Abstract
Background Impacted morselized bone allograft is a well-established way of giving joint arthroplasties additional support in situations where there is insufficient bone stock. For long-term survival of the implant, early implant fixation is important. We hypothesized that Col-loss, a bone protein lyophilisate, might improve early implant fixation of allografted implants.
Method We inserted 4 porous-coated Ti implants in the distal femurs of 16 dogs. All implants were surrounded by a 2.5-mm gap, which was impacted with morselized allograft with or without Colloss. In each dog, the implants were treated with no Collos or low-, middle- or high-dose (0, 10, 20 and 40 mg) Colloss per cm3 allograft. The observation time was 4 weeks.
Results Mechanical implant fixation was improved for all 3 groups with Colloss-treated implants (p < 0.05). The best anchorage was seen in the middle-dose group, where fixation was improved by 100%. We saw a dramatic reduction in fibrous tissue on the surface of the Colloss-treated implants (p < 0.001). The Colloss groups showed increased ongrowth of new bone (p < 0.01) and accelerated gap remodeling (p < 0.05).
Interpretation Colloss can improve early osseointegration and fixation of allografted implants.
Impacted morselized bone allograft is a well-estab-lished way of giving joint arthroplasties additional support in situations where there is insufficient bone stock (McDonald et al. Citation1988, Gie et al. Citation1993, Hubble and Smith Citation1996, Schreurs et al. Citation1994). This is often the case in revision surgery, which accounts for up to 20% of all joint replacement operations (Havelin et al. Citation2000). Apart from initial stability, the aim is to achieve bone ingrowth and healing of interfaces and bony defects. This provides a good long-term result (Lind et al. Citation2002, Ornstein Citation2002), and also provides better bone stock conditions in case of a re-revision. However, revision arthroplasties have higher failure rates and a poorer functional outcome (Espehaug et al. Citation1998). The impacted graft is often not incorporated by bone (Linder Citation2000, van der Donk et al. Citation2002), and high rates of implant subsidence have been reported (Eldridge et al. Citation1997, Meding et al. Citation1997, Pekkarinen et al. Citation2000). It is therefore of considerable interest to enhance the bioactivity of bone allograft.
Colloss is a lyophilized complex of the extracellular matrix proteins extracted from diaphyseal bovine bone. In animal studies, Colloss has shown bone regenerating properties in orthotopic application sites (Schlegel et al. Citation2004, Wiltfang et al. Citation2004), and also osteoinductive properties (Walboomers and Jansen Citation2005). In a porcine spine model for caged interbody fusion, Colloss gave the same fusion rates as autograft (Li et al. Citation2005). It is currently employed in clinical applications as bone void filler, for fracture healing and to increase bone volume in the vicinity of maxillofacial implants.
The Colloss material has a cotton-like structure, takes up practically no volume when mixed with other substances, and has no mechanical strength. It is therefore not expected to be of benefit by itself in closing large defects around implants in need of mechanical support. Its bone remodeling properties have never been investigated in combination with allograft, and there is also little information on its tissue regenerating effects on a clinically relevant titanium implant surface.
The purpose of the present experiment was to study the effect of Colloss on the fixation of allografted implants. The implant fixation was evaluated by mechanical testing, and by histomorphometrical assessment of the tissue on the implant surface and in the allografted gap around the implant. Different doses of Colloss were compared.
We hypothesized that Colloss would improve the overall implant fixation. For experimental purposes, we defined this as improving the mechanical fixation and enhancing new bone formation in the gap and on the implant surface, while at the same time maintaining a controlled bone graft resorption.
Animals and methods
Design
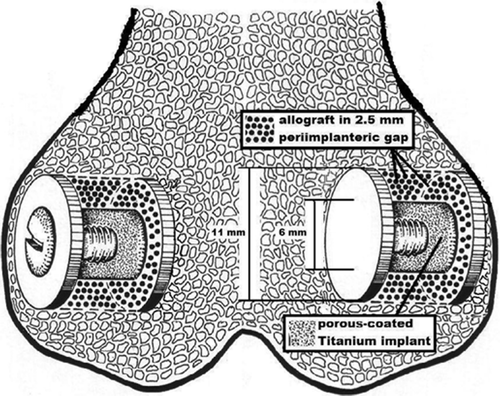
The experiment was designed as a paired animal study with 16 dogs. Each dog received 4 gap implants in the distal femurs (). Using impaction technique, the gap around each implant was filled with allograft with or without Colloss. In each dog, the implants were treated with no Col-loss or low-, middle- or high-dose (0, 10, 20 and 40 mg) Colloss per cm3 allograft. The implantation site of each group was alternated systematically with random start. The observation time was 4 weeks.
Implants
We used 64 porous-coated titanium alloy (Ti-6A1-4V) implants for the experiment, manufactured by Biomet Inc. (Warsaw, IN). The implants were cylindrical with a height of 10 mm and 6 mm in diameter. The porous coating was plasma-sprayed, giving a mean pore diameter of 480 μm and a porosity of 44% (as specified by the manufacturer). A footplate of 11 mm in diameter was attached to one end of each implant. When inserted into an 11mm drill hole, this centered the implant and provided a uniform 2.5-mm gap around it. After grafting of the gap, an 11-mm diameter top-washer was mounted on the outer end of the implant to ensure stability and bone graft containment ().
Fresh-frozen allograft
The bone graft was harvested immediately post mortem under sterile conditions, from two dogs not included in the study. The proximal humerus, the proximal tibia and the distal femur were used. All soft tissue and cartilage was removed. The bone was then morselized with a standard bone mill on fine setting, creating bone chips of 1–4 mm. The chips from the different bones were mixed together, swabbed, portioned in duplicate sterile containers and stored at –80°C. At surgery, bone chips larger than approximately 2 mm were not used. The graft was divided into four portions of 1 cm3 in a standardized container. Each portion of 1 cm3 allograft was added to 0, 10, 20 or 40 mg Colloss.
Animals
16 Labrador dogs with a mean weight of 29 (23– 35) kg and 14–15 months old were included in the study. Skeletal maturity was verified by radiography. 2 additional dogs served as bone graft donors. The dogs were bred for scientific purposes, and the experiment was approved and monitored by the Danish Animal Research Committee.
Surgical procedure
Under general anesthesia and using sterile conditions, the femoral epicondyles were exposed, starting with a medial incision. The joint capsule was opened, identifying the collateral ligament. A 2.5-mm guide wire was placed perpendicular to the epicondylar surface, 18 mm from the distal edge of the condyle and 14 mm from the anterior edge of the condyle. A cannulated drill bit of 11-mm diameter was then used to create a 12-mm-deep drill hole. A drill speed of 2 rotations per second was used to avoid thermal damage to the bone. The implants with footplates were inserted with a specially designed impaction tool, to ensure uniform central placement. The same tool was then used to impact 1 cm3 of bone allograft with or without Col-loss into the peri-implanteric gap. Finally, the top washer was mounted, and the soft tissues closed in layers. The procedure was repeated for the lateral side, and then for the opposite femur. Pre- and postoperatively, the dogs were given one dose of dicloxacillin, 1 g intravenously, as antibiotic prophylaxis. A Fentanyl transdermal patch (75 μg/h) lasting 3 days was given as postoperative analgesic treatment. The dogs were allowed unlimited activity. After 4 weeks of observation time, they were sedated and killed with an overdose of hypersaturated barbiturate.
Specimen preparation
The distal femurs were frozen and stored at –20°C immediately after retrieval. The outermost 0.5 mm of the implant-bone specimen was cut off and discarded. The rest of the implant with surrounding bone was divided into two sections perpendicular to the long axis of the implant with a water-cooled diamond band saw (Exact Apparatebau, Nordenstedt, Germany).
The outermost section was cut to a thickness of 3.5 mm and stored at –20°C pending mechanical testing (Linde and Sorensen Citation1993).
The innermost section was cut to a thickness of 5.5 mm and prepared for histomorphometry. The specimens were dehydrated in graded ethanol (70– 100%) containing basic fuchsin, and embedded in methylmetacrylate (Technovit 7200 VCL; Exact Apparatbau). Using vertical sectioning technique (Baddeley et al. Citation1986), each specimen was cut into four 30-μm thick histological sections with a microtome (KDG-95; MeProTech, Heerhugowaard, Holland). Finally, these were surface counterstained with 2% light green for 2 min, rinsed and mounted on glass. This preparation provides red staining of non-calcified tissue and green staining of calcified tissue, and the different types of calcified tissues such as woven bone and lamellar bone can be discriminated based on their morphological characteristics (Gotfredsen et al. Citation1989).
Mechanical testing
The thawed specimens were tested to failure by axial push-out test on an Instron Universal test machine (Instron Ltd., High Wycombe, UK). Testing was performed blinded and in one session. The specimens were placed with the cortical side facing up on a metal support jig with the implant centered over a 7.4-mm opening and under a cylindrical test probe of 5-mm diameter. A preload of 2 N defined the contact position for the start of the test. The implants were then pushed out of the surrounding tissue in the direction of the implant axis at a velocity of 5 mm/min. Load versus implant displacement data was continuously recorded. From these data, the mechanical implant fixation parameters as described earlier (Soballe Citation1993) were calculated: ultimate shear strength, apparent shear stiffness, and total energy absorption.
Histological evaluation
Blinded histomorphometry was performed using the stereological software C.A.S.T. Grid (Olympus Denmark AS, Ballerup, Denmark). With the aid of the software, we defined two regions of interest: zone 1 from the innermost parts of the implant surface and 500 μm into the gap, and zone 2 in the 500–2,500-μm part of the gap. In these gap zones, volume fractions of new bone, allograft, fibrous tissue and marrow space were quantified by point-counting technique (Gundersen et al. Citation1988, Sumner et al. Citation1990). On the implant surface, the area fractions of the same tissues were quantified by line-interception technique (Baddeley et al. Citation1986).
Statistics
We used the Intercooled STATA 8.0 software (StataCorp, College Station, TX).
The mechanical data followed a normal distribution, and fulfilled the assumptions for one-way ANOVA followed by paired t-test. The data are presented as means with 95% confidence intervals (CIs). Differences between means were considered statistically significant for p-values less than 0.05.
All histological datasets were not normally distributed, as some parameters were close to zero for certain groups. For simplicity, all parameters were evaluated by Friedman’s nonparametric analysis of paired data, followed by Wilcoxon signed rank test. The data are presented as medians and inter-quartile ranges. Differences between medians were considered statistically significant for p-values less than 0.05.
Results
Methodological results
Cultures from the morselized allograft showed no bacterial growth. No postoperative complications were seen, and all dogs were fully weight bearing within 3 days of surgery. All animals completed the 4-week observation period. There were no clinical signs of infection at the time of death, and cultures from the implantation sites at this time point were negative for pathogens.
Mechanical results (, )
All 3 Colloss-treated groups were better anchored in the tissue than the control group. This was the case for all mechanical parameters, except for 1 parameter in the high-dose group. The best mechanical fixation was seen in the middle-dose Colloss group, where the mechanical fixation of the implant was increased by up to 100%. There was no difference between the low-dose (10 mg) and the middle-dose (20 mg) groups. In the high-dose (40 mg) group, we saw a reduction in mechanical implant fixation compared to the middle-dose group. This was the case for all mechanical parameters.
Table 1. Results from mechanical push-out test; mean (95% CI)
Histological results (, and )
Figure 2. Characteristic histomorphometry of control implant with fibrous tissue covering the implant surface. Wov: woven bone; Lam: lamellar bone; Fib: fibrous tissue; Mar: marrow space.
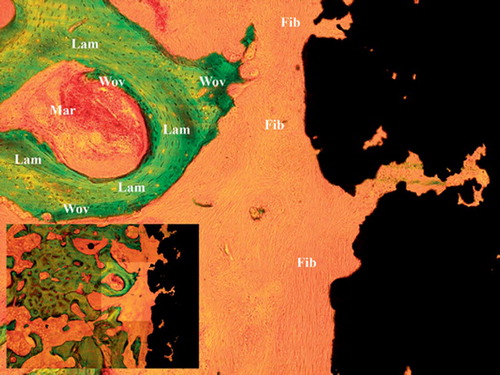
Figure 3. Characteristic histomorphometry of treated implant with no fibrous tissue and increased bone-implant contact. Note also the increased new bone formation along with increased allograft resorption. Wov: woven bone; Lam: lamellar bone; Fib: fibrous tissue, Mar: marrow space.
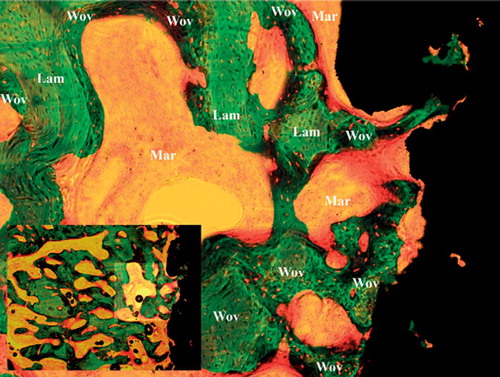
Table 2. Results from histomorphometrical analysis; median (interquartile range)
The mean surface intercept count for each implant was 535. The mean gap volume point count for zone 1 was 490, and for zone 2 it was 1,404.
Implant surface ongrowth
On the implant surface, the extent of new bone coverage was more than double in all Colloss-treated groups. The median fibrous tissue coverage of the implant surface was 39% in the control group and 0% in the treated groups.
Ingrowth in gap zone 1
In the innermost part of the gap—zone 1 (0–500 μm)—we also saw an almost complete elimination of fibrous tissue in the Colloss-treated implants. In the control group, the median volume fraction of fibrous tissue was 11% whereas it was under 1% in all 3 treatment groups. Colloss-treated implants had more new bone and less allograft in zone 1 than the control implants. There was no difference in total bone density (allograft plus new bone) between control and treatment groups (p = 0.4).
Ingrowth in gap zone 2
In the outer part of the gap—zone 2 (500–2,500 μm)—fibrous tissue was reduced from 6% in the control group to less than 1% in the treatment groups. As in zone 1, there was an increase in new bone formation in the Colloss-treated groups and a decrease in the volume fraction of allograft compared to the control group.
There was also a notable difference in zone 2 in the treatment groups. There was less allograft in the high-dose group than in the low- and middle-dose groups, indicating an increased rate of resorption. Moreover, this was not compensated for by an equivalent increase in new bone formation, giving a reduced total bone density (allograft plus new bone) in zone 2 for the high-dose group.
Discussion
Ideally, impacted bone allograft should be biocompatible and provide sufficient mechanical support. Over time, it should be replaced by new bone to provide a living, osseous anchorage of the implant. Based on radiographic appearance, this remodeling process appears to take place (Gie et al. Citation1993), but histological examinations of autopsy specimens have shown that the graft is commonly invaded by fibrous tissue rather than bone (Linder Citation2000, van der Donk et al. Citation2002). Other experimental studies have shown that impaction inhibits the growth of new bone into the allograft (Tägil and Aspenberg Citation1998). Although one would presume that new bone growth into the graft material is better than fibrous tissue, it has also been suggested that infiltration of fibrous tissue increases the mechanical strength of impacted allograft pending remodeling (Tägil and Aspenberg Citation2001).
The osteoinductive properties of demineralized bone matrix were described by Urist in the 1960s (Urist Citation2002). Commercially available purified bone morphogenetic proteins (BMPs) are used clinically in fracture healing, and have shown varying osteogenic properties in animal implant models (Lind et al. Citation2000, Citation2001, Soballe et al. Citation2004). Their effects on implant fixation are controversial. Massive bone resorption was observed in a pilot study using OP1 as bone growth stimulator in posterolateral spine fusion (Laursen et al. Citation1999). OP-1 increased new bone formation around the implant in a canine model, but also accelerated graft resorption and mechanical weakening (Jensen et al. Citation2002). Accelerated remodeling has presumably resulted in a transient period with the combination of weakened graft and immature woven bone, causing mechanical failure as postulated by Jeppson and Aspenberg (Jeppsson et al. Citation2003). It is unclear whether these findings are due to dosage, carrier issues or the monotherapy approach of using a single purified BMP.
The Colloss material consists of the collagenous and non-collagenous proteins of bone. Collagen by itself is not osteoinductive (Murata et al. Citation2000, van den Dolder et al. Citation2003), and the exact composition of the non-collagenous proteins in Colloss has not yet been determined. Still unpublished mass spectroscopy data has shown the presence of TGFβ-1, TGFβ-2 and BMP3 in similarly processed bone (O N Jensen, Protein Research Group, SDU, Odense Denmark. Personal communication, June 30, 2005). One study using immunohistochemistry found a high initial expression of BMP-2 at Colloss-treated implantation sites (Schlegel et al. Citation2004). This may indicate the presence of BMP-2 in Colloss, but could also mean that Colloss had stimulated production of BMP-2 in the surrounding tissue by another pathway.
In the present study, we examined the effects of Colloss on porous-coated Ti implants in an impaction allograft setting. We used dogs as experimental animals because their epiphyseal cancellous bone closely resembles the bone in which most joint implant components are fixed. Canine bone reflects the composition, density and quality of human bone better than that of most other animals (Aerssens et al. Citation1998), and substantial bone-implant research has been done in dogs. However, no animal experiment can give complete information about the effects of a given treatment in humans: canine bone remodels three times faster than human bone, and all implants were put in the healthy bone of young dogs.
For the experiment, we chose a non-weight-bear-ing model using a 2.5-mm-gap implant. In contrast to the weight-bearing model, it lacks clinically relevant influences such as joint fluid pressure and direct load. Furthermore, it does not provide the revision environment of compromised bone as replicated in the micromotion model of Bechtold and Soballe (Bechtold et al. Citation2001). However, this basic experimental model is well controlled and has less “noise” than the weight-bearing model (Soballe Citation1993). It also permits a gap large enough for simulation of impaction grafting, and it permits paired comparison of up to four treatment groups.
Push-out test revealed a markedly improved mechanical implant fixation in all Colloss-treated groups. The best mechanical results were seen in the middle-dose group of 20 mg/cm3 allograft, where energy absorption and resistance to shear forces was almost double. When the dose of Col-loss was doubled to 40 mg/cm3 in the high-dose group, there was a reduction in mechanical fixation compared to the middle-dose group.
The histological findings were in full accordance with the mechanical results. Whereas the control implants were partly covered by a dense fibrous membrane, fibrous tissue was almost eliminated in all of the treatment groups. The treated implants were better osseointegrated, and there was more new bone formation in the impaction-allografted gap. There was also increased allograft resorption in the treated groups. This seemed to be dose-dependent, as the high-dose group had less remaining allograft than the low- and middle-dose groups.
The effect of Colloss on fibrous tissue formation is interesting, as implants embedded in a fibrous capsule represent a clinical problem. The elasticity of such fibrous tissue can result in excessive micromotion of the implant and subsequent loosening (Van der Vis et al. Citation1999). There is more wear particle movement along a fibrous implant interface (Rahbek et al. Citation2000), which may promote implant loosening further through inflammatory foreign-body responses.
One explanation for the reduction in fibrous tissue may be that bone formation is stimulated directly through growth factors present in the Colloss material, or by Colloss-mediated release of growth factors from the surrounding tissue. Another explanation could be stimulation of angioneogenesis, giving more vascularization and better conditions for highly differentiated tissues such as bone. However, this experimental model is limited to detection of the biological responses to implants and adjuvant therapies in terms of mechanical fixation and tissue distribution. No conclusions regarding the specific interaction between Colloss and cells can be made from this study.
The implants treated with high doses of Colloss were not as firmly anchored as the middle-dose group. We believe that the reason for this was overstimulation of the remodeling process. The volume fraction of new bone remained constant throughout the gap for all three treatment groups, but in zone 2 of the high-dose group we saw a reduction in the volume fraction of allograft. This may indicate that stimulation beyond a certain level has less effect on new bone formation, but a continuous effect on accelerating osteoclastic bone resorption.
The finding of an optimum dosage range of 10–20 mg Colloss per cm3 allograft may also indicate that excessive stimulation can actually cause weakening of the implant fixation, just as has been observed using purified monotherapy BMPs and rhTGFβ−1 (Lind et al. Citation1996). This further suggests that the adverse effects on implant fixation that have been seen previously with these substances may have been due to overdosing.
Our findings are encouraging. We have shown that the Colloss material can improve mechanical implant fixation, almost eliminate fibrous tissue formation, and increase the osseous anchorage and bone remodeling of allografted porous-coated Ti implants. Further research is warranted and currently under way. The results are certainly promising, and may prove useful for improving the longevity and clinical outcome of allografted revision joint implants.
The authors wish to thank laboratory technicians Jane Pauli, Lisa Feng and Anette Milton for excellent laboratory work with the histological sections, and Niels Trolle Andersen for guidance with statistics.
Colloss was donated unconditionally by Ossacur AG, Germany. The implants were provided unconditionally by Biomet Inc., Warsaw, IN. Unconditional support for the work was provided by the Augustinus Fund, Denmark, Danfoss A/S, Nordborg, Denmark, the Hartmann Fund, Denmark, and through the Interdiciplinary Research Group “Nanoscience and Biocompatibility” which is funded by the Danish Research Council (2052-01-006).
Contributions of authors
JB design, surgery, evaluation, statistics, manuscript, discussion. AL, TBJ, BE, KS design, surgery, manuscript comments, discussion.
- Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 1998; 139: 663–70
- Baddeley A J, Gundersen H J, Cruz-Orive L M. Estimation of surface area from vertical sections. J Microsc 1986; 142(Pt 3)259–76
- Bechtold J E, Mouzin O, Kidder L, Soballe K. A controlled experimental model of revision implants: Part II. Implementation with loaded titanium implants and bone graft. Acta Orthop Scand 2001; 72: 650–6
- Eldridge J D, Smith E J, Hubble M J, Whitehouse S L, Lear-month I D. Massive early subsidence following femoral impaction grafting. J Arthroplasty 1997; 12: 535–40
- Espehaug B, Havelin L I, Engesaeter L B, Langeland N, Vollset S E. Patient satisfaction and function after primary and revision total hip replacement. Clin Orthop 1998, 351: 135–48
- Gie G A, Linder L, Ling R S, Simon J P, Slooff T J, Timperley A J. Contained morselized allograft in revision total hip arthroplasty. Surgical technique. Orthop Clin North Am 1993; 24: 717–25
- Gotfredsen K, Budtz-Jorgensen E, Jensen L N. A method for preparing and staining histological sections containing titanium implants for light microscopy. Stain Technol 1989; 64: 121–7
- Gundersen H J, Bendtsen T F, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, Sorensen F B, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988; 96: 379–94
- Havelin L I, Engesaeter L B, Espehaug B, Furnes O, Lie S A, Vollset S E. The Norwegian Arthroplasty Register: 11 years and 73,000 arthroplasties. Acta Orthop Scand 2000; 71: 337–53
- Hubble M J, Smith E J. Revision of failed total hip replacement. Br J Hosp Med 1996; 55: 432–6
- Jensen T B, Overgaard S, Lind M, Rahbek O, Bunger C, Soballe K. Osteogenic protein 1 device increases bone formation and bone graft resorption around cementless implants. Acta Orthop Scand 2002; 73: 31–9
- Jeppsson C, Åstrand J, Tägil M, Aspenberg P. A combination of bisphosphonate and BMP additives in impacted bone allografts. Acta Orthop Scand 2003; 74: 483–9
- Laursen M, Hoy K, Hansen E S, Gelineck J, Christensen F B, Bunger C E. Recombinant bone morphogenetic pro-tein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur Spine J 1999; 8: 485–90
- Li H, Zou X, Woo C, Ding M, Lind M, Bunger C. Experimental anterior lumbar interbody fusion with an osteoinductive bovine bone collagen extract. Spine 2005; 30: 890–6
- Lind M, Overgaard S, Soballe K, Nguyen T, Ongpipattanakul B, Bunger C. Transforming growth factor-beta 1 enhances bone healing to unloaded tricalcium phosphate coated implants: an experimental study in dogs. J Orthop Res 1996; 14: 343–50
- Lind M, Overgaard S, Song Y, Goodman S B, Bunger C, Soballe K. Osteogenic protein 1 device stimulates bone healing to hydroxyapaptite-coated and titanium implants. J Arthroplasty 2000; 15: 339–46
- Lind M, Overgaard S, Jensen T B, Song Y, Goodman S B, Bunger C, Soballe K. Effect of osteogenic protein 1/colla-gen composite combined with impacted allograft around hydroxyapatite-coated titanium alloy implants is moderate. J Biomed Mater Res 2001; 55: 89–95
- Lind M, Krarup N, Mikkelsen S, Horlyck E. Exchange impaction allografting for femoral revision hip arthroplasty: results in 87 cases after 3.6 years’ follow-up. J Arthroplasty 2002; 17: 158–64
- Linde F, Sorensen H C. The effect of different storage methods on the mechanical properties of trabecular bone. J Biomech 1993; 26: 1249–52
- Linder L. Cancellous impaction grafting in the human femur: histological and radiographic observations in 6 autopsy femurs and 8 biopsies. Acta Orthop Scand 2000; 71: 543–52
- McDonald D J, Fitzgerald R H, Jr., Chao E Y. The enhancement of fixation of a porous-coated femoral component by autograft and allograft in the dog. J Bone Joint Surg (Am) 1988; 70: 728–37
- Meding J B, Ritter M A, Keating E M, Faris P M. Impaction bone-grafting before insertion of a femoral stem with cement in revision total hip arthroplasty. A minimum two-year follow-up study. J Bone Joint Surg (Am) 1997; 79: 1834–41
- Murata M, Maki F, Sato D, Shibata T, Arisue M. Bone augmentation by onlay implant using recombinant human BMP-2 and collagen on adult rat skull without periosteum. Clin Oral Implants Res 2000; 11: 289–95
- Ornstein E. Hip revisions with impacted morselized allograft bone and cement. Patient outcome, prosthetic fixation and risks. Acta Orthop Scand 2002; 73(Suppl 306)1–66
- Pekkarinen J, Alho A, Lepisto J, Ylikoski M, Ylinen P, Paavilainen T. Impaction bone grafting in revision hip surgery. A high incidence of complications. J Bone Joint Surg (Br) 2000, 82: 103–7
- Rahbek O, Overgaard S, Jensen T B, Bendix K, Soballe K. Sealing effect of hydroxyapatite coating: a 12-month study in canines. Acta Orthop Scand 2000; 71: 563–73
- Schlegel K A, Donath K, Rupprecht S, Falk S, Zimmermann R, Felszeghy E, Wiltfang J. De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials 2004; 25: 5387–93
- Schreurs B W, Buma P, Huiskes R, Slagter J L, Slooff T J. Morsellized allografts for fixation of the hip prosthesis femoral component. A mechanical and histological study in the goat. Acta Orthop Scand 1994; 65: 267–75
- Søballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand 1993, Suppl 255: 1–58
- Søballe K, Jensen T B, Mouzin O, Kidder L, Bechtold J E. Differential effect of a bone morphogenetic protein-7 (OP-1) on primary and revision loaded, stable implants with allograft. J Biomed Mater Res A 2004; 71: 569–76
- Sumner D R, Bryan J M, Urban R M, Kuszak J R. Measuring the volume fraction of bone ingrowth: a comparison of three techniques. J Orthop Res 1990; 8: 448–52
- Tägil M, Aspenberg P. Impaction of cancellous bone grafts impairs osteoconduction in titanium chambers. Clin Orthop 1998, 352: 231–8
- Tägil M, Aspenberg P. Fibrous tissue armoring increases the mechanical strength of an impacted bone graft. Acta Orthop Scand 2001; 72: 78–82
- Urist M R. Bone: formation by autoinduction. 1965. Clin Orthop 2002, 395: 4–10
- van den Dolder J, Bancroft G N, Sikavitsas V I, Spauwen P H, Mikos A G, Jansen J A. Effect of fibronectin- and collagen I-coated titanium fiber mesh on proliferation and differentiation of osteogenic cells. Tissue Eng 2003; 9: 505–15
- van der Donk S, Buma P, Slooff T J, Gardeniers J W, Schreurs B W. Incorporation of morselized bone grafts: a study of 24 acetabular biopsy specimens. Clin Orthop 2002, 396: 131–41
- Van der Vis H M, Aspenberg P, Tigchelaar W, Van Noorden C J. Mechanical compression of a fibrous membrane surrounding bone causes bone resorption. Acta Histochem 1999; 101: 203–12
- Walboomers X F, Jansen J A. Bone tissue induction, using a COLLOSS-filled titanium fibre mesh-scaffolding material. Biomaterials 2005; 26: 4779–85
- Wiltfang J, Kloss F R, Kessler P, Nkenke E, Schultze-Mosgau S, Zimmermann R, Schlegel K A. Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects. An animal experiment. Clin Oral Implants Res 2004; 15: 187–93