensity of 20 mW cm−2 was performed. According to mechanical analysis, an increase of up to 3.64 kPa of the compression moduli and an increased G′ from 1.06 kPa for 0.005 mg mL−1 GO to 1.61 kPa for 1.4 mg mL−1 GO was observed. Further, an increase in cell viability was also recorded with increasing GO content [Citation130]. A similar study by Jiang et al. reinforced 8% GelMA with GO concentrations ranging from 0.5 to 2 mg mL−1 with a cell density of 2 × 106 cells·mL−1 MSCs. Extrusion was performed through a 260 µm nozzle, followed by UV cross-linking for 30 s and results indicated that higher concentrations resulted in increased osteocalcin levels and alkaline phosphatase activity, with concentrations up to 2.0 mg mL−1 exhibiting no cytotoxic effect [Citation131].
In a different approach, Zhang et al. exploited reduced graphene oxide (rGO) to engineer a scaffold with the potential to promote neuronal differentiation within the newly formed bone structure. Reduced graphene oxide is a bioactive nanomaterial that is produced by reducing GO and exhibits both higher stability and better electrical properties than GO in vivo. In this paper, inks containing 0.05–0.1% rGO, 10% GelMA and 0.25% LAP were extruded at a pressure of 950 kPa, a speed of 180 mm·min−1, through a 200 µm nozzle and photocrosslinking was performed at 405 nm for 60 s. The study showed that a concentration greater than 0.05% rGO prevents GelMA photocrosslinking leading to a decrease in mechanical properties. Concentrations of 0.05% resulted in both higher compressive modulus and osteogenesis with neurogenesis 2 months post-implantation in animal models [Citation132]. Cernencu et al. achieved a multicomponent ink with increased printing fidelity, high mechanical properties and adequate cellular response using 8% GelMA and 2% methacrylated pectin double reinforced with 1.2% oxidised nanocellulose fibres (CNF) and 0.25–1% graphene oxide. The ink was printed at a 30–35 kPa pressure, a printing speed of 600 mm·min−1 and photocrosslinking at 365 nm for 5 s. They determined that a concentration of 0.5% GO and 1.2% CNF resulted in the achievement of adequate rheologic behaviour for high-resolution printing without affecting cell viability or GelMA photocrosslinking. Moreover, the 0.5% GO reinforced construct also exhibited the lowest enzymatic degradation rate of only 24% after 7 days of incubation. These printing samples and test results are represented in [Citation133].
Figure 5. (A) Photographs of (a) GPC_000 – with 0% GO, (b) GPC_025 – with 0.25% GO, (c) GPC_050 – with 0.5% GO and (d) GPC_100 – with 1% GO, 3D structures designed as 15 mm discs with 15% lattice filling in 10 layers with top view (*) and side view (#) insets. (B) Printing fidelity on mesh area and (C) Printing fidelity on height. (D) The modulus of elasticity calculated at a deformation of 2% for the 3D printed hydrogel scaffolds. (E) Biocompatibility assessment of 3D hydrogel scaffolds with L929 murine fibroblasts. (a) Cell viability by MTT assay after 2 and 6 days. (b) GPG cytotoxicity on contact with L929 fibroblasts by lactate dehydrogenase (LDH) assay after 2 and 6 days. (c) Live/Dead assay of L929 fibroblasts after 2 and 6 days. Scale bar – 100 μm. *p < 0.05; ***p < 0.001 by 2-way ANOVA, Bonferroni post-test [Citation133].
![Figure 5. (A) Photographs of (a) GPC_000 – with 0% GO, (b) GPC_025 – with 0.25% GO, (c) GPC_050 – with 0.5% GO and (d) GPC_100 – with 1% GO, 3D structures designed as 15 mm discs with 15% lattice filling in 10 layers with top view (*) and side view (#) insets. (B) Printing fidelity on mesh area and (C) Printing fidelity on height. (D) The modulus of elasticity calculated at a deformation of 2% for the 3D printed hydrogel scaffolds. (E) Biocompatibility assessment of 3D hydrogel scaffolds with L929 murine fibroblasts. (a) Cell viability by MTT assay after 2 and 6 days. (b) GPG cytotoxicity on contact with L929 fibroblasts by lactate dehydrogenase (LDH) assay after 2 and 6 days. (c) Live/Dead assay of L929 fibroblasts after 2 and 6 days. Scale bar – 100 μm. *p < 0.05; ***p < 0.001 by 2-way ANOVA, Bonferroni post-test [Citation133].](/cms/asset/13c40862-2bea-4e20-b7ac-08d86d04e513/nvpp_a_2378003_f0005_oc.jpg)
Pursuing the development of a bioink with a lower GelMA concentration that would support cell elongation and differentiation, Boularaoui et al. studied two reinforcement methods, one with 0.05–1 mg mL−1 gold nanorods and one with 0.05–3 mg mL−1 two-dimensional transition metal carbide (MXene) for a 2% GelMA bioink. They aimed to create a microenvironment that would promote cell differentiation by modulating mechanical, rheological and electrical conductivity properties. Thus, C2C12 cells at a density of 5 × 106 cells·mL−1 were incorporated in the reinforced formulation and bioprinted through a 210 µm nozzle at a printing speed of 300 mm·min−1. The UV crosslinking was carried at 365 nm wavelength for 240 s, using 0.1% LAP as photoinitiator. Their findings indicated that both gold nanoparticles and MXene enhance GelMA's ability to conduct electrical signals and to promote C2C12 cell differentiation [Citation134]. Similarly, Lee et al. also published a study in 2023 investigating the ability of GelMA and HAMA-based multicomponent inks reinforced with 0–200 µg mL−1 MXene to promote differentiation of hMSCs into osteoblasts. The extrusion was carried out through a 590 µm nozzle at a pressure of 20 kPa and a printing speed of 360 mm·min−1. They showed how MXene exhibits a strong binding affinity to serum proteins and calcium ions, thus promoting osteogenic activity [Citation135].
A bioink with osteogenic properties was also designed by Moghimi et al. in 2024, incorporating 7–10% GelMa, 1–3% ALG and 8–35 mg mL−1 polylactic acid (PLA) microfibers, coated and uncoated with HAp nanoparticles. Scaffolds were bioprinted into a core shell design, with the shell consisting of a bioink reinforced with HAp-coated PLA microfibers and MC3T3 cells, and the core reinforced with PLA and platelet-rich fibrin extracted from human blood. Bioprinting was performed through a 530 µm nozzle at a print speed of 120 mm·min−1, and for photocrosslinking a concentration of 0.5% Irgacure 2959 and a 25 mW cm−2 source was employed for 20–60 s at 365 nm wavelength. Based on the printability results it was determined that the optimal concentration was 8% GelMA, 2% ALG and 20 mg mL−1 PLA fibres, and the mechanical analysis showed that reinforcement with 20 mg mL−1 PLA microfibers resulted in an increase of the compression modulus from 1.3a to 3.1 kPa. Moreover, the employment of HAp-coated microfibres resulted in an even higher increase of the compression modulus up to 3.4 kPa. Furthermore, a comparison was performed between scaffolds with an empty core, a core with platelet-rich fibrin and a core with platelet-rich fibrin and PLA microfibers. The evaluation results demonstrated superior tissue regeneration for the latter, including both early and late osteogenic differentiation [Citation136]. Yu et al. also developed two separate bioinks for producing one scaffold, but in this study, they focused on bioprinting a multicellular biphasic substitute for osteochondral regeneration. For the cartilage composition 10% GelaMA with a cell density of 2 × 106 cells·mL−1 BMSCs and articular chondrocytes were employed and for the subchondral bone the bioink consisted of 10% GelMA, 0.05% strontium substituted xonotlite (Sr-CSH) and a cell density of 2 × 106 cells·mL−1 BMSCs. The choice of Sr is attributed to its dual effect on bone metabolism, since it both promotes osteogenic differentiation and inhibits osteoclast mediated bone resorption activity. The extrusion was carried out at a pressure of 150–200 kPa, through a 410 µm nozzle, at a speed of 480 mm·min−1. Additionally, a concentration of 0.5% LAP was used as photoinitiator for both bioinks and photocrosslinking was performed at 405 nm for 60 s. In vitro and in vivo results indicated that these biphasic multicellular scaffolds are capable of successful regeneration of osteochondral tissue, leading to new tissue formation in drilling defects after only 12 weeks postoperatively. Mechanical testing revealed that Sr-CSH reinforcement resulted in an increase in elastic modulus from 1.2 kPa to 3.3 kPa, and the biphasic scaffolds exhibited enhanced mechanical strength of 3.5 kPa, however these values remain inferior to the properties of natural osteochondral tissues [Citation137].
Regardless of the reinforcing agent, nanocomposite formulations have indicated the best results in extrusion bioprinting, allowing the printing of bone regeneration scaffolds with superior mechanical strength, capable of osteogenic differentiation and bone cell proliferation. The most prominent results in terms of mechanical properties are shown for inks reinforced with nanoscale agents such as nano-HAp [Citation123], nano-ATP [Citation127], or rGO [Citation132], while in terms of biological properties, laponite-reinforced structures [Citation128] exhibited the highest cell proliferation, osteogenic and angiogenic differentiation.
7.2. Composite and multicomponent GelMA inks/bioinks for vat photopolymerisation-based bioprinting
The selection of DLP for GelMA-based composite and multicomponent bioinks is justified by the superiority of the technique in terms of contrast ratio and high resolution. In 2021 Su et al. proposed the reinforcement of a 5% GelMA bioink with 1–3% silanised hydroxyapatite (Si-HAp) and fabrication by a visible light curing DLP system. The photocrosslinking was achieved after the light was projected at 440 nm, with an intensity of 8 mW cm−2, VA-086 being employed as photoinitiator. In addition, two types of cells were encapsulated in the bioink, MG63 cells at a cell density of 1 × 106 cells·mL−1 and hMSCs at a density of 1 × 107 cells·mL−1. The obtained ink product displayed low cytotoxicity and expression of osteogenesis-specific genes, as displayed in [Citation138]. Likewise, by using nano-HAp Song et al. were able to reinforce 30% GelMA and 0–30% methacrylic anhydride silk fibroin (SilMA) and print multicomponent structures by DLP technique. For nano-HAp, a concentration of 30 w/v% (relative to the mass of GelMA) was employed and photonitiation was obtained with the aid of 0.5% LAP. Additionally, poly(ethylene oxide) was added to the composition in order to obtain an aqueous two-phase emulsion able to provide the adequate microporous structure. The printing was performed with a 15 mW cm−2 intensity, for three layers, each being exposed for 9–10 s. Data indicated an increase in G′, G″ and elastic modulus with increasing polymer concentration and the addition of HAp in the inks. Thus, G′ increased from 42.7 kPa for 15% GelMA and 15% SilMA to 73.3 kPa for 30% GelMA and 30% SilMA, and elastic modulus increased from 51.4 kPa to 118.2 kPa for the same concentartions [Citation139].
Figure 6. Mechanical and biological properties of GelMA-HAp and GelMA-Si-HAp composite hydrogels. (A) Schematic representation of the preparation of composite hydrogel constructs. (B) Stress and strain curve of GelMA-HAp (blue) and GelMA-Si-HAp (red) hydrogels (3% fillers). (C) Elastic modulus of GelMA-HAp and GelMA-Si-HAp hydrogels. (D) Live/dead cell viability assay of human MG63 cells and MSCs on day 14; green-calcein AM, red-ethidium homodimer-1. (E) MTT assay of human MG63 and MSCs on day 1, day 7, day 14 (G: 15% GelMA, G1H-G3H: 15% GelMA with 1–3% HAp, G1Si–G3Si: 15% GelMA with 1–3% Si-HAp). (F) The expressions of alkaline phosphatase (ALP), collagen type 1, alpha 1 (COL1A1), osteocalcin (OC), and osteopontin (OPN) in MG63 cell-laden composite hydrogels were quantified by qPCR on day 1 and day 7. Fold change was normalized to the day 1 GelMA group. (G) Four osteogenic markers in MSCs laden composite hydrogels were quantified by qPCR on day 7. MSCs were cultured with a growth medium or osteogenic induction medium. Gene expression was normalized to the GelMA group with growth medium. G: 15% GelMA, G3H: 15% GelMA with 3% HAp, G3Si: 15% GelMA with 3% Si-HAp. *p < 0.05, ***p < 0.001. Reprinted from [Citation138] under CC by 4.0 Licence.
![Figure 6. Mechanical and biological properties of GelMA-HAp and GelMA-Si-HAp composite hydrogels. (A) Schematic representation of the preparation of composite hydrogel constructs. (B) Stress and strain curve of GelMA-HAp (blue) and GelMA-Si-HAp (red) hydrogels (3% fillers). (C) Elastic modulus of GelMA-HAp and GelMA-Si-HAp hydrogels. (D) Live/dead cell viability assay of human MG63 cells and MSCs on day 14; green-calcein AM, red-ethidium homodimer-1. (E) MTT assay of human MG63 and MSCs on day 1, day 7, day 14 (G: 15% GelMA, G1H-G3H: 15% GelMA with 1–3% HAp, G1Si–G3Si: 15% GelMA with 1–3% Si-HAp). (F) The expressions of alkaline phosphatase (ALP), collagen type 1, alpha 1 (COL1A1), osteocalcin (OC), and osteopontin (OPN) in MG63 cell-laden composite hydrogels were quantified by qPCR on day 1 and day 7. Fold change was normalized to the day 1 GelMA group. (G) Four osteogenic markers in MSCs laden composite hydrogels were quantified by qPCR on day 7. MSCs were cultured with a growth medium or osteogenic induction medium. Gene expression was normalized to the GelMA group with growth medium. G: 15% GelMA, G3H: 15% GelMA with 3% HAp, G3Si: 15% GelMA with 3% Si-HAp. *p < 0.05, ***p < 0.001. Reprinted from [Citation138] under CC by 4.0 Licence.](/cms/asset/12725e10-ed08-42af-8a6a-a6177e528883/nvpp_a_2378003_f0006_oc.jpg)
In a different research, Anada et al. were able to obtain vascularised constructs that promote both osteogenesis and angiogenesis by employing octacalcium phosphates (OCP). OCP is a biodegradable material capable of transitioning into HAp thermodynamically under physiological conditions. The formulations contained 5–10% GelMA, 0.25% LAP and 0–30% OCP. During DLP the ink was exposed to visible light for 120 s for photocrosslinking. OCP-reinforced GelMA has been found to stimulate the differentiation of multipotent mouse C3H10T1/2 cells into osteoblasts and to have a positive effect on angiogenesis [Citation140]. GelMA-based printed scaffolds can support both bone tissue regeneration and controlled drug delivery, as demonstrated by Choi et al. in their study published in 2023 in which they obtained a bioprintable GelMA hydrogel reinforced with zinc-based metal–organic framework (MOFs) nanoparticles that can sustain vancomycin release. By adding 0.5–3 mg mL−1 MOFs in 5–20% GelMA, bioprinting with a cell density of 2 × 106 cells·mL−1 of human adipose-derived MSCs was performed by DLP at 405 nm with a source intensity of 10–30 mW cm−2 for 8 s per layer exposure. Results indicated that smaller GelMA concentrations affected printability as it limited the crosslinking efficiency. In contrast, the bioinks reinforced with MOFs exhibited good bioprintability, enhanced compressive modulus of 128.1 kPa, promotion of osteogenic differentiation and favourable vancomycin release of 87.5% in 48 h with significant antibacterial effect [Citation141].
Although, from a mechanical strength point of view, nanocomposite scaffolds obtained by DLP stereolithography did not present results as significant as those bioprinted by extrusion, they indicated enhanced compressive modulus values, increased cell viability and osteogenic differentiation. Furthermore, such structures have demonstrated the ability to support controlled drug delivery, a feature that indicates the potential for future structures of releasing different agents or drugs in a targeted and supervised manner.
8. Future perspectives and conclusion
Bone TE encounters many challenges both from an architectural standpoint and in terms of fabricating complex geometries able to maintain high cell viability and even differentiation. The 3D bioprinting method represents a technological achievement that allows the production of complex geometric structures with high resolution. In order to obtain biocompatible and suitable structures for bone tissue replacement, the materials, the bioprinting technology and the printing parameters, that exhibit a strong co-dependency, must be carefully selected considering the final application. GelMA-based hydrogels are among the most common inks and bioinks used in the medical field due to their ability to promote biological interactions through their RGD sequence and due to their photocrosslinking capacity achieved through gelatin methacrylation.
As previously stated, GelMA-based hydrogels have been proven to exhibit a great potential in the manufacture of structures for bone tissue replacement, but their capabilities extend much further, such inks or bioinks being capable of controlled release of drugs and growth factors. Numerous studies have focused on the release of active substances from GelMA-based scaffolds, examples including antibiotics such as gentamicin [Citation142], cefazolin [Citation143], rifampicin and vancomycin [Citation144]. One of the future challenges is to optimise the integration of such drugs into GelMA-based bioinks able to bioprint bone scaffolds without affecting the viability of the encapsulated cells.
Another research direction for the future lies in the shift from 3D printing to 4D bioprinting, the novelty arising from the introduction of a space–time axis. Currently, 4D printing, which is considered the latest additive manufacturing technology, is recognised as a cornerstone of scientific advancement that warrants investigation beyond the constraints of conventional manufacturing methods (3D printing) in top class domains like regenerative medicine. 4D printing is foreseen to allow the creation of adaptive structures when used in conjunction with smart materials able to generate a response to predetermined stimulus e.g. humidity, thermal energy, electromagnetic fields (light, electricity, magnetic fields), osmotic pressure, mechanical incentives, chemical catalysts or biological cues. Considering GelMA functionalities, chemical and structural versatility smart formulation with shape memory based on GelMA/GelMA nanocomposites/GelMA multicomponent formulations could be easily fabricated and by further association to 4D printing would grant the fabrication of hierarchical structures with shape-changing behaviour or magnetic stimulated shape memory that evolve over time into a superior 3D item (postmanufacture optimisation under trigger stimulation). Such smart formulation would provide intricate combinations of micro- and macro-features which work together to provide robust mechanical, rheological, electrical, or chemical stability properties needed to support physiological events of tissue regeneration but also high resolution and dimensional accuracy [Citation145]. In addition, a fifth dimension can be added for a 5D printing process, the last dimension being information. Information is defined as any species that will bring environmental changes after implantation, such as biomolecules, growth factors, nanoparticles or genes [Citation146]. These promising results pave the way to a future research direction involving the transition from 3D towards 5D bioprinting by employing smart materials and encapsulating growth factors or drugs with different applications, such as antimicrobial, anticancer or for genetic or epigenetic therapy.
Epigenetics addresses the molecular modification that can modify gene activity independently of the DNA sequence, the main mechanisms being DNA methylation, histone modifications and non-coding RNAs (ncRNAs) [Citation147]. It is recognised that bone regeneration scaffolds have an impact on epigenetics not solely due to the chemical properties of the used biomaterial, but also through its geometry at the micro and nanoscale (e.g. pore size and shape) which directly modulate cell mechanosensing and therefore gene expression associated with mesenchymal stem cell differentiation [Citation148]. However, recent studies move towards a modification of GelMA-based hydrogels for an enhanced epigenetic effect. For instance, Man et al. studied the ability of a GelMA-poly(ethylene glycol)-terephthalate/poly(butylene terephthalate) scaffold to deliver epigenetically activated MSCs that promote osteogenesis and the results proved favourable [Citation149]. Additionally, a research team led also by Man demonstrated the efficacy of a GelMA hydrogel reinforced with laponite nanoclay in the controlled delivery of extracellular vesicles with epigenetically activated osteoblasts for promoting stem cell mineralisation [Citation150]. These studies outline a new research direction in which bioprinted GelMA constructs will be able to induce epigenetic modifications that will overcome the current limitations in bone tissue regeneration.
Nonetheless, arguably the most promising future prospect resides in the standardisation of the machine learning systems in the bioprinting process. These techniques have developed dramatically in recent years and tailored systems already exist for different bioprinting techniques. Probably the most important contributions reside in the optimisation of the bioprinting process and quality control. Systems of this type incorporate algorithms capable of forming associations between the rheological properties of the materials and the printing results, therefore enabling them to predict and optimise the parameters needed to achieve the desired outcomes in terms of both structure and biocompatibility [Citation151]. Moreover, such models can determine, depending on the viscoelastic properties of the ink, the printing parameters needed to prevent nozzle clogging, one of the notable drawbacks of the extrusion-based methods [Citation152]. Therefore, by employing these technologies, we could determine the appropriate printing parameters for minimising the cytotoxic effects on cells caused by shear stress or UV light exposure without compromising significantly the mechanical stability of the structures [Citation153]. These types of machine learning systems are leading the way towards personalised medicine that will allow the development of bioprinted tissues tailored to the specific needs of the patient, increasing the prospects of matching the tissue defect and the acceptance by the patient's body. We are confident that through these techniques we will be able to surpass the current limitations in a considerably shorter timeline and achieve FDA and EMA approved medical products based on bioinks for clinical applications.
Summarising, this review paper encompassed from commercial GelMA-based inks, to the most widely used crosslinking methods in biomedical applications for GelMA and proceeded with an outline of formulations with not only GelMA, but also GelMA-polysaccharides, GelMA-synthetic polymers as well as GelMA in multicomponent inks and bioinks. We summarised the latest developments in bioink formulations based on GelMA and classified by printing method for obtaining complex structures for bone tissue regeneration. It has been observed that the commonly adopted biofabrication method is the extrusion-based one, while inkjet is rarely used for bioprinting with GelMA hydrogels, as this biomaterial requires additional treatments to be suitable with the inkjet method. When GelMA is used alone in formulations, structures with good printability and adequate biological activity are obtained. However, the obtained structures present limitations in terms of mechanical stability and GelMA-only bioprinted scaffolds cannot match the mechanical properties of bone tissue, therefore the involvement of various other biomaterials is required for achieving such performances. The stability of the scaffolds and the printing fidelity is improved with the development of GelMA-polysaccharide and GelMA-synthetic inks and bioinks. Nevertheless, we concluded that, for the achievement of bone regeneration constructs with the necessary mechanical properties, the employment of reinforcing agents is mandatory, and the most notable results have been observed by reinforcing with nano-hydroxyapatite, nano-silica, nano-attapulgite and graphene oxides. Currently, none of the 3D bioprinting methods is ideal, and future research directions will focus on developing GelMA methods and bioinks capable of combining osteoconductive, osteoinductive and osteogenic properties to meet all the requirements for the achievement of optimal bone tissue regeneration. However, the current research results are promising and the use of GelMA-based bioprinted scaffolds in clinical applications for bone tissue regeneration is imminent.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Chandra PK, Soker S, Atala A. Tissue engineering: current status and future perspectives. In: Lanza R, Langer R, Vacanti JP, Atala A, editors. Principles of tissue engineering. London: Academic Press; 2020. pp. 1–35. doi:10.1016/B978-0-12-818422-6.00004-6
- Donati D, Zolezzi C, Tomba P, et al. Bone grafting: historical and conceptual review, starting with an old manuscript by vittorio putti. Acta Orthop. 2007;78(1):19–25. doi:10.1080/17453670610013376
- Urist MR. Bone: formation by autoinduction. Science (1979). 1965;150(3698):893–899. doi:10.1126/science.150.3698.893
- Chen X, Li H, Ma Y, et al. Calcium phosphate-based nanomaterials: preparation, multifunction, and application for bone tissue engineering. Molecules. 2023;28(12):4790. doi:10.3390/molecules28124790
- Gao C, et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci. 2014;15(3):4714–4732. doi:10.3390/ijms15034714
- Xu H, et al. 3D bioprinting advanced biomaterials for craniofacial and dental tissue engineering – a review. Mater Des. 2024;241:112886. doi:10.1016/j.matdes.2024.112886
- Ozdil D, Aydin HM. Polymers for medical and tissue engineering applications. J Chem Technol Biotechnol. 2014;89(12):1793–1810. doi:10.1002/jctb.4505
- Schicker M, Seitz H, Drosse I, et al. Biomaterials as scaffold for bone tissue engineering. Eur J Trauma. 2006;32(2):114–124. doi:10.1007/s00068-006-6047-8
- Pilia M, Guda T, Appleford M. Development of composite scaffolds for load-bearing segmental bone defects. BioMed Res Int. 2013;2013:458253. doi:10.1155/2013/458253
- Wang C, Huang W, Zhou Y. et al. 3D printing of bone tissue engineering scaffolds. Bioactive Mater. 2020;5(1):82–91. doi:10.1016/j.bioactmat.2020.01.004
- Tappa K, Jammalamadaka U. Novel biomaterials used in medical 3D printing techniques. J Funct Biomater. 2018;9(1). doi:10.3390/jfb9010017
- Pugliese R, Beltrami B, Regondi S, et al. Polymeric biomaterials for 3D printing in medicine: an overview. Ann 3D Print Med. 2021;2:100011. doi:10.1016/j.stlm.2021.100011
- Ng WL, Chua CK, Shen Y-F. Print me an organ! Why we are not there yet. Prog Polym Sci. 2019;97:101145. doi:10.1016/j.progpolymsci.2019.101145
- Nacu I, Bercea M, Niță LE, et al. 3D bioprinted scaffolds based on functionalized gelatin for soft tissue engineering. React Funct Polym. 2023;190:105636. doi:10.1016/j.reactfunctpolym.2023.105636
- Datta P, Barui A, Wu Y, et al. Essential steps in bioprinting: from pre- to post-bioprinting. Biotechnol Adv. 2018;36(5):1481–1504. doi:10.1016/j.biotechadv.2018.06.003
- Alexander AE, Wake N, Chepelev L, et al. A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print Med. 2021;7(1):8. doi:10.1186/s41205-021-00098-5
- Lee JM, Sing SL, Zhou M, et al. 3D bioprinting processes: a perspective on classification and terminology. Int J Bioprint. 2018;4(2):1–10. doi:10.18063/ijb.v4i2.151
- Koch F, Tröndle K, Finkenzeller G, et al. Generic method of printing window adjustment for extrusion-based 3D-bioprinting to maintain high viability of mesenchymal stem cells in an alginate-gelatin hydrogel. Bioprinting. 2020;20:e00094. doi:10.1016/j.bprint.2020.e00094
- Matai I, Kaur G, Seyedsalehi A, et al. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi:10.1016/j.biomaterials.2019.119536
- Udiger Landers R, Ubner B UH, Schmelzeisen R, et al. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering; 2002.
- Wilson WC, Boland T. Cell and organ printing 1: protein and cell printers. Anat Rec Pt A Discover Mol Cell Evol Biol. 2003;272(2):491–496. doi:10.1002/ar.a.10057
- Boland T, Mironov V, Gutowska A, et al. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anat Rec Pt A Discover Mol Cell Evol Biol. 2003;272(2):497–502. doi:10.1002/ar.a.10059
- Dhariwala B, Hunt E, Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004;10(9–10):1316–1322. doi:10.1089/ten.2004.10.1316
- Lee SC, Gillispie G, Prim P, et al. Physical and chemical factors influencing the printability of hydrogel-based extrusion bioinks. Chem Rev. 2020;120(19):10834–10886. doi:10.1021/acs.chemrev.0c00015
- Ouyang L. Pushing the rheological and mechanical boundaries of extrusion-based 3D bioprinting. Trends Biotechnol. 2022;40(7):891–902. doi:10.1016/j.tibtech.2022.01.001
- Karvinen J, Kellomäki M. Design aspects and characterization of hydrogel-based bioinks for extrusion-based bioprinting. Bioprinting. 2023;32:e00274. doi:10.1016/j.bprint.2023.e00274
- Yang Y, Jia Y, Yang Q, et al. Engineering bio-inks for 3D bioprinting cell mechanical microenvironment. Int J Bioprint. 2022;9(1):632. doi:10.18063/ijb.v9i1.632
- Amorim PA, d’Ávila MA, Anand R, et al. Insights on shear rheology of inks for extrusion-based 3D bioprinting. Bioprinting. 2021;22:e00129. doi:10.1016/j.bprint.2021.e00129
- Schwab A, Levato R, D’Este M, et al. Printability and shape fidelity of bioinks in 3D bioprinting. Chem Rev. 2020;120(19):11028–11055. doi:10.1021/acs.chemrev.0c00084
- Bedell ML, Navara AM, Du Y, et al. Polymeric systems for bioprinting. Chem Rev. 2020;120(19):10744–10792. doi:10.1021/acs.chemrev.9b00834
- You S, Xiang Y, Hwang HH, et al. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci Adv. 2023;9(8). doi:10.1126/sciadv.ade7923
- Lepowsky E, Muradoglu M, Tasoglu S. Towards preserving post-printing cell viability and improving the resolution: past, present, and future of 3D bioprinting theory. Bioprinting. 2018;11:e00034. doi:10.1016/j.bprint.2018.e00034
- Li X, Liu B, Pei B, et al. Inkjet bioprinting of biomaterials. Chem Rev. 2020;120(19):10793–10833. doi:10.1021/acs.chemrev.0c00008
- Li Y, Zhang X, Zhang X, et al. Recent progress of the Vat photopolymerization technique in tissue engineering: a brief review of mechanisms, methods, materials, and applications. Polymers (Basel). 2023;15(19):3940. doi:10.3390/polym15193940
- Zheng Z, Eglin D, Alini M, et al. Visible light-induced 3D bioprinting technologies and corresponding bioink materials for tissue engineering: a review. Engineering. 2021;7(7):966–978. doi:10.1016/j.eng.2020.05.021
- Goodarzi Hosseinabadi H, Dogan E, Miri AK, et al. Digital light processing bioprinting advances for microtissue models. ACS Biomater Sci Eng. 2022;8(4):1381–1395. doi:10.1021/acsbiomaterials.1c01509
- Derakhshanfar S, Mbeleck R, Xu K, et al. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater. 2018;3(2):144–156. doi:10.1016/j.bioactmat.2017.11.008
- Liu W, Heinrich MA, Zhou Y, et al. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv Healthc Mater. 2017;6(12). doi:10.1002/adhm.201601451
- Asim S, Hayhurst E, Callaghan R, et al. Ultra-low content physio-chemically crosslinked gelatin hydrogel improves encapsulated 3D cell culture. Int J Biol Macromol. 2024;264:130657. doi:10.1016/j.ijbiomac.2024.130657
- Mendoza-Cerezo L, Rodríguez-Rego JM, Macías-García A, et al. Three-Dimensional bioprinting of GelMA hydrogels with culture medium: balancing printability, rheology and cell viability for tissue regeneration. Polymers (Basel). 2024;16(10):1437. doi:10.3390/polym16101437
- Yin J, Yan M, Wang Y, et al. 3D bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl Mater Interfaces. 2018;10(8):6849–6857. doi:10.1021/acsami.7b16059
- Gungor-Ozkerim PS, Inci I, Zhang YS, et al. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018;6(5):915–946. doi:10.1039/C7BM00765E
- Mobaraki M, Ghaffari M, Yazdanpanah A, et al. Bioinks and bioprinting: a focused review. Bioprinting. 2020;18:e00080. doi:10.1016/j.bprint.2020.e00080
- Ghorbani F, Ghalandari B, Khajehmohammadi M, et al. Photo-cross-linkable hyaluronic acid bioinks for bone and cartilage tissue engineering applications. Int Mater Rev. 2023;68(7):901–942. doi:10.1080/09506608.2023.2167559
- Kurian AG, Singh RK, Patel KD, et al. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact Mater. 2022;8:267–295. doi:10.1016/j.bioactmat.2021.06.027
- Zhang Y, Lv J, Zhao J, et al. A versatile GelMA composite hydrogel: designing principles, delivery forms and biomedical applications. Eur Polym J. 2023;197:112370. doi:10.1016/j.eurpolymj.2023.112370
- Guo A, Zhang S, Yang R, et al. Enhancing the mechanical strength of 3D printed GelMA for soft tissue engineering applications. Mater Today Biol. 2024;24:100939. doi:10.1016/j.mtbio.2023.100939
- Mamidi N, Ijadi F, Norahan MH. Leveraging the recent advancements in GelMA scaffolds for bone tissue engineering: an assessment of challenges and opportunities. Biomacromolecules. 2023;25(4):2075–2113. doi:10.1021/acs.biomac.3c00279
- Koepff P, Braumer K, Babel W. U.S. Pat. Appl US 5,733,994 (1998); p. 3.
- Van Den Bulcke AI, Bogdanov B, De Rooze N, et al. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1(1):31–38. doi:10.1021/bm990017d
- Agarwal K, Srinivasan V, Lather V, et al. Insights of 3D bioprinting and focusing the paradigm shift towards 4D printing for biomedical applications. J Mater Res. 2023;38(1):112–141. doi:10.1557/s43578-022-00524-2
- Huang G, Zhao Y, Chen D, et al. Applications, advancements, and challenges of 3D bioprinting in organ transplantation. Biomater Sci. 2024;12(6):1425–1448. doi:10.1039/D3BM01934A
- Glaeser JD, Bao X, Kaneda G, et al. iPSC-neural crest derived cells embedded in 3D printable bio-ink promote cranial bone defect repair. Sci Rep. 2022;12(1):18701. doi:10.1038/s41598-022-22502-8
- Salg GA, Poisel E, Neulinger-Munoz M, et al. Toward 3D-bioprinting of an endocrine pancreas: a building-block concept for bioartificial insulin-secreting tissue. J Tissue Eng. 2022;13:204173142210910. doi:10.1177/20417314221091033
- Schmidt SK, Schmid R, Arkudas A, et al. Tumor cells develop defined cellular phenotypes after 3D-bioprinting in different bioinks. Cells. 2019;8(10):1295. doi:10.3390/cells8101295
- Yang Y, Xu R, Wang C, et al. Recombinant human collagen-based bioinks for the 3D bioprinting of full-thickness human skin equivalent. Int J Bioprint. 2022;8(4):611. doi:10.18063/ijb.v8i4.611
- Bedell ML, Melchiorri AJ, Aleman J, et al. A high-throughput approach to compare the biocompatibility of candidate bioink formulations. Bioprinting. 2020;17:e00068. doi:10.1016/j.bprint.2019.e00068
- Young AT, White OC, Daniele MA. Rheological properties of coordinated physical gelation and chemical crosslinking in gelatin methacryloyl (GelMA) hydrogels. Macromol Biosci. 2020;20(12):2000183. doi:10.1002/mabi.202000183
- Chen S, Wang Y, Lai J, et al. Structure and properties of gelatin methacryloyl (GelMA) synthesized in different reaction systems. Biomacromolecules. 2023;24(6):2928–2941. doi:10.1021/acs.biomac.3c00302
- Kočí Z, Sridharan R, Hibbitts AJ, et al. The use of genipin as an effective, biocompatible, anti-inflammatory cross-linking method for nerve guidance conduits. Adv Biosyst. 2020;4(3):1900212. doi:10.1002/adbi.201900212
- Trengove A, Duchi S, Onofrillo C, et al. Microbial transglutaminase improves ex vivo adhesion of gelatin methacryloyl hydrogels to human cartilage. Front Med Technol. 2021;3:773673. doi:10.3389/fmedt.2021.773673
- Chansoria P, Asif S, Polkoff K, et al. Characterizing the effects of synergistic thermal and photo-cross-linking during biofabrication on the structural and functional properties of gelatin methacryloyl (GelMA) hydrogels. ACS Biomater Sci Eng. 2021;7(11):5175–5188. doi:10.1021/acsbiomaterials.1c00635
- Xu H, Casillas J, Krishnamoorthy S, et al. Effects of irgacure 2959 and lithium phenyl-2,4,6-trimethylbenzoylphosphinate on cell viability, physical properties, and microstructure in 3D bioprinting of vascular-like constructs. Biomed Mater (Bristol). 2020;15(5):82–91. doi:10.1088/1748-605X/ab954e
- Kielbassa C. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis. 1997;18(4):811–816. doi:10.1093/carcin/18.4.811
- Kong X, Mohanty SK, Stephens J, et al. Comparative analysis of different laser systems to study cellular responses to DNA damage in mammalian cells. Nucleic Acids Res. 2009;37(9):e68. doi:10.1093/nar/gkp221
- Fedorovich NE, Oudshoorn MH, van Geemen D, et al. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30(3):344–353. doi:10.1016/j.biomaterials.2008.09.037
- Noshadi I, Hong S, Sullivan KE, et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater Sci. 2017;5(10):2093–2105. doi:10.1039/C7BM00110J
- Occhetta P, Visone R, Russo L, et al. VA-086 methacrylate gelatine photopolymerizable hydrogels: a parametric study for highly biocompatible 3D cell embedding. J Biomed Mater Res A. 2015;103(6):2109–2117. doi:10.1002/jbm.a.35346
- Biazar E, Najafi S M, Heidari K S, et al. 3D bio-printing technology for body tissues and organs regeneration. J Med Eng Technol. 2018;42(3):187–202. doi:10.1080/03091902.2018.1457094
- Xu H-Q, Liu J-C, Zhang Z-Y, et al. A review on cell damage, viability, and functionality during 3D bioprinting. Mil Med Res. 2022;9(1):70. doi:10.1186/s40779-022-00429-5
- Adhikari J, Roy A, Das A, et al. Effects of processing parameters of 3D bioprinting on the cellular activity of bioinks. Macromol Biosci. 2021;21(1):2000179. doi:10.1002/mabi.202000179
- Gironi P, Petraro L, Santoni S, et al. A computational model of cell viability and proliferation of extrusion-based 3D-bioprinted constructs during tissue maturation process. Int J Bioprint. 2023;9(4):741. doi:10.18063/ijb.741
- Thakare K, Jerpseth L, Pei Z, et al. Green bioprinting with layer-by-layer photo-crosslinking: a designed experimental investigation on shape fidelity and cell viability of printed constructs. J Manufact Mater Process. 2022;6(2):45. doi:10.3390/jmmp6020045
- Chand R, Muhire BS, Vijayavenkataraman S. Computational fluid dynamics assessment of the effect of bioprinting parameters in extrusion bioprinting. Int J Bioprint. 2022;8(2):545. doi:10.18063/ijb.v8i2.545
- Lim KS, Galarraga JH, Cui X, et al. Fundamentals and applications of photo-cross-linking in bioprinting. Chem Rev. 2020;120(19):10662–10694. doi:10.1021/acs.chemrev.9b00812
- Elkhoury K, Zuazola J, Vijayavenkataraman S. Bioprinting the future using light: a review on photocrosslinking reactions, photoreactive groups, and photoinitiators. SLAS Technol. 2023;28(3):142–151. doi:10.1016/j.slast.2023.02.003
- Billiet T, Gevaert E, De Schryver T, et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. doi:10.1016/j.biomaterials.2013.09.078
- Bertassoni LE, Cardoso JC, Manoharan V, et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014;6(2):024105. doi:10.1088/1758-5082/6/2/024105
- Liu W, Heinrich MA, Zhou Y, et al. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv Healthc Mater. 2017;6(12):1601451. doi:10.1002/adhm.201601451
- Wang W, Zhu Y, Liu Y, et al. 3D bioprinting of DPSCs with GelMA hydrogel of various concentrations for bone regeneration. Tissue Cell. 2024;88:102418. doi:10.1016/j.tice.2024.102418
- Madrid-Sánchez A, Duerr F, Nie Y, et al. Fabrication of large-scale scaffolds with microscale features using light sheet stereolithography. Int J Bioprint. 2022;9(2):650. doi:10.18063/ijb.v9i2.650
- Levato R, Dudaryeva O, Garciamendez-Mijares CE, et al. Light-based vat-polymerization bioprinting. Nat Rev Methods Primer. 2023;3(1):47. doi:10.1038/s43586-023-00231-0
- Ng WL, Lee JM, Zhou M, et al. Vat polymerization-based bioprinting—process, materials, applications and regulatory challenges. Biofabrication. 2020;12(2):022001. doi:10.1088/1758-5090/ab6034
- Ruskowitz ER, DeForest CA. Proteome-wide analysis of cellular response to ultraviolet light for biomaterial synthesis and modification. ACS Biomater Sci Eng. 2019;5(5):2111–2116. doi:10.1021/acsbiomaterials.9b00177
- Sun W, Liu Z, Xu J, et al. 3D skin models along with skin-on-a-chip systems: a critical review. Chin Chem Lett. 2023;34(5). doi:10.1016/j.cclet.2022.107819
- Shi H, Li Y, Xu K, et al. Advantages of photo-curable collagen-based cell-laden bioinks compared to methacrylated gelatin (GelMA) in digital light processing (DLP) and extrusion bioprinting. Mater Today Bio. 2023;23:100799. doi:10.1016/j.mtbio.2023.100799
- Li W, Wang M, Ma H, et al. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience. 2023;26(2):106039.
- Sarker MD, Naghieh S, Sharma NK, et al. Bioprinting of vascularized tissue scaffolds: influence of biopolymer, cells, growth factors, and gene delivery. J Healthc Eng. 2019;2019:9156921. doi:10.1155/2019/9156921
- Wang Z, Jin X, Dai R, et al. An ultrafast hydrogel photocrosslinking method for direct laser bioprinting. RSC Adv. 2016;6(25):21099–21104. doi:10.1039/c5ra24910d
- Wang Z, Kumar H, Tian Z, et al. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting. ACS Appl Mater Interfaces. 2018;10(32):26859–26869. doi:10.1021/acsami.8b06607
- Kumar H, Sakthivel K, Mohamed MGA, et al. Designing gelatin methacryloyl (GelMA)-based bioinks for visible light stereolithographic 3D biofabrication. Macromol Biosci. 2021;21(1):2000317. doi:10.1002/mabi.202000317
- Ng WL, Huang X, Shkolnikov V, et al. Polyvinylpyrrolidone-based bioink: influence of bioink properties on printing performance and cell proliferation during inkjet-based bioprinting. Biodes Manuf. 2023;6(6):676–690. doi:10.1007/s42242-023-00245-3
- Ng WL, Huang X, Shkolnikov V, et al. Controlling droplet impact velocity and droplet volume: key factors to achieving high cell viability in sub-nanoliter droplet-based bioprinting. Int J Bioprint. 2021;8(1):424. doi:10.18063/ijb.v8i1.424
- Ng WL, Shkolnikov V. Optimizing cell deposition for inkjet-based bioprinting. Int J Bioprint. 2024;10(2):2135. doi:10.36922/ijb.2135
- Suntornnond R, Ng WL, Huang X, et al. Improving printability of hydrogel-based bio-inks for thermal inkjet bioprinting applications via saponification and heat treatment processes. J Mater Chem B. 2022;10(31):5989–6000. doi:10.1039/D2TB00442A
- Afewerki S, Sheikhi A, Kannan S, et al. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: towards natural therapeutics. Bioeng Transl Med. 2019;4(1):96–115. doi:10.1002/btm2.10124
- Krishnamoorthy S, Zhang Z, Xu C. Biofabrication of three-dimensional cellular structures based on gelatin methacrylate–alginate interpenetrating network hydrogel. J Biomater Appl. 2019;33(8):1105–1117. doi:10.1177/0885328218823329
- Aldana AA, Valente F, Dilley R, et al. Development of 3D bioprinted GelMA-alginate hydrogels with tunable mechanical properties. Bioprinting. 2021;21:e00105. doi:10.1016/j.bprint.2020.e00105
- Melchels FPW, Dhert WJA, Hutmacher DW, et al. Development and characterisation of a new bioink for additive tissue manufacturing. J Mater Chem B. 2014;2(16):2282–2289. doi:10.1039/c3tb21280g
- Mouser VHM, Melchels FPW, Visser J, et al. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication. 2016;8(3):035003. doi:10.1088/1758-5090/8/3/035003
- Chen Y, Le Y, Yang J, et al. 3D bioprinted xanthan hydrogels with dual antioxidant and chondrogenic functions for post-traumatic cartilage regeneration. ACS Biomater Sci Eng. 2024;10(3):1661–1675. doi:10.1021/acsbiomaterials.3c01636
- Baniasadi H, Kimiaei E, Polez RT, et al. High-resolution 3D printing of xanthan gum/nanocellulose bio-inks. Int J Biol Macromol. 2022;209:2020–2031. doi:10.1016/j.ijbiomac.2022.04.183
- Taniguchi Nagahara MH, Caiado Decarli M, Inforçatti Neto P, et al. Crosslinked alginate-xanthan gum blends as effective hydrogels for 3D bioprinting of biological tissues. J Appl Polym Sci. 2022;139(28):e52612. doi:10.1002/app.52612
- Iervolino F, Belgio B, Bonessa A, et al. Versatile and non-cytotoxic GelMA-xanthan gum biomaterial ink for extrusion-based 3D bioprinting. Bioprinting. 2023;31:e00269. doi:10.1016/j.bprint.2023.e00269
- Garcia-Cruz MR, Postma A, Frith JE, et al. Printability and bio-functionality of a shear thinning methacrylated xanthan–gelatin composite bioink. Biofabrication. 2021;13(3):035023. doi:10.1088/1758-5090/abec2d
- Rastin H, Ormsby RT, Atkins GJ, et al. 3D bioprinting of methylcellulose/gelatin-methacryloyl (MC/GelMA) bioink with high shape integrity. ACS Appl Bio Mater. 2020;3(3):1815–1826. doi:10.1021/acsabm.0c00169
- Lam T, Dehne T, Krüger JP, et al. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J Biomed Mater Res B Appl Biomater. 2019;107(8):2649–2657. doi:10.1002/jbm.b.34354
- Shopperly LK, Spinnen J, Krüger JP, et al. Blends of gelatin and hyaluronic acid stratified by stereolithographic bioprinting approximate cartilaginous matrix gradients. J Biomed Mater Res B Appl Biomater. 2022;110(10):2310–2322. doi:10.1002/jbm.b.35079
- Yoon S, Park JA, Lee H, et al. Inkjet–spray hybrid printing for 3D freeform fabrication of multilayered hydrogel structures. Adv Healthc Mater. 2018;7(14):1800050. doi:10.1002/adhm.201800050
- Kumar S. Synthetic polymer-derived single-network inks/bioinks for extrusion-based 3D printing towards bioapplications. Mater Adv. 2021;2(21):6928–6941. doi:10.1039/D1MA00525A
- Kim SH, Park SJ, Xu B, et al. Development of polycaprolactone grafts with improved physical properties and body stability using a screw extrusion-type 3D bioprinter. Int J Bioprint. 2023;9(2):652. doi:10.18063/ijb.v9i2.652
- Yeong WY, Sudarmadji N, Yu HY, et al. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010;6:2028–2034. doi:10.1016/j.actbio.2009.12.033
- Buyuksungur S, Hasirci V, Hasirci N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J Biomed Mater Res A. 2021;109(12):2425–2437. doi:10.1002/jbm.a.37235
- Jia W, Gungor-Ozkerim PS, Zhang YS, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi:10.1016/j.biomaterials.2016.07.038
- Tilton M, Camilleri ET, Potes MDA, et al. Visible light-induced 3D bioprinted injectable scaffold for minimally invasive tissue regeneration. Biomater Adv. 2023;153. doi:10.1016/j.bioadv.2023.213539
- Gao J, Li M, Cheng J, et al. 3D-Printed GelMA/PEGDA/F127DA scaffolds for bone regeneration. J Funct Biomater. 2023;14(2):96. doi:10.3390/jfb14020096
- Gao J, Wang H, Li M, et al. DLP-printed GelMA-PMAA scaffold for bone regeneration through endochondral ossification. Int J Bioprint. 2023;9(5):754. doi:10.18063/ijb.754
- Kyriakides TR, Kim H-J, Zheng C, et al. Foreign body response to synthetic polymer biomaterials and the role of adaptive immunity. Biomed Mater. 2022;17(2):022007. doi:10.1088/1748-605X/ac5574
- Bhatia S. Natural polymers vs synthetic polymer. In: Bhatia S, editor. Natural polymer drug delivery systems. Cham: Springer International Publishing; 2016. p. 95–118. doi:10.1007/978-3-319-41129-3_3
- Gao G, Schilling AF, Hubbell K, et al. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol Lett. 2015;37(11):2349–2355. doi:10.1007/s10529-015-1921-2
- Leu Alexa R, Cucuruz A, Ghițulică CD, et al. 3D printable composite biomaterials based on GelMA and hydroxyapatite powders doped with cerium ions for bone tissue regeneration. Int J Mol Sci. 2022;23(3):1841. doi:10.3390/ijms23031841
- Wenz A, Borchers K, Tovar GEM, et al. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication. 2017;9(4):044103. doi:10.1088/1758-5090/aa91ec
- Osi AR, Zhang H, Chen J, et al. Three-Dimensional-Printable thermo/photo-cross-linked methacrylated chitosan–gelatin hydrogel composites for tissue engineering. ACS Appl Mater Interfaces. 2021;13(19):22902–22913. doi:10.1021/acsami.1c01321
- Kosik-Kozioł A, et al. 3D bioprinted hydrogel model incorporating β-tricalcium phosphate for calcified cartilage tissue engineering. Biofabrication. 2019;11(3):035016. doi:10.1088/1758-5090/ab15cb
- Choi E, Kim D, Kang D, et al. 3D-printed gelatin methacrylate (GelMA)/silanated silica scaffold assisted by two-stage cooling system for hard tissue regeneration. Regen Biomater. 2021;8(2):rbab001. doi:10.1093/rb/rbab001
- Chimene D, Miller L, Cross LM, et al. Nanoengineered osteoinductive bioink for 3D bioprinting bone tissue. ACS Appl Mater Interfaces. 2020;12(14):15976–15988. doi:10.1021/acsami.9b19037
- Liu C, Dai T, Wu X, et al. 3D bioprinting of cell-laden nano-attapulgite/gelatin methacrylate composite hydrogel scaffolds for bone tissue repair. J Mater Sci Technol. 2023;135:111–125. doi:10.1016/j.jmst.2022.07.011
- Cidonio G, Alcala-Orozco CR, Lim KS, et al. Osteogenic and angiogenic tissue formation in high fidelity nanocomposite laponite-gelatin bioinks. Biofabrication. 2019;11(3):035027. doi:10.1088/1758-5090/ab19fd
- Wang J, Zhou D, Wang G, et al. Enhanced bone regeneration with bioprinted GelMA/bentonite scaffolds inspired by bone matrix. Virtual Phys Prototyp. 2024;19(1):e2345765. doi:10.1080/17452759.2024.2345765
- Xavier Mendes A, Moraes Silva S, O'Connell CD, et al. Enhanced electroactivity, mechanical properties, and printability through the addition of graphene oxide to photo-cross-linkable gelatin methacryloyl hydrogel. ACS Biomater Sci Eng. 2021;7(6):2279–2295. doi:10.1021/acsbiomaterials.0c01734
- Jiang Y, Zhou D, Yang B. 3D bioprinted GelMA/GO composite induces osteoblastic differentiation. J Biomater Appl. 2022;37(3):527–537. doi:10.1177/08853282221098235
- Zhang X, Zhang H, Zhang Y, et al. 3D printed reduced graphene oxide-GelMA hybrid hydrogel scaffolds for potential neuralized bone regeneration. J Mater Chem B. 2023;11(6):1288–1301. doi:10.1039/D2TB01979E
- Cernencu AI, Vlasceanu GM, Serafim A, et al. 3D double-reinforced graphene oxide – nanocellulose biomaterial inks for tissue engineered constructs. RSC Adv. 2023;13(34):24053–24063. doi:10.1039/D3RA02786D
- Boularaoui S, Shanti A, Lanotte M, et al. Nanocomposite conductive bioinks based on Low-concentration GelMA and MXene nanosheets/gold nanoparticles providing enhanced printability of functional skeletal muscle tissues. ACS Biomater Sci Eng. 2021;7(12):5810–5822. doi:10.1021/acsbiomaterials.1c01193
- Lee SH, et al. 3D bioprinting of human mesenchymal stem cells-laden hydrogels incorporating MXene for spontaneous osteodifferentiation. Heliyon. 2023;9(3):e14490. doi:10.1016/j.heliyon.2023.e14490
- Moghimi N, Kamaraj M, Zehtabi F, et al. Development of bioactive short fiber-reinforced printable hydrogels with tunable mechanical and osteogenic properties for bone repair. J Mater Chem B. 2024;12(11):2818–2830. doi:10.1039/d3tb02924g
- Yu X, Gholipourmalekabadi M, Wang X, et al. Three-dimensional bioprinting biphasic multicellular living scaffold facilitates osteochondral defect regeneration. Interdiscip Mater. 2024:1–19. doi:10.1002/idm2.12181
- Su JJ-M, Lin C-H, Chen H, et al. Biofabrication of cell-laden gelatin methacryloyl hydrogels with incorporation of silanized hydroxyapatite by visible light projection. Polymers (Basel). 2021;13(14):2354. doi:10.3390/polym13142354
- Song P, Gui X, Wu L, et al. DLP fabrication of multiple hierarchical biomimetic GelMA/SilMA/HAp scaffolds for enhancing bone regeneration. Biomacromolecules. 2024;25(3):1871–1886. doi:10.1021/acs.biomac.3c01318
- Anada T, Pan C, Stahl AM, et al. Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int J Mol Sci. 2019;20(5):1096. doi:10.3390/ijms20051096
- Choi C-E, Chakraborty A, Adzija H, et al. Metal organic framework-incorporated three-dimensional (3D) bio-printable hydrogels to facilitate bone repair: preparation and in vitro bioactivity analysis. Gels. 2023;9(12):923–2023. doi:10.3390/gels9120923
- Izgordu MS, Ayran M, Ulag S, et al. Fabrication of gentamicin sulfate-loaded 3D-printed polyvinyl alcohol/sodium alginate/gelatin-methacryloyl hybrid scaffolds for skin tissue replacement. Macromol Mater Eng. 2023;308(12):2300151. doi:10.1002/mame.202300151
- Vigata M, O’Connell CD, Cometta S, et al. Gelatin methacryloyl hydrogels for the localized delivery of cefazolin. Polymers (Basel). 2021;13(22):3960. doi:10.3390/polym13223960
- Martínez-Pérez D, Guarch-Pérez C, Purbayanto MAK, et al. 3D-printed dual drug delivery nanoparticleloaded hydrogels to combat antibiotic-resistant bacteria. Int J Bioprint. 2023;9(3):683. doi:10.18063/ijb.683
- Shahbazi M, Jäger H, Ettelaie R, et al. Multimaterial 3D printing of self-assembling smart thermo-responsive polymers into 4D printed objects: a review. Addit Manuf. 2023;71:103598. doi:10.1016/j.addma.2023.103598
- Lai J, Wang M. Developments of additive manufacturing and 5D printing in tissue engineering. J Mater Res. 2023;38(21):4692–4725. doi:10.1557/s43578-023-01193-5
- Li Y. Modern epigenetics methods in biological research. Methods. 2021;187:104–113. doi:10.1016/j.ymeth.2020.06.0221
- Han P, Gomez GA, Duda GN, et al. Scaffold geometry modulation of mechanotransduction and its influence on epigenetics. Acta Biomater. 2023;163:259–274. doi:10.1016/j.actbio.2022.01.020
- Man K, Alcala C, Mekhileri NV, et al. GelMA hydrogel reinforced with 3D printed PEGT/PBT scaffolds for supporting epigenetically-activated human bone marrow stromal cells for bone repair. J Funct Biomater. 2022;13(2):41. doi:10.3390/jfb13020041
- Man K, Barroso A, Brunet MY, et al. Controlled release of epigenetically-enhanced extracellular vesicles from a GelMA/nanoclay composite hydrogel to promote bone repair. Int J Mol Sci. 2022;23(2):832. doi:10.3390/ijms23020832
- Oh D, Shirzad M, Chang Kim M, et al. Rheology-informed hierarchical machine learning model for the prediction of printing resolution in extrusion-based bioprinting. Int J Bioprint. 2023;9(6):1280. doi:10.36922/ijb.1280
- Sun J, Yao K, An J, et al. Machine learning and 3D bioprinting. Int J Bioprint. 2023;9(4):717. doi:10.18063/ijb.717
- Ng WL, Goh GL, Goh GD, et al. Progress and opportunities for machine learning in materials and processes of additive manufacturing. Adv Mater. 2024:2310006. doi:10.1002/adma.202310006