Abstract
Background Osteonecrosis (ON) of the femoral head is a serious complication in patients who have undergone organ transplantation. Introduction of cyclosporin A has resulted in lower-dosage steroid treatment and a decrease in the occurrence of ON. We examined the effect of cyclosporin A on the development of ON in rabbits.
Methods In experiment A, rabbits were given cyclosporin A and 20 mg/kg methylprednisolone acetate. The control group was given 20 mg/kg methylprednisolone acetate only. Experiment B was then performed to mimic the clinical situation in which the use of cyclosporin A and lower steroid doses resulted in a decrease in occurrence of ON. In Experiment C, the effects of treatment with cyclosporin A only on development of ON were examined. 4 weeks after injection, bilateral femora and humeri were examined histopathologically for ON.
Results Cyclosporin A increased the incidence of ON in rabbits when given in combination with steroid (p = 0.04). No ON lesions were observed in rabbits treated with cyclosporin A alone.
Interpretation Our findings suggest that the clinically reported reduction in occurrence of ON following the use of cyclosporin A is probably attributable to the lower steroid doses used.
Osteonecrosis (ON) of the femoral head is a serious complication in patients undergoing organ transplantation—including kidney, bone marrow and liver allografts (Landmann et al. Citation1987, Kubo et al. Citation1997, Lieberman et al. Citation2000, Torii et al. Citation2001). MRI analysis has demonstrated an ON incidence of 21% and 19% for kidney and bone marrow transplantation, respectively (Kubo et al. Citation1997, Torii et al. Citation2001).
The precise etiology of posttransplant ON remains unclear. Steroid treatment following transplantation is generally considered to be a cause of ON, as steroid dosage has been found to be correlated to the incidence of ON after organ transplantation (Fink et al. Citation1998, Kubo et al. Citation1998). Several different kinds of possible pathogenesis have been suggested for steroid-induced ON by experimental studies, including thrombophilic and hypofibrinolytic coagulation abnormalities and hyperlipidemia (Glueck et al. Citation1994, Jones JP, Jr Citation1993, Yamamoto et al. Citation1997, Miyanishi et al. Citation2002).
Cyclosporin A (CsA) is a potent immunosuppressive drug that is widely used to prevent the rejection of transplanted organs (Powles et al. Citation1980). The conversion from high-dose steroid treatment to combined CsA and low-dose steroid treatment resulted in a reduction in the incidence of ON (Landmann et al. Citation1987). We examined the effects of CsA on development of ON in rabbits, alone and together with steroid treatment.
Animals and methods
We used a rabbit model of steroid-induced ON (Yamamoto et al. Citation1997). No surgery was performed in the rabbits. Administration of a single high dose (20 mg/kg) of methylprednisolone acetate (Upjohn, Tokyo, Japan), simulating a dose of human steroid pulse therapy, reproducibly causes ON lesions in this model. All experiments were reviewed by the Common Ethics Committee for Animal Experiments at our university, and were conducted in accordance with the Guidelines for Animal Experimentation, the Law (no. 105), and notification (no. 6) of the government and the Committee on Ethics in Japan.
Animals
90 adult (i.e. with the growth plate already closed) male Japanese white rabbits (Kyudo, Tosu, Japan) weighing 3.3–3.9 kg were used at the Animal Center of our university and maintained on a standard laboratory diet and water. Their ages ranged from 28 to 32 weeks.
Experimental design
Experiment A.
22 rabbits were given 25 mg/kg/day of CsA (Novartis, Tokyo, Japan) intramuscularly for 2 days (Green et al. Citation1978, Dunn et al. Citation1979, Durak et al. Citation1998), and also a single dose (20 mg/ kg) of methylprednisolone acetate after the second CsA injection. 43 rabbits were given a single dose (20 mg/kg) of methylprednisolone acetate only intramuscularly as a control. Different numbers of rabbits were used for the experimental and control groups due to limitation of the amount of CsA available.
Experiment B.
15 rabbits were given 25 mg/kg/ day of CsA intramuscularly for 2 days, together with a single dose of 8.8 mg/kg methylprednisolone acetate after the second CsA injection. The 43 rabbits given a single dose (20 mg/kg) of methylprednisolone acetate only in experiment A were also used as controls in this experiment.
Experiment C.
We examined the effect of treatment with CsA alone on development of ON. 10 rabbits were given CsA (25 mg/kg/day) intramuscularly for 2 days (Green et al. Citation1978, Dunn et al. Citation1979, Durak et al. Citation1998).
Tissue preparation
4 weeks after injection of methylprednisolone acetate (experiments A and B) or the first CsA injection (experiment C), animals were anesthetized with an intravenous injection of pentobarbital sodium (25 mg/kg of body weight) (Abbott Laboratories, Abbott Park, USA) and then killed by exsanguination via aortectomy. For light microscopic examination, both femora and humeri (for a total of 4 bone samples per rabbit) were obtained at the time of death and fixed in a 10% formalin-0.1M phosphate buffer (pH 7.4) for 1 week. Bone samples were decalcified with 25% formic acid for 3 days and then neutralized with 0.35 M sodium sulfate for 3 days. Samples were then cut along the coronal plane in the proximal one-third and axial plane in the distal part (condyle). Lastly, the specimens were embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin.
Evaluation of osteonecrosis
Whole areas of the proximal one-third and distal condyles of both femora and humeri (for a total of 8 regions) were examined histopathologically for the presence of ON. Diagnosis of ON was made in blinded fashion by 3 authors (KM, TY, TI), based on the diffuse presence of empty lacunae or pyknotic nuclei of osteocytes in the bone trabeculae, accompanied by cell necrosis in the surrounding bone marrow (Yamamoto et al. Citation1997, Miyanishi et al. Citation2002). The 3 examiners independently made a diagnosis of ON for each sample without knowing the group from which the sample had come. Rabbits that had at least one osteonecrotic lesion in the 8 areas examined were considered to be rabbits with ON. The numbers of rabbits with ON and number of osteonecrotic regions per rabbit (maximum 8 regions) were determined.
Hematological examination
Blood samples were obtained from fasted rabbits prior to the experiment and at 1, 2, 3 and 4 weeks after injection of methylprednisolone acetate (experiments A and B) or the first CsA injection (experiment C). Hematological evaluations of low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) plasma levels were carried out using the turbidimetric method (Kawai et al. Citation1978).
Whole blood concentrations of CsA were measured with a fluorescence polarization immunoassay kit (Abbott Laboratories, Abbott Park, USA). Measurements were taken 1 and 2 weeks after methylprednisolone acetate injection in experiment A, because we have found that the 1–2 week period after methylprednisolone acetate injection is critical for development of ON in this rabbit model (Yamamoto et al. Citation1997, Miyanishi et al. Citation2002).
Statistics
Numbers of ON-positive rabbits were compared using Fisher's exact test (experiment A) or chi-square test (experiment B). Numbers of ON regions per rabbit and hematological data were compared using the Mann-Whitney U-test and unpaired Student's t-test, respectively. Statistical analyses were performed using StatView J-5.0 (SAS Institute Inc., Cary, NC). P-values ≤ 0.05 were considered significant.
Results
5 rabbits died during the experiments. 2 of the rabbits treated with both methylprednisolone acetate and CsA and 3 of the rabbits receiving methylprednisolone acetate alone died 2 weeks after injection of methylprednisolone acetate. These 5 rabbits were excluded from the study. The dead rabbits with combined CsA and steroid treatment all belonged to experiment A.
Histopathological features
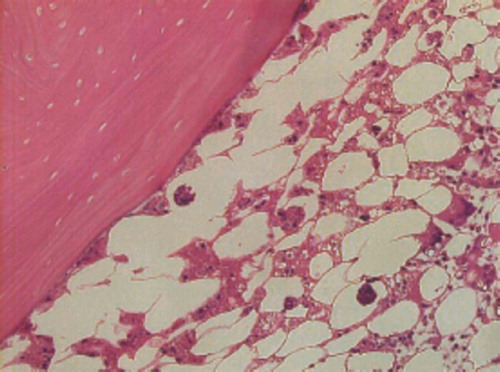
Macroscopically, osteonecrosis appeared as yellowish-colored areas. Histologically, ON lesions showed an accumulation of bone marrow cell debris and bone trabeculae with empty lacunae (). For experiments A and B, these findings were consistent in all osteonecrotic tissues. All 3 examiners agreed on the diagnosis of ON in every sample. In experiment C, the histological findings for bone marrow cells and bone trabeculae were almost normal in rabbits given cyclosporin A alone. Quantitative assessment of thrombus and lipid embolus formation was sometimes difficult due to variation in size, and was not performed.
Incidence of osteonecrosis
Experiment A.
There was an increase in the incidence of ON in rabbits treated with methylprednisolone acetate and CsA relative to rabbits receiving methylprednisolone acetate alone (p = 0.04; ). The number of ON regions per rabbit was 4.4 (SD 1.3) in rabbits receiving methylprednisolone acetate and CsA whereas it was 2.5 (SD 1.6) in those receiving methylprednisolone acetate alone (p < 0.001; ).
Figure 2. Number of osteonecrotic regions per rabbit. A. Rabbits were given cyclosporin A (CsA) and a single dose of 20 mg/kg methylprednisolone acetate (MPSL). B. Rabbits were given CsA and a reduced dose of MPSL (8.8 mg/kg). Proximal and distal parts of bilateral femora and humeri (for a total of 8 regions) were examined for the presence of ON 4 weeks after the MPSL injection. Rabbits receiving 20 mg/kg MPSL alone served as a control.
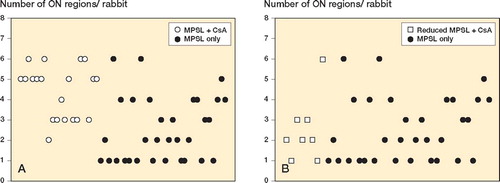
Table 1. Incidence of ON in experiment A
Experiment B.
The incidence of ON decreased in rabbits receiving reduced doses of methylprednisolone acetate together with CsA relative to rabbits receiving the original high dose of methylprednisolone acetate alone (p = 0.05) (). However, no differences between the two groups were observed in the number of ON regions per rabbit (p = 1; ).
Table 2. Incidence of ON in experiment B
Experiment C.
No ON lesions were observed in rabbits treated with CsA alone.
Hematological examination
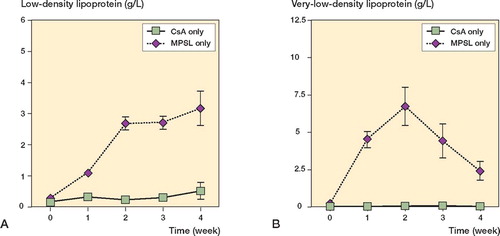
In experiment A, the serum levels of LDL and VLDL were higher at 2 weeks in rabbits receiving methylprednisolone acetate and CsA than in those receiving methylprednisolone acetate alone (p < 0.001; ). There was no statistical difference in LDL and VLDL levels between the two groups at any time points examined, except at 2 weeks. In experiment B, the serum LDL levels were lower at 2, 3, and 4 weeks in rabbits receiving a reduced dose of methylprednisolone acetate and CsA than in those receiving the original dose of methylprednisolone acetate alone (p < 0.001; ). The rabbits with a reduced dose of methylprednisolone acetate and CsA also showed lower serum VLDL levels at 1, 2, and 3 weeks as compared to control rabbits (p < 0.001; ). In experiment C, LDL and VLDL levels remained unchanged throughout the experimental period ().
Figure 3. Sequential changes in serum levels of low-density lipoprotein (A) and very low-density lipoprotein (B) in experiment A. Rabbits receiving cyclosporin A (CsA) were also given a single dose of 20 mg/kg methylprednisolone acetate (MPSL). Rabbits receiving 20 mg/kg MPSL alone served as a control.
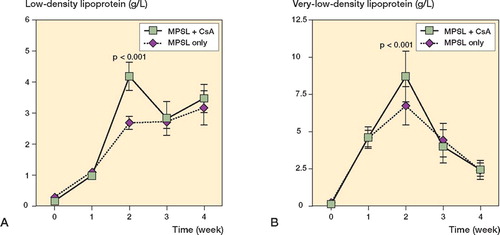
Figure 4. Sequential changes in serum levels of low-density lipoprotein (A) and very low-density lipoprotein (B) in experiment B. Rabbits given cyclosporin A (CsA) were also given a reduced dose of MPSL (8.8 mg/kg). Rabbits receiving a single dose of 20 mg/kg MPSL alone served as a control.
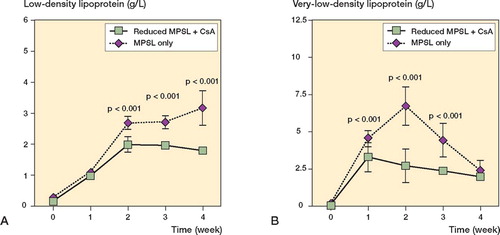
Figure 5. Sequential changes in serum levels of low-density lipoprotein (A) and very low-density lipoprotein (B) in experiment C. Rabbits were treated with cyclosporin A (CsA) alone. Data from rabbits receiving 20 mg/kg MPSL alone are also shown as a reference.
Blood concentrations of CsA in rabbits given methylprednisolone acetate and CsA were 225 (SD 15) ng/mL and 167 (SD 13) ng/mL 1 and 2 weeks after methylprednisolone acetate injection, respectively. These concentrations are all within the immunosuppressive range, as determined on the basis of a previous study (Andersen et al. Citation1994).
Discussion
In this study, the dose of CsA was determined on the basis of previous studies (Green et al. Citation1978, Dunn et al. Citation1979, Durak et al. Citation1998). The dosage of immunosuppressant and duration of treatment used here produced blood drug levels falling within the human therapeutic range 1 and 2 weeks after methylprednisolone acetate injection, which represents a period that is critical for development of osteonecrosis in rabbits (Andersen et al. Citation1994, Yamamoto et al. Citation1997, Miyanishi et al. Citation2002).
To the best of our knowledge, the effects of immunosuppressive drugs on the development of ON in humans have not yet been fully elucidated. An association between immune complexes and ON development has been demonstrated in rabbits (Nakata et al. Citation1996). In this respect, immunosuppressants may be protective in ON. On the other hand, ON has been increasingly recognized as an important complication in patients infected with human immunodeficiency virus (Allison et al. Citation2003), which may suggest a possible stimulatory effect of immunosuppressants on osteonecrosis. To address this question, experiment A was performed under conditions in which the same steroid dose was given to each group.
CsA therapy has been found to be responsible for increased incidence of thromboembolic complications such as renal thrombotic microangiopathy (Guillemain et al. Citation1990) and thrombus formation in systemic venous vessels (Vanrenterghem et al. Citation1985). Hyperlipidemia is another frequent complication of CsA treatment following organ transplantation (Colak et al. Citation2002). Our observation of higher serum levels of LDL and VLDL in CsAtreated rabbits at 2 weeks corroborates this finding. One possible explanation—which could partly account for the increased incidence of ON in rabbits treated with methylprednisolone acetate and CsA in experiment A—may be that CsA enhances a steroid-induced procoagulant and hyperlipidemic plasma state.
It is worth noting that an increase in the incidence of ON was observed only when CsA was given in the presence of steroids; no ON lesions resulted from treatment with CsA alone. One study of the interaction between CsA and steroids has documented reduced clearance of steroids in CsAtreated patients (Langhoff et al. Citation1985).
Experiment B was carried out in order to mimic the clinical situation in which the use of combined CsA and low-dose steroid treatment ultimately results in a reduction in occurrence of ON (Landmann et al. Citation1987). The reduced steroid dose used here was determined on the basis of a clinical steroid dose reduction rate reported previously (Landmann et al. Citation1987). One important limitation of experiment B was that the rabbits given 20 mg/kg methylprednisolone acetate only were not a proper control in the strict sense of the term, since they received a much higher dose of methylprednisolone acetate than the experimental group. However, this was used as a reference point, relative to which the effects of combinations of cyclosporin A and low-dose steroid given here could be evaluated.
In conclusion, we found that cyclosporin A increased the incidence of ON in rabbits when given in combination with steroid. The results suggest that the clinically reported decrease in occurrence of ON following the introduction of cyclosporin A is most likely attributable to the lower steroid doses used.
This work was supported in part by a grant from the Nakatomi Foundation, a grant-in-aid for JSPS fellows, and a grant for intractable diseases from the Ministry of Health and Welfare of Japan.
No competing interests declared.
Contributions of authors
KM, TY, SJ and YI designed the research. KM, TY, TI, AY and GM did the experiment and collected and analyzed the data. KM, TY and TI made the histological examinations. KM wrote the draft manuscript. TY, SJ and YI revised the draft manuscript.
- Allison G T, Bostrom M P, Glesby M J. Osteonecrosis in HIV disease: epidemiology, etiologies, and clinical management. Aids 2003; 17: 1–9
- Andersen H, Madsen G, Nordestgaard B G, Hansen B F, Kjeldsen K, Stender S. Cyclosporin suppresses transplant arteriosclerosis in the aorta-allografted, cholesterol-clamped rabbit. Suppression preceded by decrease in arterial lipoprotein permeability. Arterioscler Thromb 1994; 14: 944–50
- Colak T, Karakayali H, Yagmurdur M C, Moray G. Effect of conversion from cyclosporine to tacrolimus on lipid profiles in renal transplant recipients. Transplant Proc 2002; 34: 2081–2
- Dunn D C, White D J, Herbertson B M, Wade J. Prolongation of kidney survival during and after cyclosporin A therapy. Transplantation 1979; 27: 359–61
- Durak I, Karabacak H I, Buyukkocak S, Cimen M Y, Kacmaz M, Omeroglu E, Ozturk H S. Impaired antioxidant defense system in the kidney tissues from rabbits treated with cyclosporine. Protective effects of vitamins E C. Nephron 1998; 78: 207–11
- Fink J C, Leisenring W M, Sullivan K M, Sherrard D J, Weiss N S. Avascular necrosis following bone marrow transplantation: a case-control study. Bone 1998; 22: 67–71
- Glueck C J, Freiberg R, Glueck H I, Henderson C, Welch M, Tracy T, Stroop D, Hamer T, Sosa F, Levy M. Hypofibrinolysis: a common, major cause of osteonecrosis. Am J Hematol 1994; 45: 156–66
- Green C J, Allison A C. Extensive prolongation of rabbit kidney allograft survival after short-term cyclosporin-A treatment. Lancet 1978; 1(8075)1182–3
- Guillemain R, Farge D, Amrein C, Vulser C, Bruneval P, Jacquot C, Carpentier A. Thrombotic microangiopathy with reversible acute renal failure in a cardiac transplant recipient under cyclosporin. Clin Nephrol 1990; 34: 237–8
- Jones J P, Jr. Fat embolism, intravascular coagulation and osteonecrosis. Clin Orthop 1993, 292: 294–308
- Kawai T, Sakurabayashi I, Koide A. Fractional quantitation of serum beta-lipoprotein by a simple turbidimetric method. Protides of the biological fluids. Pergamon Press, Oxford and New York 1978; 415
- Kubo T, Yamazoe S, Sugano N, Fujioka M, Naruse S, Yoshimura N, Oka T, Hirasawa Y. Initial MRI findings of non-traumatic osteonecrosis of the femoral head in renal allograft recipients. Magn Reson Imaging 1997; 15: 1017–23
- Kubo T, Fujioka M, Yamazoe S, Yoshimura N, Oka T, Ushijima Y, Hasegawa Y, Hirasawa Y. Relationship between steroid dosage and osteonecrosis of the femoral head after renal transplantation as measured by magnetic resonance imaging. Transplant Proc 1998; 30: 3039–40
- Landmann J, Renner N, Gachter A, Thiel G, Harder F. Cyclosporin A and osteonecrosis of the femoral head. J Bone Joint Surg (Am) 1987; 69: 1226–8
- Langhoff E, Madsen S, Flachs H, Olgaard K, Ladefoged J, Hvidberg E F. Inhibition of prednisolone metabolism by cyclosporine in kidney-transplanted patients. Transplantation 1985; 39: 107–9
- Lieberman J R, Scaduto A A, Wellmeyer E. Symptomatic osteonecrosis of the hip after orthotopic liver transplantation. J Arthroplasty 2000; 15: 767–71
- Miyanishi K, Yamamoto T, Irisa T, Yamashita A, Jingushi S, Noguchi Y, Iwamoto Y. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone 2002; 30: 185–90
- Nakata K, Masuhara K, Nakamura N, Shibuya T, Sugano N, Matsui M, Ochi T, Ohzono K. Inducible osteonecrosis in a rabbit serum sickness model: deposition of immune complexes in bone marrow. Bone 1996; 18: 609–15
- Powles R L, Clink H M, Spence D, Morgenstern G, Watson J G, Selby P J, Woods M, Barrett A, Jameson B, Sloane J, Lawler S D, Kay H E, Lawson D, McElwain T J, Alexander P. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet 1980; 1(8164)327–9
- Torii Y, Hasegawa Y, Kubo T, Kodera Y, Minami S, Morishita Y, Yamada Y, Iwata H. Osteonecrosis of the femoral head after allogeneic bone marrow transplantation. Clin Orthop 2001, 382: 124–32
- Vanrenterghem Y, Roels L, Lerut T, Gruwez J, Michielsen P, Gresele P, Deckmyn H, Colucci M, Arnout J, Vermylen J. Thromboembolic complications and haemostatic changes in cyclosporin-treated cadaveric kidney allograft recipients. Lancet 1985; 1(8436)999–1002
- Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues. Arthritis Rheum 1997; 40: 2055–64