Abstract
Background Different fracture fixation techniques and fracture environment influence bone formation in healing fractures. However, the influence on the development of biomechanical properties has not been clear described. We evaluated the influence of fracture fixation stability and fracture environment on mechanical properties in healing femoral fractures in rats.
Methods Animals were treated surgically with external fixation: 1 group (27 rats) with 0-mm fracture gap size with bone ends touching, corresponding to an axial stiffness of 265 (SD 34) N/mm, and a second group (27 rats) with 2-mm fracture gap size corresponding to an axial stiffness of 30 (SD 2.1) N/mm. From each group, 6–7 animals were killed at 2, 4, 6 and 12 weeks. Torsional test revealed a delay in torsional stiffness in fractures in group 2 compared to group 1. In group 2, the torsional stiffness of the contralateral femora was found to be greater at 12 weeks than the torsional stiffness in group 1.
Interpretation We found that during fracture healing, the development of torsional stiffness corresponds to the magnitude of endochondral ossification and late response of bone formation. A significantly increased torsional stiffness in the non-fractured leg of rats with delayed fracture healing was also found, possibly indicating a response to loading conditions or a systemic stimulation of bone mass.
A number of clinical and experimental studies have illustrated how factors such as fracture type, gap condition, fixation rigidity, loading and biological environment influence stability in fracture repair (Lewallen et al. Citation1984, Forwood and Parker Citation1986, Claes et al. Citation1998, Chao et al. Citation1989, Meadows et al. Citation1990). Rigidly fixated undisplaced fractures have been shown to lead to direct osteonal healing, whereas intramembranous and/or endochondral ossification occurs in less rigidly fixated displaced fractures (Einhorn Citation1998).
To explain the mechanical influence on differentiation of skeletal tissue during fracture repair, several mechano-biological concepts have been developed, mainly finite element models (Carter Citation1998). Basically, these concepts assume that in the initial phase of tissue regeneration in fracture healing, bone formation is permitted in areas of low to moderate tensile strain, fibrous tissue is promoted in areas of moderate to high tensile strain, and that there is chondrogenesis in areas with hydrostatic compressive stress (Carter Citation1998). In addition, poor vascularity and low oxygen tension, possibly explained by increased hydrostatic pressure, have been shown to stimulate undifferentiated mesenchymal cells into a chondrogenic pathway (Carter Citation1998). The mechanical properties of different fracture fixations has been related to later mechanical and histological site-specific properties in healing fractures (Augat et al. Citation1998). However, it is not known whether changes in site-specific histological properties during fracture healing can influence fracture stability.
We investigate the possible influence of fracture environment and stability of fracture fixation on mechanical properties during fracture healing.
Animals and methods
Animals and anesthesia
We used 59 male Sprague-Dawley rats weighing 350–450 g. The animals were anesthetized by intraperitoneal injection of 1 mL of a solution of Ketalar (50 mg/mL, Park Davis AB, Solna, Sweden) and Stesolid (5 mg/mL, Dumex Alpharma AB, Stockholm, Sweden) in a proportion of 4:1. Before surgery Dalacin, a broad-spectrum antibiotic (Pharmacia and Upjohn Sverige AB, Solna, Sweden; 4 mg/kg body weight) was given intramuscularly and Temgesic (Meda Sverige AB, Göteborg, Sweden; 0.01–0.05 mg/kg body weight) was given subcutaneously after surgery. Postoperatively, the animals were caged in pairs; unrestricted weight bearing and activity were allowed. Water was provided ad libitum and the rats were monitored daily. 5 animals were excluded, 3 because of infection at the skin-penetration sites of the fixator pins, 1 because of unexplained death, and 1 because of mechanical test failure. We obtained research ethics committee approval for the study.
Surgical protocol
Mark et al. (Citation2003) have described a reliable and reproducible method for experimental external fixation of fractures in the femur of rats, which we used in this study. Briefly, a curved incision was made through the skin from the tail to the knee, creating a skin flap followed by dissection between the quadriceps and the hamstrings. A tunnel was dissected around the mid-portion of the femur and a selflocking nylon strap was pulled through, temporary fixing a drill guide on the lateral aspect of the bone. Four 0.8-mm holes were drilled and tapped. Four 1.2-mm pins were inserted through the holes in the bone and were cannulated through the skin flap. A fixator was fastened to the pins at a preset distance from the bone surface. An osteotomy was done between the two middle pins using a reciprocating saw. Using a screw for distraction or compression of the fixator, the bone fragments were moved to a preset fixator setting such as fracture gap size.
The osteotomies were stabilized with 1.2-mm pins and an offset of 6 mm. We used 2 fixator settings (fracture gap sizes). Group 1 consisted of 27 animals with 0-mm fracture gap size, and with bone ends touching. This has been shown to correspond to an axial fixation stiffness of 265 (SD 34) N/mm. Group 2 consisted of 27 animals with 2-mm fracture gap size, corresponding to an axial fixation stiffness of 30 (SD 2.1) N/mm (Mark et al. Citation2003). From each group, 6–7 animals were killed at 2, 4, 6, and 12 weeks of fracture healing.
Mechanical testing
We dissected the fractured and contralateral leg, keeping the surrounding soft tissue (muscle, periosteum and fascia) of the femora intact. The fixator was removed but the pin-bone interface was kept intact. The specimens were stored frozen (−20°C) until the time of analysis. Before measurement, specimens were thawed and carefully cleared from the remaining soft tissue. We took care to standardize the time of thawing and not to dry the specimens. The positions of the pins and the fracture were measured and marked at the corresponding position on the contralateral femur of the same animal.
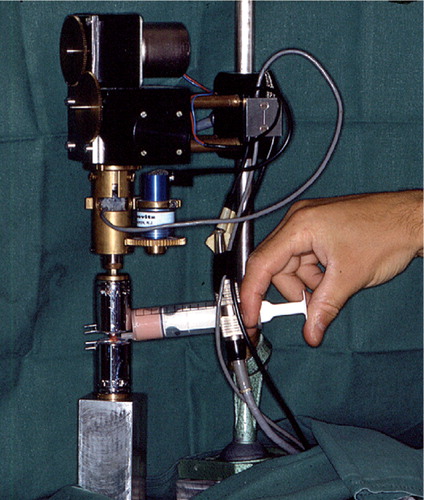
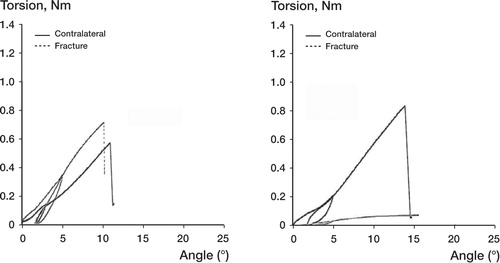
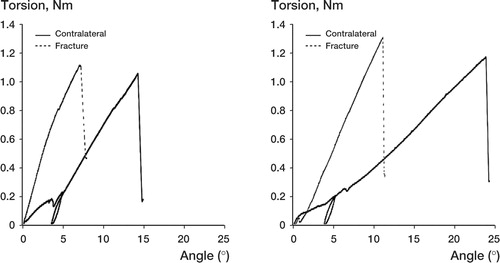
At first, the knee joint was fixed in a holding clamp that allowed the bone to be lowered and centered into a testing jig. A polymer monomer compound (ProBase Cold, Ivoclar Ag, Schaan, Liechtenstein) was poured up to the level of the pin close to the fracture, or the corresponding mark on contralateral bones. When the polymer monomer compound was set, we fastened the jig upside down into the torque sensor of the test equipment. The procedure could be repeated to fasten the proximal end of the bone in another jig (). Specially developed torsion test equipment was used, with accuracies of ± 2% for torque and ± 0.1% for angular displacement (Brånemark and Skalak Citation1998). We applied the torque at a rate of 2°/min until failure occurred, a procedure of unload and reload was performed when the angle was 5°, and recording was performed until failure occurred. A computerized torque-angle displacement graph was drawn up ( and ). We calculated maximum torque (at bone failure), torsion angle (number of degrees of torsion deformation in the test bone just prior to failure), and the torsion stiffness (the ratio of the maximum torque and angle at that point) (White et al. Citation1977, Nordin and Frankel Citation2001).
Statistics
We used Fisher's nonparametric permutation test for comparison between groups. In order to eliminate the influence of one extreme outlier in group 2 with 2-mm fracture defects at 4 weeks, we used the Mann Whitney nonparametric U-test. This test allows calculations of rank, which reduces the influence of extreme values, and is more appropriate in this particular case than Fisher's permutation test. All significance tests were two-tailed and conducted at the 5% significance level.
Results
Fractured leg
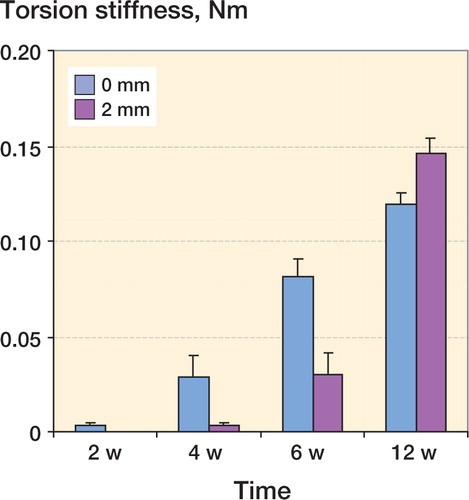
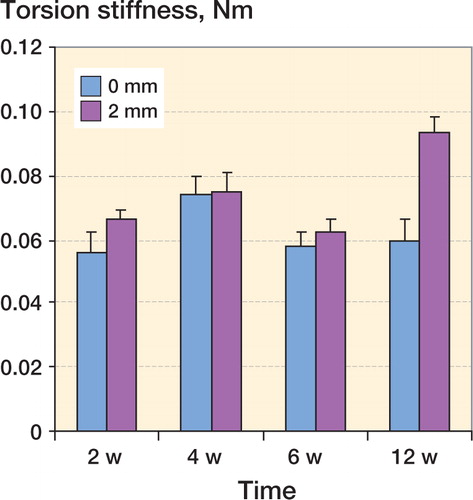
In group 1, the mean torsional stiffness of the fractures was higher than in group 2 at 2 weeks of healing (0.003, SD 0.002 Nm/°, and 0.00 Nm/°, respectively; p = 0.01), 4 weeks of healing (0.028, SD 0.011 Nm/° and 0.004, SD 0.001 Nm/°; p = 0.04) and 6 weeks of healing (0.082, SD 0.009 Nm/° and 0.03, SD 0.011 Nm/°; p = 0.007) ( and ). At 12 weeks of healing, the mean torsional stiffness of the fractures was higher in group 2 than in group 1 (0.15, SD 0.008 Nm/° and 0.12, SD 0.006 Nm/°; p = 0.04) ( and ). Maximum torque in group 1 was higher than in group 2 at 2 weeks of healing (0.019, SD 0.008 Nm and 0.003, SD 0.001 Nm; p = 0.009), 4 weeks of healing (0.17, SD 0.045 Nm and 0.027, SD 0.007 Nm; p = 0.007), and 6 weeks of healing (0.47, SD 0.056 Nm and 0.16, SD 0.047 Nm; p = 0.002). We found no difference in maximum torque between 0-mm and 2-mm fracture defects at 12 weeks of healing. There were no differences in failure angle between bones with 0-mm and 2-mm fracture defects at 2, 4, 6 and 12 weeks of healing.
Figure 3. Torsional stiffness in fractured bone with 0-mm and 2-mm fracture gap at 2, 4, 6 and 12 weeks of healing. The bars represent mean ± SE. In bone with 0-mm fracture defects, the torsion stiffness of the fractures was statistically significantly higher than in bone with 2-mm fracture defects at 2 weeks of healing (p = 0.01), 4 weeks of healing (p = 0.04), and 6 weeks of healing (p = 0.007). Due to one extreme value recorded in the test at 4 weeks, Mann-Whitney U-test was used. At 12 weeks of healing, the torsion stiffness of the fractures was statistically significantly higher in bones with 2-mm fracture defects than in bones with 0-mm fracture defects (p = 0.04) (Table 1).
Contralateral leg
There was no statistical difference in torsional stiff(). The torsional stiffness of unfractured contralateral bone in group 2 was higher at 12 weeks than torsional stiffness of unfractured contralateral bone in group 1 (0.093, SD 0.005 Nm/° and 0.059, SD 0.007 Nm/°; p = 0.006) ( and ). We found no differences in maximum torque and failure angle between the unfractured contralateral bone of rats in groups 1 and 2.
Figure 5. Torsional stiffness in the contralateral unfractured bone at 2, 4, 6 and 12 weeks in rats with 0-mm and 2-mm fracture defects. The bars represent mean ± SE. The torsion stiffness of unfractured contralateral bone in rats with 2-mm fractured defects was statistically significantly higher at 12 weeks of healing than in unfractured contralateral bone of rats with 0-mm fractured defects at 12 weeks (p = 0.006) (Table 2).
Discussion
We found a great difference in stability between the two types of fractures, i.e. the torsional stiffness in group 1 was higher than in group 2 at 2, 4 and 6 weeks of healing (). Also, the stiffness of fractures in group 1 was about 50% of the stiffness of the corresponding contralateral leg after 2 weeks, whereas the stiffness of fractures in group 2 did not reach 50% of the stiffness in the contralateral leg until after 6 weeks of healing ( and ).
These measurements are similar to those of an earlier study (Mark et al. 2004). In that study, the peak of total new bone formation (periosteal and intramedullary) occurred earlier in 0-mm fractures (2 weeks) than in 2-mm fractures (6 weeks). Also, in that study there was a substantial increase in chondroid tissue in 2-mm fractures compared to 0-mm fractures at 2, 3, 4 and 6 weeks of healing. Woven bone could also be seen bridging the 0-mm fracture gap after 1 week, whereas in a 2-mm fracture, this occurred after 4 weeks. Consequently, these data showed a later response in bone formation and prolonged time for full ossification in 2-mm fractures compared to 0-mm fractures, and we suggest that the difference in magnitude of chondroid tissue is the major reason to the differences in fracture stability that we found.
The torsional stiffness was increased compared to the contralateral leg after 6 weeks of healing in group 1, and after 12 weeks in group 2 ( and ). Thus, at these time points the stiffness of the callus is greater than the stiffness of the non-fractured contralateral leg. In the earlier study by Mark et al. (Citation2004), there was no apparent histological difference between the different fractures after 12 weeks of healing. However, in the present study we found a significant increase in torsional stiffness in group 2 compared to group 1 at 12 weeks (). Theoretically, the remodeling process is more advanced in a 0-mm fracture gap than in a 2-mm fracture after 12 weeks; hence, a possible difference in cross-sectional area may exist. To examine this further, detailed morphometrical analyses of cross sections are required.
We also tested the mechanical properties of the contralateral legs and found no differences in torsional stiffness, either at 2, 4 or 6 weeks of healing. However, at 12 weeks, torsional stiffness of the contralateral legs in group 2 was increased compared to the contralateral legs in group 1 (). In a previous study by Bak and Jensen (Citation1992) in animals with tibial fractures, no such increase in stiffness in the nonfractured legs was found during the healing period. It is reasonable to assume that animals in group 2 will, over time, load the contralateral leg more than animals in group 1; thus, the present observation is most likely explained by differences in loading pattern (Wolff's law; Turner Citation1992). The response is a slow osteoblast/osteoclast remodeling process, which explains why the difference cannot be found before 12 weeks of healing. Another explanation could be that biological factors are released, stimulating growth of the entire bone mass. Such factors might be released as a result of a general biomechanical stimulus, or by the presence of tissue-specific components in these animals.
In conclusion, we found that during fracture healing, the development of torsional stiffness in experimental fractures corresponds to the magnitude of endochondral ossification and late response of bone formation, as shown earlier by Mark and co-workers (Citation2004). We also found an increased torsional stiffness in the nonfractured legs of rats with delayed fracture healing, possibly indicating a response to loading conditions or a systemic stimulation of bone mass.
This work was perfomed at the Lundberg Laboratory for Orthopedic Research, Göteborg University, Sweden, and was funded by grants from the IngaBritt and Arne Lundberg Research Foundation and Gothenburg Medical Society. The work was supported by a grant from Gothenburg Medical Society. We wish express our thanks to Dr. Rickard Brånemark and Mrs. Julie Matthews, Integrum AB, for expert biomechanical evaluation, Nils-Gunnar Pehrson, Statistiska Konsultgruppen, Gothenburg, for statistical advice and Dr. Ulf Nannmark for valuable comments.
- Augat P, Margevicius P A K, Wolf J S S, Suger G, Claes L. Local tissue properties in bone healing: influences of size and stability of the osteotomy gap. J Orthop Res 1998; 16: 475–81
- Bak B, Jensen K S. Standardization of tibial fractures in the rat. Bone 1992; 13: 289–95
- Brånemark R, Skalak R. An in-vivo method for biomechanical characterization of bone anchored implants. Med Eng Phys 1998; 20: 216–9
- Carter D A. Mechanobiology of skeletal regeneration. Clin Orthop 1998, Suppl 355: S41–S55
- Chao E Y S, Aro H T, Lewallen D G, Kelly P J. The effect of rigidity on fracture healing in external fixation. Clin Orthop 1989, 241: 24–35
- Claes L E, Heigele C A, Neidlinger-Wilke C, Kaspar D, Seidle W, Margevicius K J, Augat P. Effects of mechnical factors on the fracture healing process. Clin Orthop 1998, Suppl 355: S132–S47
- Einhorn T A. The cell and molecular biology of fracture healing. Clin Orthop 1998, Suppl 355: S7–S21
- Forwood M R, Parker W A. Effects of exercise on bone morphology. Acta Orthop Scand 1986; 57: 204–7
- Lewallen D G, Chao E Y S, Kasman R A, Kelly P J. Comparison of the effects of compression plates and external fixators on early bone-healing. J Bone and Joint Surg (Am) 1984; 66: 1084–91
- Mark H, Bergholm J, Nilsson A, Rydevik B, Strömberg L. An external fixation method and device to study fracture healing in rats. Acta Orthop Scand 2003; 74(4)476–82
- Mark H, Nilsson A, Nannmark U, Rydevik B. Effects of fracture fixation stability on ossification in healing fractures. Clin Orthop 2004, 419: 245–50
- Meadows T H, Bronk J T, Chao E Y S, Kelly P J. Effect of weight-bearing on healing of cortical defects in the canine tibia. J Bone and Joint Surg (Am) 1990; 72: 1074–80
- Nordin M, Frankel V H. Basic biomechanics of the musculoskeletal system. Lippingcott Williams & Wilkins, Philadelphia, PA 2001
- Turner C H. Editorial: Functional determinants of bone structure: Beyond Wolff's law of bone transformation. Bone 1992; 13: 403–9
- White A A, Panjabi M M, Southwick W O. The four biomechanical stages of fracture repair. J Bone and Joint Surg (Am) 1977; 59: 188–92