Abstract
Background Electrothermally-assisted capsular shrinkage has been gaining increased acceptance in the treatment of shoulder instability. Its indication in ACL-deficient knees has been discussed recently.
Methods We examined the influence of immobilization on cell homeostasis of healing collagenous tissue after radiofrequency energy was applied to the patellar tendon in 23 rabbits. The animals were killed immediately after surgery (n = 6) or 3 weeks after surgery (n = 17). 10 rabbits were allowed normal cage activity, whereas the treated hind limb of 7 animals was immobilized for 3 weeks in a cast. Feulgen staining was used to stain the DNA of cell nuclei. Cells undergoing apoptosis were identified by the TUNEL method. Quantitative histological assessment was performed using imaging analysis software.
Results Severe cellular damage in RF-treated collagenous tissue was partly induced by the immediate onset of apoptosis. At 3 weeks after surgery, non-immobilized tendon showed increased cellularity and apoptosis, whereas immobilization prevented the increase in cellularity and apoptosis significantly. The calculated ratio of apoptosis was not influenced by any postoperative treatment.
Interpretation Diminished cellularity and apoptosis during tissue remodeling, due to immobilization, may protect the shortened collagenous scaffold from stretching and further optimize the clinical outcome after radiofrequency shrinkage. To stabilize the shrunken tissue, proliferation during postoperative wound healing should be minimized by careful rehabilitation.
The use of radiofrequency energy to shrink redundant collagenous tissue (RF shrinkage) has become increasingly popular for the treatment of shoulder instability because of encouraging short-term to mid-term results (Levy et al. Citation2001, Wong and Williams, Citation2001, Favorito et al. Citation2002, Frostick et al. Citation2003). Experimental studies on the viscoelastic properties of shrunken tissue did not mimic the clinical success, however. The potential risk of recurrent stretching-out at postoperative physiological loading, a predetermining factor of recurrent instability, has been reported by several investigators (Pullin et al. Citation1997, Schaefer et al. Citation1997, Wallace et al. Citation2002). Preliminary studies concerning RF shrinkage of anterior cruciate ligaments (ACL) reported not only mechanical failures, but also complete rupture (Carter et al. Citation2002, Lopez and Markel Citation2003).
Over the last 2 decades, the use of postoperative immobilization in the treatment of capsule, ligament or tendon injuries has changed in favor of accelerated rehabilitation (Woo et al. Citation1982, Gamble et al. Citation1984). Although much research has been done, the loading conditions that are necessary to improve and accelerate the healing process without causing damage or adverse biomechanical effects remain unclear (Steadman et al. Citation1989, Zeichen et al. Citation2000, Bosch et al. Citation2002, Skutek et al. Citation2003).
It is known that mechanical stretching induces signaling pathways that lead to increased gene expression, protein synthesis, mitogenesis and apoptosis (Banes et al. Citation1995, Zeichen et al. Citation2000, Bosch et al. Citation2002). Correspondingly, progressive rehabilitation causes increased fibroblast proliferation and collagen synthesis in various animal models of tendon and ligament healing, but intensive proliferation and hypercellularity seem to have adverse effects on the mechanical properties of healing tissue (Woo et al. Citation1982, Frank et al. Citation1991, Kamps et al. Citation1994, Atkinson et al. Citation1999). These results have generated controversy concerning the role of mechanical loading, especially since cell necrosis and subsequent intensive fibroblastic response after RF shrinkage have been reported (Hayashi et al. Citation1999, Hecht et al. Citation1998, Pullin et al. Citation1997, Schaefer et al. Citation1997).
Our study assessed the influence of postoperative immobilization on cellularity and apoptosis after RF treatment of collagenous tissue. To our knowledge, no study has been conducted with special regard to cellularity or apoptosis during immobilization. The assessment of cell homeostasis may help to illustrate the controversy about the effect of mobilization and mechanical loading on the healing properties of RF-treated collagenous tissues.
Animals and methods
Animal model and tissue preparation
We used 32 mature female New Zealand White rabbits, ranging in weight from 3.5 to 5.5 kg. The study was approved by the local institutional committee dealing with animal use and care. All animals were purchased from a single supplier and were allowed to acclimatize for at least 1 week before surgery.
The animals were anesthetised by an intramuscular injection of ketamine hydrochloride (15 mg/kg) and xylazine (2 mg/kg). The right hind limb of each rabbit was shaved and then aseptically prepared for surgery. To maintain anesthesia, the injection of ketamine hydrochloride was repeated every 20 min during the operation. Using aseptic techniques, the patella tendon of the right hind limb of each rabbit was approached via a medial parapatellar incision. The tendon was marked with a surgical marker every 5 mm between its patellar origin and its insertion onto the tibia, to allow a consistent energy application.
Radiofrequency energy was delivered to the patella tendon with a radiofrequency generator (VAPR II, MITEK Division Ethicon, Norderstedt, Germany) at a temperature setting of 70°C and a power setting of 40 W, coupled with a bipolar, temperature-controlled probe (VAPR TC, MITEK, Ethicon). The surfaces of the tendon were treated in a uniform manner using the premarked grid pattern, whereas the RF probe was moved from lateral to medial at a velocity of approximately 1–2 mm/sec with the parallel passes spaced approximately 5 mm. The knee joint was entirely covered with saline solution. Using the technique of Schaefer et al. (Citation1997), we were able to monitor the shortening of the tendon exactly. The RF treatment was stopped after shrinkage of the tendon length by 5%. The incision was closed with simple interrupted 3-0 vicryl sutures (VICRYL, Ethicon).
Postoperatively, the rabbits were randomly assigned to one of the following groups: group 1: 9 untreated contralateral specimens (randomly chosen among each following animal group) served as control; group 2: 6 rabbits were killed immediately postoperatively; group 3: 10 rabbits were killed after 3 weeks (normal cage activity without any immobilization); group 4: 7 rabbits were killed after 3 weeks of permanent immobilization in a cast.
After killing, the patellar tendon, including patella and proximal tibia, was immediately harvested as one unit from the hind limbs of each rabbit. The specimens were separated from the surrounding tissue and immediately frozen at −80 °C. After thawing, a section of 0.6 mm × 0.3 mm × 0.3 mm was cut out of each patellar tendon close to its tibial insertion. The specimens were fixed in neutral-buffered 7% formalin, embedded in paraffin and sectioned along the longitudinal axis of the tendon. Two consecutive sections of each tissue specimen were stained with both Feulgen staining (unselective DNA staining, based on Schiff's reaction) and in situ DNA labeling assay (selective fragmented DNA staining). Two other consecutive sections of each specimen had a minimum distance of 60 μm and were treated in the same manner.
Feulgen staining
The Feulgen reaction proposed by Feulgen and Rossenbeck is one of the cytohistochemical reactions most widely used in biology and medicine. It allows DNA to be specifically stained in situ, based on the reaction of Schiff or Schiff-like reagents with aldehyde groups engendered in the deoxyribose molecules by HCl-mediated hydrolysis. The method has been extensively modified over the last 75 years (Chieco and Derenzini Citation1999). In its initial formulation, the histochemical reaction was used simply for the detection of DNA in the nucleus, but since the demonstration that it is both specific and stoichiometric for DNA, it has become the most important means of staining nuclear DNA for densitometric quantification (Schulte Citation1991).
In situ end-labeling of fragmented DNA (TUNEL)
One of the early features of apoptosis is DNA cleavage in cell nuclei (Wyllie et al. Citation1980, Gavrieli et al. Citation1992). To assess the extent of DNA cleavage in our study, we used the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end-labeling reaction (TUNEL). The TUNEL method preferentially labels the 3'-OH ends exposed by double-strand breaks in the DNA, the main form of cleavage during apoptosis (Wyllie et al. Citation1980, Van Cruchten and Van Den Citation2002). The labeling was performed using a commercial kit (Oncogene TdT-FragEL, Boston, MA). Paraffin sections of tendon tissue (5 μm thick) were carefully dewaxed with xylene and graded ethanol, and dehydrated in TBS buffer (20 mM Tris-HCl, pH 7.6, 140 mM NaCl) for 1 min. The tissue was digested with 20 μg/mL proteinase K for 20 min at room temperature, incubated with 3% H2O2 to inactivate endogenous peroxidase for 5 min, and equilibrated with TdT buffer (1 M sodium cacodylate, 0.15 M Tris, 1.5 mg/mL BSA, 3.75 mM CoCl2, pH 6.6) for 20 min. Fragmented nuclear DNA was end-labeled with biotinylated dUTP using TdT for 90 min at 37°C in a humidified chamber. Each section was covered with Parafilm during the incubation. The labeling reaction was terminated by using the stop solution for 5 min. After incubation with streptavidin-HRP for 30 min, the color was developed with DAB solution for 10 min at room temperature. Sections were counterstained with methyl green. For negative control sections, the TdT was omitted.
Quantification of cells (digital imaging)
Light microscopic analysis using bright-field illumination was conducted with a Zeiss-Photomikroskop-III (Zeiss, Germany) using final magnifications of up to × 825. Images were obtained with a high-resolution camera (Cool SNAP-Pro Digital Kit, Version 4.0, Media Cybernetics, MD) and quantified using imaging software (Image-Pro Plus, Media Cybernetics). The number of fibroblast nuclei was assessed by two researchers who were blinded as to the source of tissue. Counts were performed using two representative areas (0.03 mm2) per section. After the threshold level was selected, the nuclei were automatically counted. Each object sized greater than 100 pixels and within the threshold density was then counted and outlined. Any non-nuclear objects, automatically outlined, were manually excluded by two blinded investigators.
Statistics
All values in the figures are expressed as median and inter-quartile ranges (IR) per area (0.03 mm2). Because the control group (group 1) was represented by a random selection of untreated contra-lateral tendons, we had to use an unpaired statistical test. As the sample size could not be assumed to be normally distributed, we used the non-parametric Mann-Whitney test for comparisons between the different treatment groups. Confidence limits were predetermined at an alpha level of 0.05.
Results
Cellularity analysis by Feulgen staining of DNA
Groups 1 and 2. Cell nuclei stained dark purple. Most cell nuclei had a fibroblast-like appearance, independent of the time of investigation. Fibroblasts in control sections obtained from the contralateral limbs had a typical spindle-shaped appearance with no alterations of cell nuclei and cell orientation.
At time 0 after the operation, alterations of chromatin distribution—characterized by chromatin condensation within the nuclei—were evident in all tissue samples. The median cellularity of the postoperative group (43/IR 29) did not differ significantly (p = 0.3) from the untreated control tissue sections (37/IR 18) ().
Figure 1. Feulgen staining (nuclei per 0.03 mm2). Bars marked with an asterisk are significantly different from untreated controls.
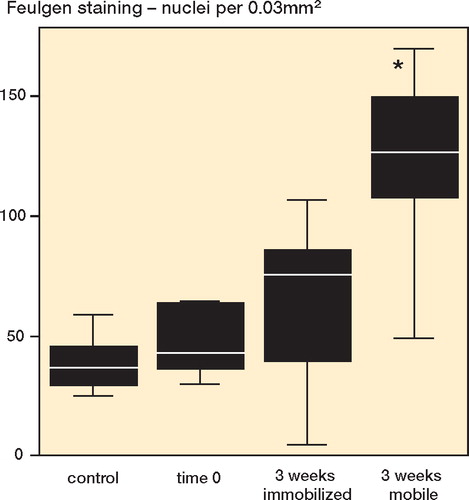
Groups 3 and 4. 3 weeks after RF treatment, the median quantity of cells increased 2.1 fold on average (p = 0.1) in immobilized limbs (76/IR 58) compared to the untreated controls. The median quantity of cells in the non-immobilized tendon (127/IR 47) increased 3.5 fold (p = 0.001) in the same period of time ().
Direct comparison between non-immobilized (group 3) and immobilized (group 4) tendon at 3 weeks post-surgery showed a higher level of cells if no immobilization was performed (p = 0.006) ( and ).
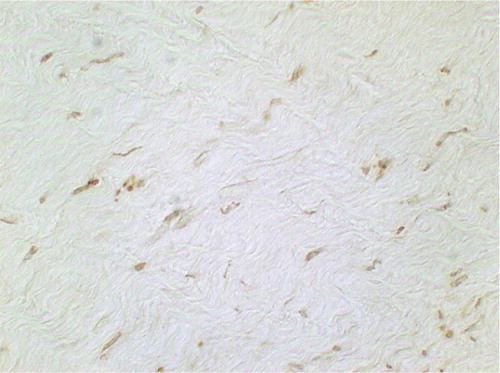
Figure 2. Feulgen staining at 3 weeks after radiofrequency treatment, permanently immobilized. Cellularity appears to be slightly increased (not significant) (original magnification: × 750).
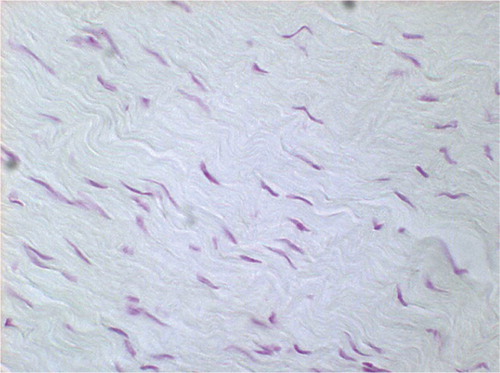
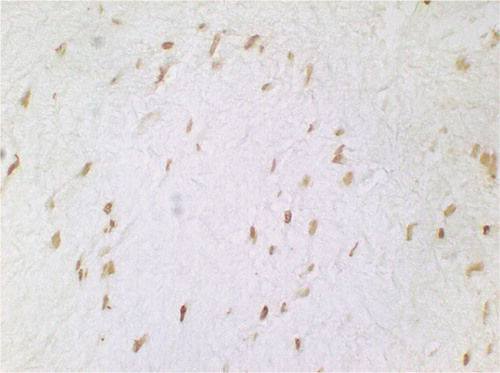
Figure 3. Feulgen staining at 3 weeks after radiofrequency treatment without immobilization. Cellularity was significantly increased. The fibroblasts appeared large and rounded/oval in shape, which was combined with intense depth of staining. The histomorphology indicates a vast amount of DNA and cell activity (original magnification: × 750).
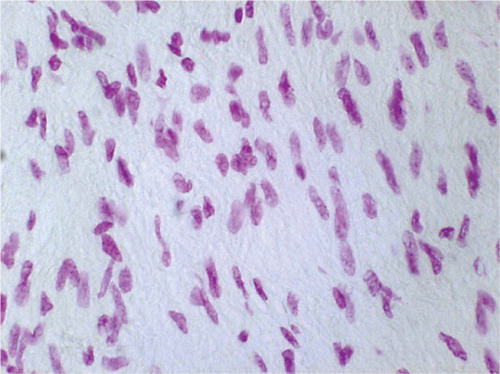
The nuclei of non-immobilized tendon were oval to round in shape with enhanced intensity of staining of DNA, whereas the fibroblasts of immobilized tissue were spindle-shaped and stained slightly more prominently than the controls ( and ).
Apoptosis detection by in situ end-labeling of fragmented DNA (TUNEL)
Groups 1 and 2. With the DNA end-labeling method, apoptotic cells stained brown, and negative cells and collagen bundles stained weak green. The TdT-omitted sections showed no brown nuclei, confirming that no specific binding had occurred in negative control sections.
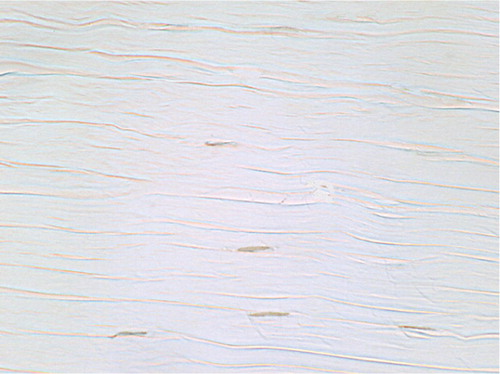
Control tissue sections had a normal appearance of wavy collagen bundles interspersed with spindle-shaped fibroblast-like cells ().
Figure 4. TUNEL-positive cells in untreated contralateral control: wavy collagen bundles interspersed with normal spindle-shaped fibroblasts (original magnification: × 750).
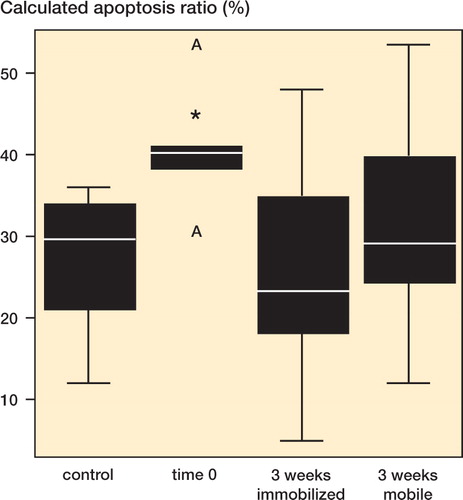
Computer-assisted cell counts revealed an average of 9.8/IR 4.6 TUNEL-positive cells per 0.03 mm2 in the control tendon. With regard to the total cell amount, determined by Feulgen staining, an apoptosis ratio of 30%, IR 15% was calculated ().
Figure 5. Calculated apoptosis ratio (in %). Bars marked with an asterisk are significantly different from untreated controls.
The number of positively TUNEL-labeled fibroblasts, indicating DNA strand breaks, increased (p = 0.02) from 9.8/ IR 4.6 (group 1) to 20/IR 13 (group 2) immediately after the application of radiofrequency energy (). Alterations in the distribution of hybrid-labeled DNA—characterized by clumpy staining within the nuclei—were evident in all tissue samples ().
Figure 6. TUNEL-positive cells (nuclei per 0.03 mm2). Bars marked with an asterisk are significantly different from untreated controls.
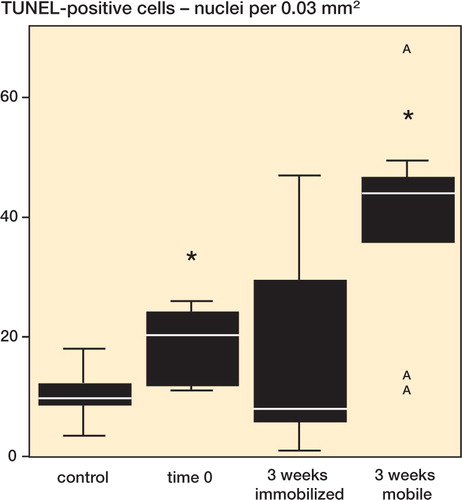
Figure 7. TUNEL-positive cells at time 0 after RF treatment: the staining pattern of fibroblast nuclei appears clumpy and they appear more intensely stained than the controls, indicating thermal damage (original magnification: × 850).
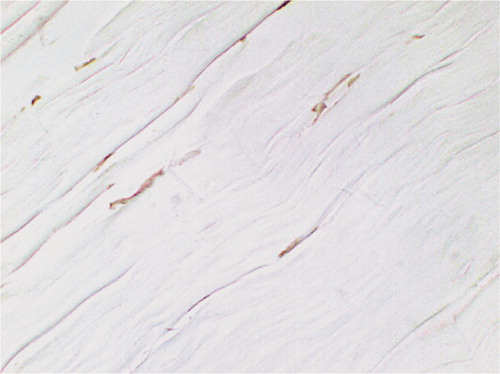
The calculated ratio of apoptotic cells was 40%, IR 7.9 % immediately after the specimens were harvested (). This boost in the apoptosis ratio was statistically significant (p = 0.01) compared to the controls. Fibroblast-like apoptotic cells were evenly distributed within the extracellular matrix (ECM) ().
Groups 3 and 4. 3 weeks postoperatively, the number of nuclei undergoing apoptosis increased by 4.5 fold, up to 44/IR 24 cells per 0.03 mm2 in non-immobilized limbs (group 3) relative to untreated controls. This increase was statistically significant (p = 0.05). No difference (p = 0.4) was seen among the immobilized limbs (8.0/IR 32) for the same period of time (). 3 weeks post-surgery, there was a higher level of apoptotic cells if no immobilization was performed (p = 0.04).
The ratio of fibroblasts undergoing apoptosis in the immobilized group (group 4) (23%, IR 28%) was no different (p = 0.4) from the corresponding mean in the non-immobilized group (group 3) (29%, IR 21%) ().
Round-shaped TUNEL staining patterns in fibroblast nuclei were visible in non-immobilized limbs. If immobilization was performed, the ECM was interspersed with spindle-shaped fibroblast-like cells mainly ( and ). In general, no microscopic differences in cellularity between the superficial and deep layers of the tendon were recognized.
Discussion
The healing of thermally treated tissue, with special regard to its histological condition, has been described by several authors. In a canine model, Pullin et al. (Citation1997) found substantial inflammation, necrosis and hypercellularity 6 weeks after laser-assisted shrinkage. In a rabbit model, Schaefer et al. (Citation1997) described a generalized fibroblastic response characterized by a marked increase in cellularity and vascularity 8 weeks postoperatively. Hecht et al. (Citation1998) examined monopolar radiofrequency energy effects in a sheep model with postoperative follow-up times of 0, 2, 6 and 12 weeks and found immediate postoperative collagen hyalinization and cell necrosis followed by an active tissue repair beyond the sixth week. Hayashi et al. (Citation1999) performed a histological evaluation of the glenohumeral joint capsule after laser-assisted shrinkage in 42 patients with shoulder instability. The sampled tissue specimens mainly contained necrotic cells at time 0 and a significantly increased cellularity during a follow-up of more than 6 months.
According to the literature, Feulgen staining provides histological evidence of initial cellular damage after radiofrequency-induced thermal shrinkage. Apoptosis in the patellar tendon has not been described. Yuan et al. (Citation2002) found that 13% of fibroblasts had undergone apoptosis in control sections of the subscapularis tendon. Apoptosis in radiofrequency-treated collagenous tissue at time 0 may occur due to ischemia, hypoxia, free radical generation, heat shock, physical damage or combinations of these factors (Jennings and Reimer Citation1991, Biagas Citation1999, Shackelford et al. Citation2000, Skutek et al. Citation2003). The onset of nuclear fragmentation is considered to be relatively short (1–3 h), but the exact onset of apoptosis after thermal treatment remains unclear (Gavrieli et al. Citation1992, Smith et al. Citation2000). In our study, the tendon remained in situ at least 60 min due to length measurement, killing and tissue preparation, until the tissue was harvested (time 0). However, we found a surprisingly high percentage of TUNEL-positive cells (30%) in control sections. The postoperative increase in both apoptosis ratio and total number of TUNEL-positive nuclei indicates the potential for deleterious effects from RF energy.
The recent nomenclature dealing with mechanisms of cell death precisely defines the order of and relationship between apoptosis and subsequent necrosis (Van Cruchten and Van Den Citation2002). Previously reported necrosis, karyorrhexis and cellular shrinkage (Pullin et al. Citation1997, Schaefer et al. Citation1997, Hecht et al. Citation1998, Hayashi et al. Citation2000) after RF energy may therefore reflect characteristic post-apoptotic conditions. Our findings mimic previously reported data, but give further information on the pathomechanism of premortal processes caused by RF treatment.
Although the number of apoptotic cells in non-immobilized tendons increased significantly, the apoptosis ratios of both treatment modalities were similar. The cell cycle of fibroblasts is partly modulated by poorly understood mechano-transduction pathways (Banes et al. Citation1995, Bosch et al. Citation2002). An in vitro modulation of stretch-induced pathways, leading to apoptosis in a time-dependent manner, was reported by Skutek et al. (Citation2003). Short stretch periods seemed to induce apoptosis, whereas longer stress periods resulted in reduced apoptosis. On the other hand, hind limb immobilization for 2 and 6 days also increased the proportion of TUNEL-positive myonuclei in the soleus muscle (Smith et al. Citation2000).
Our results suggest that 3 weeks after RF treatment, cell homeostasis may already have adapted to the prevailing mechanical environment, reflected by a similar ratio of apoptosis. The higher total numbers of apoptotic fibroblasts after 3 weeks without immobilization (group 3) may nevertheless cause a higher release of chemotactic agents and subsequent phagocytosis (Moodley et al. Citation2003). This assumption is consistent with simultaneously increased cellularity. Further investigations should concentrate on this aspect of tendon healing.
In our study, Feulgen cell counts indicate that cast immobilization for 3 weeks prevents excessive proliferation and metabolic activity. The influence of immobilization on cellularity and tissue remodeling has been investigated in various animal models. Atkinson et al. (Citation1999) reported that patellar tendon augmentation after removal of its central third led to a significant reduction in tissue proliferation and, concomitantly, less tissue remodeling accompanied by biomechanical advantages. Takahashi et al. (Citation2002) have been the only investigators to investigate immobilization after thermal shrinkage in a patellar tendon model. They found that 12 months after irradiation, the non-immobilized ligaments had become longer than the intact controls, whereas initial immobilization prevented the ligament from stretching out. Gamble et al. (Citation1984) concluded that fibroblasts switch from an anabolic to a catabolic state during immobility. These data support our hypothesis that after RF treatment, diminished cell numbers and subsequent prevention of excessive degradation and synthesis may contribute to initially stabilize the shortened collagenous scaffold (Hayashi et al. Citation2000).
Our study has several limitations. Firstly, the patella tendon in this rabbit model is different to the shoulder capsule or to the ACL in humans; the morphological difference between tendons and (capsular) ligaments may be of especial importance. Secondly, it is difficult to compare cast immobilization of a rabbit's hind limb to the immobilization of a human knee or shoulder, but the differences between the non-immobilized and immobilized limbs demonstrate a significant effect of our immobilization technique. Another important difference is that tendons are attached to muscles, and even during immobilization the muscles may fire and generate mechanical loading. Furthermore, differences exist between the posttreatment behaviour of intact or pathological collagenous tissue. Thirdly, despite its apparent simplicity, the TUNEL technique has led to disappointment because of its limitations in sensitivity and specificity (Labat-Moleur et al. Citation1998). In fact, many known causes of false-positive DNA breaks can be listed: DNA recombination, replication, repair or compaction-relaxation during mitosis, autolysis, fixation, paraffin embedding, cutting, pretreatment with H2O2, detergent, proteinase K and microwaves (Eastman and Barry Citation1992). However, the use of TUNEL staining in thermally treated collagenous tissue has not been evaluated. To further optimize the differential staining between apoptotic and non-apoptotic DNA, we followed latest recommendations and combined both: homogeneous small tissue blocks and standardized proteinase pretreatment (Labat-Moleur et al. Citation1998). The sensitivity and specificity of the Feulgen reaction is gemerally considered to be excellent, except for staining depth of differences in cell types or cycle. Since we quantified the cellularity by means of cell counts instead of densitometric analysis, the major shortcoming of the Feulgen method could be excluded (Schulte Citation1991, Chieco and Derenzini Citation1999). Lastly, quantification of cell nuclei in this study was not applied to whole tissue sections, but to two representative areas. We tried to minimize this methodological limitation using two blinded investigators, who also corrected the cell counts manually in cases of artificial interference.
In summary, this histological in vivo study using a rabbit model demonstrates that RF shrinkage causes severe cell death from the immediate induction of apoptosis. Hypercellularity and concomitant tissue remodeling—seen physiologically during tendon and ligament healing—and also a significant induction of apoptosis, were prevented by immobilization. To stabilize the initially shortened collagenous scaffold, extensive remodeling of collagen initiated by immigration, activation and proliferation of fibroblasts should be minimized by immobilization. Based on these results, careful rehabilitation after electrothermally-assisted surgical procedures is strongly recommended. Clinical evaluation may further clarify whether a change in immobilization policy may be beneficial—especially after thermal treatment.
The authors are grateful for the professional technical assistance of Claudia Kemming and Mirko Brandes. This work was supported by grants from the German Research Society (DFG) and from MITEK Division Ethicon, Norderstedt, Germany.
- Atkinson P J, Oyen-Tiesma M, Zukosky D K, DeCamp C E, Mackenzie C D, Haut R C. Patellar tendon augmentation after removal of its central third limits joints tissue changes. J Orthop Res 1999; 17(1)28–36
- Banes A J, Tsuzaki M, Yamamoto J, Fischer T, Brigman B, Brown T, Miller L. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol 1995; 73(7-8)349–65
- Biagas K. Hypoxicischemic brain injury: advancements in the understanding of mechanisms and potential avenues for therapy. Curr Opin Pediatr 1999; 11(3)223–8
- Bosch U, Zeichen J, Skutek M, Albers I, van Griensven M, Gassler N. Effect of cyclical stretch on matrix synthesis of human patellar tendon cells. Unfallchirurg 2002; 105(5)437–42
- Carter T R, Bailie D S, Edinger S. Radiofrequency electrothermal shrinkage of the anterior cruciate ligament. Am J Sports Med 2002; 30(2)221–6
- Chieco P, Derenzini M. The Feulgen reaction 75 years on. Histochem Cell Biol 1999; 111(5)345–58
- Eastman A, Barry M A. The origins of DNA breaks: a consequence of DNA damage, DNA repair, or apoptosis?. Cancer Invest 1992; 10(3)229–40
- Favorito P J, Langenderfer M A, Colosimo A J, Heidt R S, Jr., Carlonas R L. Arthroscopic laser-assisted capsular shift in the treatment of patients with multidirectional shoulder instability. Am J Sports Med 2002; 30(3)322–8
- Frank C, MacFarlane B, Edwards P, Rangayyan R, Liu Z Q, Walsh S, Bray R. A quantitative analysis of matrix alignment in ligament scars: a comparison of movement versus immobilization in an immature rabbit model. J Orthop Res 1991; 9(2)219–27
- Frostick S P, Sinopidis C, Al Maskari S, Gibson J, Kemp G J, Richmond J C. Arthroscopic capsular shrinkage of the shoulder for the treatment of patients with multidirectional instability: Minimum 2-year follow-up. Arthroscopy 2003; 19(3)227–33
- Gamble J G, Edwards C C, Max S R. Enzymatic adaptation in ligaments during immobilization. Am J Sports Med 1984; 12(3)221–8
- Gavrieli Y, Sherman Y, Sasson Ben S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992; 119(3)493–501
- Hayashi K, Massa K L, Thabit G, III, Fanton G S, Dillingham M F, Gilchrist K W, Markel M D. Histologic evaluation of the glenohumeral joint capsule after the laser-assisted capsular shift procedure for glenohumeral instability. Am J Sports Med 1999; 27(2)162–7
- Hayashi K, Peters D M, Thabit G, III, Hecht P, Vanderby R, Jr., Fanton G S, Markel M D. The mechanism of joint capsule thermal modification in an in-vitro sheep model. Clin Orthop 2000, 370: 236–49
- Hecht P, Hayashi K, Cooley A J, Lu Y, Fanton G S, Thabit G, III, Markel M D. The thermal effect of monopolar radiofrequency energy on the properties of joint capsule. An in vivo histologic study using a sheep model. Am J Sports Med 1998; 26(6)808–14
- Jennings R B, Reimer K A. The cell biology of acute myocardial ischemia. Annu Rev Med 1991; 42: 225–46
- Kamps B S, Linder L H, DeCamp C E, Haut R C. The influence of immobilization versus exercise on scar formation in the rabbit patellar tendon after excision of the central third. Am J Sports Med 1994; 22(6)803–11
- Labat-Moleur F, Guillermet C, Lorimier P, Robert C, Lantuejoul S, Brambilla E, Negoescu A. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement critical evaluation and improvement. J Histochem Cytochem 1998; 46(3)327–34
- Levy O, Wilson M, Williams H, Bruguera J A, Dodenhoff R, Sforza G, Copeland S. Thermal capsular shrinkage for shoulder instability. Mid-term longitudinal outcome study. J Bone Joint Surg (Br) 2001; 83(5)640–5
- Lopez M J, Markel M D. Anterior cruciate ligament rupture after thermal treatment in a canine model. Am J Sports Med 2003; 31(2)164–7
- Moodley Y, Rigby P, Bundell C, Bunt S, Hayashi H, Misso N, McAnulty R, Laurent G, Scaffidi A, Thompson P, Knight D. Macrophage recognition and phagocytosis of apoptotic fibroblasts is critically dependent on fibroblast-derived thrombospondin 1 and CD36. Am J Pathol 2003; 162(3)771–9
- Pullin J G, Collier M A, Johnson L L, DeBault L E, Walls R C. Holmium: YAG laser-assisted capsular shift in a canine model: Intraarticular pressure and histologic observations. J Shoulder Elbow Surg 1997; 6(3)272–85
- Schaefer S L, Ciarelli M J, Arnoczky S P, Ross H E. Tissue shrinkage with the holmium:yttrium aluminum garnet laser. A postoperative assessment of tissue length, stiffness, and structure. Am J Sports Med 1997; 25(6)841–8
- Schulte E K. Standardization of the Feulgen reaction for absorption DNA image cytometry: a review. Anal Cell Pathol 1991; 3(3)167–82
- Shackelford R E, Kaufmann W K, Paules R S. Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med 2000; 28(9)1387–404
- Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching of human patellar tendon fibroblasts: activation of JNK and modulation of apoptosis. Knee Surg Sports Traumatol Arthrosc 2003; 11(2)122–9
- Smith H K, Maxwell L, Martyn J A, Bass J J. Nuclear DNA fragmentation and morphological alterations in adult rabbit skeletal muscle after short-term immobilization. Cell Tissue Res 2000; 302(2)235–41
- Steadman J R, Forster R S, Silferskiold J P. Rehabilitation of the knee. Clin Sports Med 1989; 8(3)605–27
- Takahashi T, Wada Y, Tanaka M, Yamanaka N, Yamamoto H. Mechanoreceptors and length of the patellar ligament after Ho-YAG laser treatment: a long-term follow-up in rabbits. Acta Orthop Scand 2002; 73(6)653–7
- Van Cruchten S, Van Den B W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol 2002; 31(4)214–23
- Wallace A L, Hollinshead R M, Frank C B. Creep behavior of a rabbit model of ligament laxity after electrothermal shrinkage in vivo. American Journal of Sports Medicine 2002; 30(1)98–102
- Wong K L, Williams G R. Complications of thermal capsulorrhaphy of the shoulder. J Bone Joint Surg (Am) 2001 83 Suppl 2 Pt 2:; 151–5
- Woo S L, Gomez M A, Woo Y K, Akeson W H. Mechanical properties of tendons and ligaments. II The relationships of immobilization and exercise on tissue remodeling. Biorheology 1982; 19(3)397–408
- Wyllie A H, Kerr J F, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol 1980; 68: 251–306
- Yuan J, Murrell G A, Wei A Q, Wang M X. Apoptosis in rotator cuff tendonopathy. J Orthop Res 2002; 20(6)1372–9
- Zeichen J, van Griensven M, Bosch U. The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. Am J Sports Med 2000; 28(6)888–92