Abstract
Background Periosteum and periosteum-derived progenitor cells have demonstrated the potential for stimulative applications in repair of various musculoskeletal tissues. It has been found that the periosteum contains mesenchymal progenitor cells that are capable of differentiating into either osteoblasts or chondrocytes, depending on the culture conditions. Anatomically, the periosteum is a heterogeneous multilayered membrane, consisting of an outer fibrous and an inner cambium layer. The present study was designed to elucidate the phenotypic characteristics of fibrous and cambium layer cells in vitro.
Methods Using a sequential enzymatic digestion method, fibrous and cambium layer cells were harvested separately from periosteum-bone explants of the proximal tibia of 6-month-old New Zealand White rabbits.
Results We found that the cells from each layer showed distinct phenotypic characteristics in a primary monolayer culture system. Specifically, the cambium cells demonstrated higher osteogenic characteristics (higher alkaline phosphatase and osteocalcin levels) than the fibrous cells. However, these differences diminished with time in vitro.
Interpretation Our findings suggest that the periosteum has phenotypically distinct heterogeneous cell populations. Care must be taken in order to identify and distinguish the intrinsic phenotypes of the heterogeneous periosteum-derived cell types in vitro.
The periosteum covers all surfaces of cortical bone except the articulating joints, and plays an important role in supporting osteoblast differentiation during the processes of bone development and healing (Jungueira et al. Citation1998). In the fracture healing process, periosteal precursor cells initially differentiate into chondrocytes and form a cartilage-like soft callus, which is eventually replaced by calcified bone matrix (McKibbin Citation1978, Sandberg et al. Citation1989). Many investigators have made use of this osteogenic potential of the periosteum or the periosteum-derived cells for tissue engineering applications. For example, a fresh periosteum explant has been used in conjunction with a host bone (Nishimura et al. Citation1997, Letts et al. Citation1998, Reynders et al. Citation1999) or a biodegradable polymer scaffold (Isogai et al. Citation1999, Thomson et al. Citation1999) to tissue-engineer a new bone. A fresh periosteum patch has also been used to enhance the healing and structural integration of tendon graft in bone tunnel (Youn et al. Citation2004). Isolated periosteal cells have also been used to induce new bone formation in vivo (Breitbart et al. Citation1998, Takushima et al. Citation1998) or to tissue-engineer a bone in a culture chamber in vitro (Isogai et al. Citation2000). In addition to osteogenesis, chondrogenesis, has also been observed with isolated periosteum or periosteum-derived cell transplants in various tissues (Poussa and Ritsila Citation1979, O'Driscoll et al. Citation1986, Citation1994, Wakitani et al. Citation1994). Many workers have investigated the autologous periosteal graft (periosteal artrhoplasty) as a means of cartilage repair in both animal models and humans (Niedermann et al. Citation1985, O'Driscoll et al. Citation1986, Korkala Citation1988, Hoikka et al. Citation1990).
Such multi-potentiality of the periosteum in osteogenesis and chondrogenesis may be related to the heterogeneous characteristics of the periosteum. Anatomically, the periosteum is a heterogeneous multilayered membrane consisting of an outer fibrous layer and an inner cambium layer (Tonna and Cronkite Citation1963, Wlodarski Citation1989). While the outer fibrous layer of the periosteum is composed of fibroblastic cells immersed in the collagenous matrix (Wlodarski Citation1989), the inner cambium layer contains a variety of cellular types including osteo-progenitor cells, osteoblasts, and osteoclasts (Poussa and Ritsila Citation1979, Burger et al. Citation1986, Koshihara et al. Citation1989). Several decades ago, it was suggested that the mechanism of bone or cartilage formation was directly related to proliferation of the cells of the cambium layer (Tonna and Cronkite Citation1963). However, Ueno et al. Citation(2001) demonstrated that when the periosteum harvested from rabbit tibia was grafted into suprahyoid muscles, the fibrous layer cells differentiated into chondrocytes and formed cartilage tissue which was later replaced by trabecular bone, whereas the cambium layer cells appeared to be necrotic and subsequently disappeared.
To understand the biological mechanisms regulating the osteogenic or chondrogenic potential of periosteum, many investigators have separated periosteum patches from the bone surface, liberated cells from the isolated periosteum using enzymatic digestion methods, and expanded the cell population on Petri dishes in an incubator (Koshihara et al. Citation1989, Deren et al. Citation1990, Nakahara et al. Citation1990 a, Citationb, Izumi et al. Citation1992, Breitbart et al. Citation1999, Bahrami et al. Citation2000, Gruber et al. Citation2001). However, such enzymatic digestion methods could result in two possible experimental artifacts. Firstly, when a periosteum patch is elevated from bone, it is very likely that the cambium layer is either damaged or separated from the periosteum and remains behind on the bone (O’Driscoll and Fitzsimmons Citation2000). Secondly, considering that the periosteum is a heterogeneous tissue, enzymatic digestion of the entire periosteum patch results in a mixed population of heterogeneous cells from both the fibrous and cambium layers. Without taking the heterogeneity in the periosteal cell populations into account, Nakahara et al. (Citation1990 a, Citationb) reported that the harvested cells from chick periosteum had the potential to differentiate into osteoblasts or chondrocytes when inoculated in vivo.
An accurate understanding of the phenotypic characteristics of periosteum is crucial for achieving controllable and predictable results in periosteum-based tissue engineering applications. Thus, the aims of the present study were to develop a new sequential enzymatic digestion method to selectively harvest the fibrous and cambium layer cells in periosteum, and to elucidate the characteristics of the heterogeneous cell population in periosteum in a monolayer culture environment using histo-morphology and biochemical assays. We hypothesized that the phenotypic characteristics of periosteal progenitor cells are distinctively specific to the fibrous and cambium layers, and that such pheno-typic distinction between the cells of the two layers would diminish with time in a standard monolayer cell culture system. The phenotypic characteristics of the cells in vitro were evaluated in terms of their morphology, their ability to proliferate, and production of alkaline phosphatase and osteocalcin.
Material and methods
Sequential enzymatic digestion method for harvesting of cells
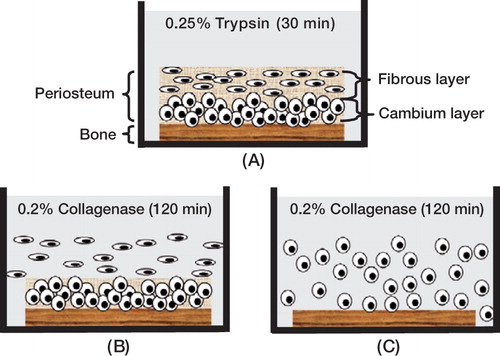
54 intact periosteum-bone blocks (about 5 × 10 mm) were harvested from the proximal medial area of both the right and left tibiae of 24 6-month-old New Zealand White (NZW) rabbits. After being completely cleaned of muscles and bone marrow, the periosteum-bone complexes were treated briefly with 0.25% trypsin for 30 min (). The samples were then digested with 0.2% Type II collagenase (17101-015, Invitrogen Corp., CA) for two sequential 2-h periods at 37°C in 5% CO2. As the collagenase reacts first on the outer fibrous layer of the periosteum, most of the cells from the fibrous layer become liberated during the first 2-h period (). The periosteal fibrous cells released during this first digestion period were harvested and transferred to 6-well plates at a density of 1 × 105 cells/well. The remaining periosteum-bone complexes were then digested with collage-nase for another 2 h (). The periosteal cells released during this second digestion period were mostly cells from the cambium layer. These cells were harvested and transferred to 6-well plates at a density of 1 × 105 cells/well. Harvested fibrous and cambium layer cells were cultured separately in α-MEM (#12561-049, Invitrogen, CA) supplemented with 10% fetal bovine serum (FBS, 16000-036, Invitrogen), 100 nM dexamethasone (D-4902, Sigma), 20 mM β-glycerol phosphate (G-9891, Sigma), and 50 μg/mL ascorbic acid (A-8960, Sigma) at 37°C in 5% CO2. The culture medium was changed every other day. Cell morphology was monitored under a phase contrast microscope.
Figure 1. Schematic diagram of sequential enzymatic digestion method.(A) Intact bone-periosteum complex was first treated with 0.2% trypsin for 30 min.(B) The majority of fibrous layer cells were liberated during the first 2-h collagenase digestion.(C) The remaining cambium layer cells were isolated after the second 2-h collagenase digestion.
In order to confirm the validity of the sequential digestion method, the periosteum-bone complexes (n = 4) were examined histologically after each digestion step. Briefly, the periosteum-bone complexes were fixed in 10% formalin overnight, decalcified with Decal (#1211A, Decal Corp., NY) for 2 days, and dehydrated through graded ethanol. After dehydration the samples were embedded in paraffin, sectioned (5 μm), and the sections were stained with hematoxylin and eosin.
Cell proliferation assay
To investigate the proliferation characteristics of the periosteal cells, the cells harvested from 6? fibrous layers and 6? cambium layers were separately cultured in 6-well plates for 3 days, and the cell proliferation was evaluated using a 5-bromo-2’-deoxyuridine (BrdU) assay kit (Model 1299964, Roche Molecular Biochemicals, IN). Briefly, the cells harvested from the fibrous layer and cambium layer were incubated separately in a bromodeoxyuridine (BrdU) labeling medium (10 μmol/L BrdU in α-MEM) for 30 min at 37°C in 5% CO2. The cells were fixed using ethanol-based Carnoy’s solution (75% absolute ethanol and 25% glacial acetic acid) at –20°C for 20 min. The fixed cells were then incubated with a mouse monoclonal anti-BrdU antibody in phosphate buffered saline (PBS) for 30 min at 37°C. After washing with PBS, the samples were incubated with alkaline phosphatase-labeled anti-mouse Ig in triethanolamine buffer for 30 min at 37°C, and were then incubated with the substrate solution (nitroblue tetrazolium salt and 5-bromo-4-chloro-3-indolyl phosphate in Tris buffer solution, pH 9.5) for 20 min at room temperature.
Alkaline phosphatase assay
Alkaline phosphatase (ALP) production by the cultured fibrous and cambium cells was quantified using a commercial ALP assay kit (ALP-10, Sigma Diagnostics) on days 0, 3, 6, and 9 of culture (n = 8 per cell type per testing day). At the time of the assay, the cells were detached from the culture well with trypsin-EDTA0.05% solution (25200-056, Invitrogen), resuspended in PBS and the cell number was determined using a hemocytometer. Resuspended cells (5 × 105) from each group were lysed by adding 500 μL of 1% (v/v) Triton X-100 (Sigma) in PBS, and were then subjected to two cycles of freeze-thawing and sonication to dissociate the ALP from the cell membrane. A 20 μL ali-quot of the lysed sample solution from each group was then mixed with 1 mL p-nitrophenyl phosphate color substrate solution in a 24-well plate, and the sample was incubated at 37°C for 5 min to allow yellow p-nitrophenol to be released due to the endogenous ALP present in the sample. The optical density of the sample solution was measured at 405 nm using a spectrophotometer (Elx800, Bio-Tek), and converted to p-nitrophenol content using a conversion factor provided by the manufacturer. The ALP content of the sample was represented quantitatively in terms of the p-nitrophenol content per incubation time and normalized using the cell number (mol p-NP/min/cell number).
The non-uniform ALP distribution within the periosteum was also examined histologically using freshly harvested periosteum and a qualitative ALP assay kit (86-R, Sigma Diagnostics). A fresh periosteum patch was harvested from the medial proximal tibia of a 6-month-old New Zealand White rabbit and fixed with 10% formalin for 24 h at 4°C. Samples were embedded in paraffin and 5 μm sections were prepared. The tissue sections were then dehydrated through graded ethanol and incubated for 2 hours at room temperature in a freshly prepared ALP detection solution. After being washed with distilled water, the sections were counter-stained with hematoxylin (GHS-3, Sigma Diagnostics) for 5 min.
Measurement of osteocalcin production
The undercarboxylated osteocalcin (Glu-OC) production was measured quantitatively using a commercial enzyme immunoassay kit (model MK118, Takara Biomedicals Inc., Japan). Briefly, the fibrous and cambium layer cells (n = 4 per cell type) were given 2 mL fresh culture medium on days 1, 4, and 7 of culture, and the conditioned culture medium was then harvested after a 48-h incubation time (on days 3, 6, and 9, respectively). At the time of harvest, the cells were detached from the culture well using trypsin-EDTA (25200-056, Invitrogen), and the cell number was determined with a hemo-cytometer. 100 μL of the samples were incubated at room temperature for 2 h in a 96-well plate pre-coated with murine monoclonal anti-Glu-OC anti-body. After being washed 3 times with PBS, the samples were incubated with a diluted solution of murine monoclonal anti-osteocalcin antibody conjugated with horseradish peroxidase (antibody-POD conjugate) at room temperature for 1 h. The samples were washed with PBS and incubated for 15 min at room temperature with a color substrate solution containing hydrogen peroxide and tetra-methylbenzidine. 100 μL 1.0 N sulphuric acid was then added to the samples, and the optical density of the sample was measured at 450 nm using a spectrophotometer (Elx800, Bio-Tek, Winooski). The Glu-OC production was determined by a calibration curve obtained using known concentrations of Glu-OC, and normalized using cell number.
Statistics
To analyze the production of ALP and osteocalcin between the fibrous layer cells and the cambium layer cells at each time point, data were analyzed using analysis of variance (ANOVA). The main factors were cell types and culture time. When a significant difference was detected, Fisher’s protected least squares difference post-hoc test was then used to examine the statistical differences between groups. Data were expressed as the mean (SD) and all tests were performed using Statview (SAS Institute, NC) at a significance level of 0.05.
Results
Cell harvest, cell morphology and cell proliferation
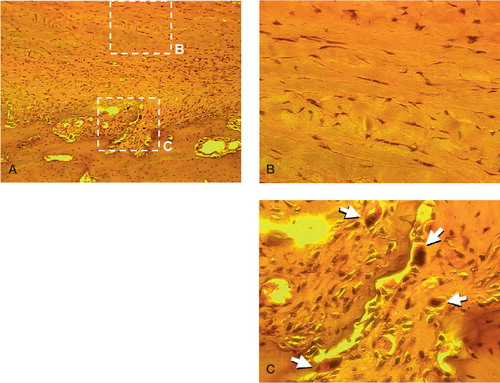
shows hematoxylin and eosin staining of an intact periosteum-bone complex harvested from the proximal tibia of a 6-month-old rabbit. In the periosteum, at least three different types of cells were identified: fibroblasts, osteoblasts, and osteo-clasts. Fibroblasts with a characteristic spindle shape were sparsely distributed in the fibrous layer of the periosteum (), while the cambium layer possessed a dense population of round cells, including osteoblast-like cells and multinucleate osteoclasts (). The surface of cortical bone appeared to be invaded by the cambium layer, where most of the osteoclasts were present, indicating an active bone remodeling process at the cambium layer-bone interface.
Figure 2. (A) Hematoxylineosin staining of bone-periosteum complex harvested from the proximal tibia of a 6-month-old rabbit (100 ×).(B) Histology of the fibrous layer in the periosteum (400 ×). Spindle-shaped fibroblasts were sparsely distributed in collagen matrix.(C) Histology of the cambium layer in the periosteum (400 ×). Osteoclasts (arrows) were found among dense osteoblast-like cells in the cambium layer.
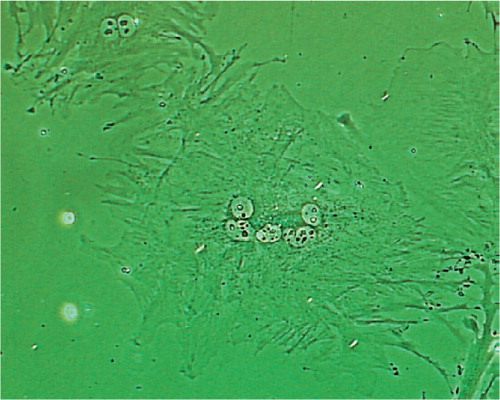
Our sequential enzymatic digestion method separated the cells of the fibrous and cambium layers effectively. is a histology picture of periosteum-bone complex after 30 min of trypsin treatment. After the first 2-h period of collagenase digestion, only cambium layer cells remained on the cortical bone (). Most of these cells were liberated during the second 2-h period of collagenase digestion. When the cells were seeded as monolayer cultures, the morphologies of the cells from the two layers were distinct. The fibrous layer cells had a fibroblastic spindle shape and showed uniform cell size (). In contrast, the cambium layer cells had a polygonal morphology and their size varied (). We also found that some cells from the cambium layer showed osteoclast-like cell morphology with multiple nuclei (), which is consistent with the above histology.
Figure 3. Histology of periosteum-bone complex during the sequential enzymatic digestion method. (A) Periosteumbone complex after 30 min of trypsin digestion. (B) After 2 h of collagenase digestion, only cambium layer cells remained on the cortical bone. (F: fibrous layer; C: cambium layer; B: bone).
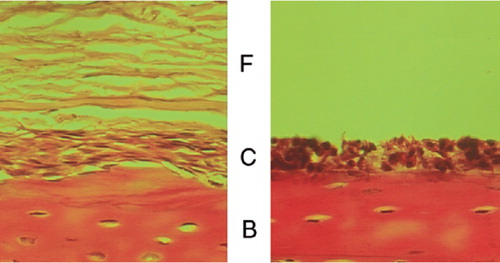
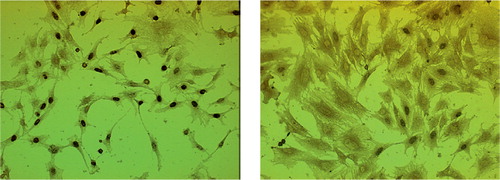
Figure 4. Morphology of the periosteal fibrous layer cells (A:day 3;C:day 6) and cambium layer cells (B: day 3; D: day 6). Magnification 200 ×.
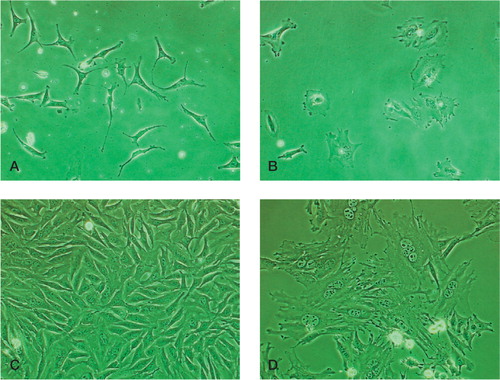
Figure 5. Osteoclast-like multinucleate cells were found in the cell population from the cambium layer (400 ×).
Cells from the fibrous and cambium layers proliferated at different rates. As shown in , a large increase in the cell population was observed in the fibrous layer cells from day 0 () to day 6 (). However, only a moderate increase in the population was observed in the cambium layer cells from day 0 () to day 6 (). This finding was validated with the bromodeoxyuridine (BrdU) cell proliferation assay. On day 3, approximately 70% of fibrous layer cells showed positive BrdU staining (), whereas only 20% of cambium layer cells showed positive BrdU staining (). We also observed that the majority of stained cambium layer cells were smaller than unstained cambium layer cells.
Alkaline phosphatase and osteocalcin production
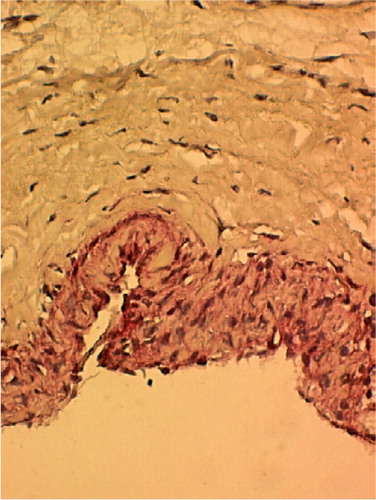
Expression of ALP and osteocalcin are useful markers for osteoblasts and for the regulation of bone formation. shows that the cambium layer had significant ALP activity (represented by dark red staining), indicating a high osteogenic potential, whereas the cells of the fibrous layer showed negligible ALP activity. In addition, the cambium layer showed a higher cell density than the fibrous layer, and the interface between the two layers was phenotypically distinctive.
Figure 7. Histology of freshly harvested periosteum stained using alkaline phosphatase histochemical assay, with hematoxylin counterstaining. The cambium layer shows a high level of alkaline phosphatase activity (shown by red color), whereas the fibrous layer shows negligible alkaline phosphatase activity (400 ×).
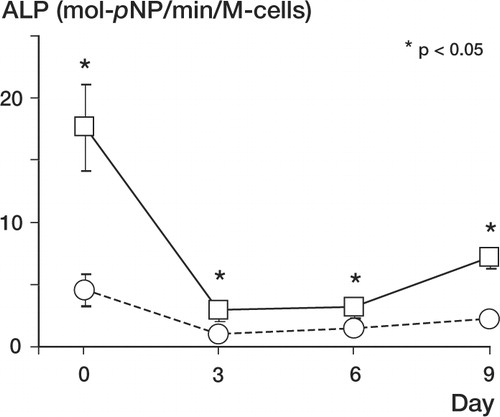
As shown in , immediately after the cell harvest (day 0), the cells from the cambium layer showed statistically higher ALP activity than the cells from the fibrous layer (p < 0.05). (Autors: Please give exact p-values, with 1 digit, not < 0.05). On day 3, however, the ALP activity of the cambium cells decreased dramatically, but was still higher than that of the fibrous layer cells (p < 0.05). The ALP activity of the cambium layer cells increased slightly on day 9, whereas that of the fibrous layer cells remained low, and no statistical differences were found among fibrous layer cells on days 3, 6 and 9.
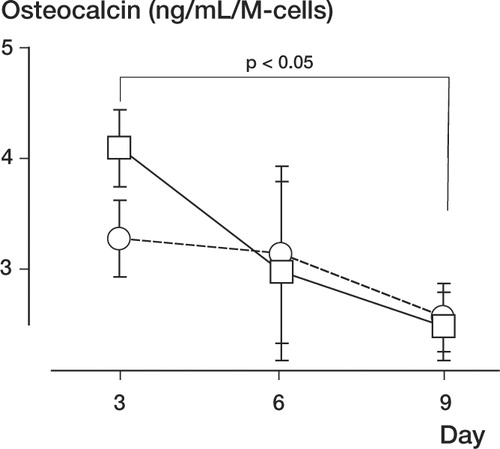
Figure 8. Quantitative alkaline phosphatase activities in fibrous layer cells (○) and lysed cambium layer (□) cells (*p < 0.05)
Although the cambium layer cells produced more osteocalcin than the fibrous layer cells on day 3, no statistical difference was found between the cell types during culture. However, the production of osteocalcin by both types of cells declined from day 3 to day 9 (p < 0.05, ).
Discussion
Using a new sequential enzymatic digestion method, we were successful in isolating the fibrous and cambium layer cells separately without damaging or losing cambium layer cells. The cells from each layer showed different phenotypes in standard monolayer culture. However, the collagenase digestion time for each layer will depend on the thickness of each layer, as it varies with the age of the specimen and the donor site (Gallay et al. Citation1994, O’Driscoll et al. Citation2001). When seeded as monolayer cultures, the proliferation rate of the fibrous layer cells was much higher than that of the cambium layer cells. This was confirmed by BrdU immunostaining. In some samples, small numbers of fibrous cells could be found among the cells harvested from the cambium layer, and these fibrous layer cells quickly multiplied to form colonies and soon outnumbered the cambium layer cells. These results contradict a previous report by Tonna and Cronkite (Citation1963) that the cambium layer showed higher cell proliferation than the fibrous layer in vivo. These contradictory findings between in vivo and in vitro results suggest that a monolayer culture of the mixed cell population from the entire periosteum may misrepresent the original differential cell populations of the two-layered periosteum.
Interestingly, osteoblast-like cells harvested from the cambium showed diverse BrdU staining and variable cell size in monolayer culture. A strong correlation was found between cell morphology and the proliferation rate of cambium layer cells, where small rounded cells showed higher proliferation activity than large rounded cells. These Day findings can be explained by the general concept from cell biology that a cell loses its proliferative lysed potential during the differentiation and enlargement stage (Brighton Citation1978). Ito et al. (Citation2001) also observed a similar nonhomogeneous cell proliferation among the cambium layer cells in periosteum explants cultured in agarose.
Initially, the cambium cells showed stronger osteogenic characteristics than fibrous cells (higher ALP and osteocalcin levels). During monolayer culture, however, these differences quickly diminished. This finding indicates that monolayer culture may not be suitable for preservation of the high osteogenic potential of cambium layer cells in the periosteum. Although no detailed data 36 9 have been included in this paper, our preliminary Day results indicate that cells from the fibrous layer of the periosteum express higher levels of ALP (by about 35%) than tendon fibroblasts. Nakahara et al. (1990) reported that whereas harvested periosteum cells showed a fibroblast-like morphology in monolayer culture, they differentiated into osteo-blasts or chondrocytes when inoculated in vivo. These findings suggest that the cells of the fibrous layer may have the potential to differentiate into osteoblast-like cells in certain environments.
Liberated periosteal cells or freshly harvested periosteum are known to have chondrogenic and osteogenic potential and are popular materials for tissue engineering. The advantage of the tissue engineering approach is that a small number of harvested periosteal cells can be expanded in various culture systems and then used for the repair of damaged musculoskeletal tissues. The harvested periosteum tissue itself can also be used as a natural scaffold material in many possible clinical applications such as repair of cartilage defects (Nakahara et al. Citation1990b, Wakitani et al. Citation1994) or enhancement of soft tissue fixation to bone (Youn et al. Citation2004). However, for clinical applications, the heterogeneous phenotypic characteristics and the functional multi-potentiality of periosteum still remain unclear. Our findings suggest that extra effort should be made to identify and maintain the intrinsic phenotypes of the heterogeneous cell types present within the periosteum. This would improve our understanding of the periosteum regarding musculoskeletal tissue repair and tissue engineering. Further studies are also required to investigate the specific role of each cell population in the milieu of osteogenesis or chondrogenesis in vivo, and the long-term phenotypic characteristics of periosteal cells in different culture environments.
The authors wish to acknowledge support from the Department of Orthopedic Surgery at Tulane University.
No competing interests declared.
- Bahrami S, Stratmann U, Wiesmann H P, Mokrys K, Bruckner P, Szuwart T. Periosteally derived osteoblast-like cells differentiate into chondrocytes in suspension culture in agarose. Anat Rec 2000; 259(2)124–30
- Breitbart A S, Grande D A, Kessler R, Ryaby J T, Fitzsimmons R J, Grant R T. Tissue engineered bone repair of calvarial defects using cultured periosteal cells. Plast Reconstr Surg 1998; 101(3)567–74, discussion 575–6.
- Breitbart A S, Grande D A, Mason J M, Barcia M, James T, Grant R T. Gene-enhanced tissue engineering: appli-cations for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg 1999; 42(5)488–95
- Brighton C T. Structure and function of the growth plate. Clin Orthop 1978, 136: 22–32
- Burger E H, Boonekamp P M, Nijweide P J. Osteoblast and osteoclast precursors in primary cultures of calvarial bone cells. Anat Rec 1986; 214(1)32–40
- Deren J A, Kaplan F S, Brighton C T. Alkaline phosphatase production by periosteal cells at various oxygen tensions in vitro. Clin Orthop 1990, 252: 307–12
- Gallay S H, Miura Y, Commisso C N, Fitzsimmons J S, O'Driscoll S W. Relationship of donor site to chondro-genic potential of periosteum in vitro. J Orthop Res 1994; 12(4)515–25
- Gruber R, Mayer C, Bobacz K, Krauth M T, Graninger W, Luyten F P, Erlacher L. Effects of cartilage-derived mor-phogenetic proteins and osteogenic protein-1 on osteo-chondrogenic differentiation of periosteum-derived cells. Endocrinology 2001; 142(5)2087–94
- Hoikka V E, Jaroma H J, Ritsila V A. Reconstruction of the patellar articulation with periosteal grafts. 4-year follow-up of 13 cases. Acta Orthop Scand 1990; 61(1)36–9
- Isogai N, Landis W, Kim T H, Gerstenfeld L C, Upton J, Vacanti P J. Formation of phalanges and small joints by tissue-engineering J. Bone Jt Surg (Am) 1999; 81(3)306–16
- Isogai N, Landis W J, Mori R, Gotoh Y, Gerstenfeld L C, Upton J, Vacanti P J. Experimental use of fibrin glue to induce site-directed osteogenesis from cultured periosteal cells. Plast Reconstr Surg 2000; 105(3)953–63
- Ito Y, Sanyal A, Fitzsimmons J S, Mello M A, O'Driscoll S W. Histomorphological and proliferative characteriza-tion of developing periosteal neochondrocytes in vitro. J Orthop Res 2001; 19(3)405–13
- Izumi T, Scully S P, Heydemann A, Bolander M E. Trans-forming growth factor beta 1 stimulates type II collagen expression in cultured periosteum-derived cells. J Bone Miner Res 1992; 7(1)115–21
- Jungueira L C, Carneiro J, Kelley R O. Basic histology. Appleton & Lange, New York 1998
- Korkala O L. Periosteal primary resurfacing of joint surface defects of the patella due to injury. Injury 1988; 19(3)216–8
- Koshihara Y, Kawamura M, Endo S, Tsutsumi C, Kodama H, Oda H, Higaki S. Establishment of human osteoblastic cells derived from periosteum in culture. In Vitro Cell Dev Biol 1989; 25(1)37–43
- Letts M, Pang E, Yang J, Carpenter B. Periosteal augmenta-tion of the acetabulum. Clin Orthop 1998, 354: 216–23
- McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg (Br) 1978; 60(2)150–62
- Nakahara H, Bruder S P, Goldberg V M, Caplan A I. In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clin Orthop 1990a, 259: 223–32
- Nakahara H, Bruder S P, Haynesworth S E, Holecek J J, Baber M A, Goldberg V M, Caplan A I. Bone and carti-lage formation in diffusion chambers by subcultured cells derived from the periosteum. Bone 1990b; 11(3)181–8
- Niedermann B, Boe S, Lauritzen J, Rubak J M. Glued peri-osteal grafts in the knee. Acta Orthop Scand 1985; 56(6)457–60
- Nishimura T, Simmons D J, Mainous E G. The origin of bone formed by heterotopic periosteal autografts. J Oral Maxillofac Surg 1997; 55(11)1265–8
- O'Driscoll S W, Fitzsimmons J S. The importance of proce-dure specific training in harvesting periosteum for chon-drogenesis. Clin Orthop 2000, 380: 269–78
- O'Driscoll S W, Keeley F W, Salter R B. The chondrogenic potential of free autogenous periosteal grafts for biologi-cal resurfacing of major full-thickness defects in joint sur-faces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg (Am) 1986; 68(7)1017–35
- O'Driscoll S W, Recklies A D, Poole A R. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J Bone Joint Surg (Am) 1994; 76(7)1042–51
- O'Driscoll S W, Saris D B, Ito Y, Fitzimmons J S. The chon-drogenic potential of periosteum decreases with age. J Orthop Res 2001; 19(1)95–103
- Poussa M, Ritsila V. The osteogenic capacity of free peri-osteal and osteoperiosteal grafts. A comparative study in growing rabbits. Acta Orthop Scand 1979; 50(5)491–9
- Reynders P, Becker J, Broos P. Osteogenic ability of free periosteal autografts in tibial fractures with severe soft tissue damage: an experimetal study. J Orthop Trauma 1999; 13(2)121–8
- Sandberg M, Aro H, Multimaki P, Aho H, Vuorio E. In situ localization of collagen production by chondrocytes and osteoblasts in fracture callus. J Bone Joint Surg (Am) 1989; 71(1)69–77
- Takushima A, Kitano Y, Harii K. Osteogenic potential of cul-tured periosteal cells in a distracted bone gap in rabbits. J Surg Res 1998; 78(1)68–77
- Thomson R C, Mikos A G, Beahm E, Lemon J C, Satterfield W C, Aufdemorte T B, Miller M J. Guided tissue fabrication from periosteum using preformed biodegradable polymer scaffolds. Biomaterials 1999; 20(21)2007–18
- Tonna E A, Cronkite E P. The periosteum. Autoradiographic studies on cellular proliferation and transformation utiliz-ing tritiated thymidine. Clin Orthop 1963, 30: 218–33
- Ueno T, Kagawa T, Mizukawa N, Nakamura H, Sugahara T, Yamamoto T. Cellular origin of endochondral ossification from grafted periosteum. Anat Rec 2001; 264(4)348–57
- Wakitani S, Goto T, Pineda S J, Young R G, Mansour J M, Caplan A I, Goldberg V M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg (Am) 1994; 76(4)579–92
- Wlodarski K H. Normal and heterotopic periosteum. Clin Orthop 1989, 241: 265–77
- Youn I, Jones D G, Andrews L P, Cook P M, Suh J-K F. Peri-osteal augmentation of tendon graft improves the tendon healing within the bone tunnel. Clin Orthop 2004, 419: 223–31