Since vacuum-assisted wound closure (VAC) was introduced independently by 2 groups (Fleischmann et al. Citation1995, Morykwas et al. Citation1997), the technique has gained wide acceptance. VAC exposes the wound bed to an under-pressure, thereby removing fluid from the extravascular space. This improves circulation and promotes wound healing and formation of granulation tissue (Morykwas et al. Citation1997, Müllner et al. Citation1997, Armstrong and Lavery Citation2005). The open-cell vacuum technique has been used in a variety of surgical disciplines, i.e. in abdominal, vascular, gynecological and plastic surgery. For orthopedic surgeons, however, VAC may be a relatively new method (Webb Citation2002). The commercially available VAC system from Kinetic Concepts Inc. (KCI, San Antonio, Texas) has been on the market for approximately 10 years and numerous reports on its use have been published. This system is expensive, and instead we have used a simplified, effective and inexpensive VAC system during the last few years.
Method and patients
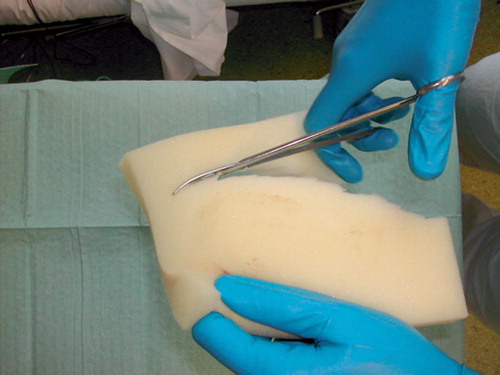
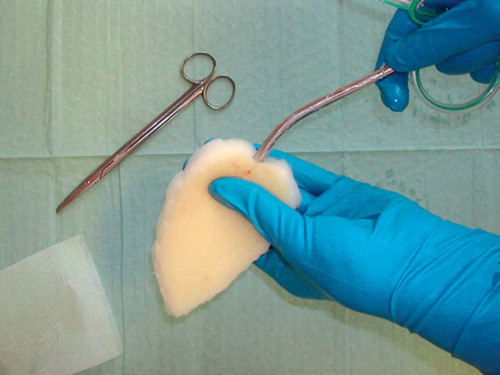
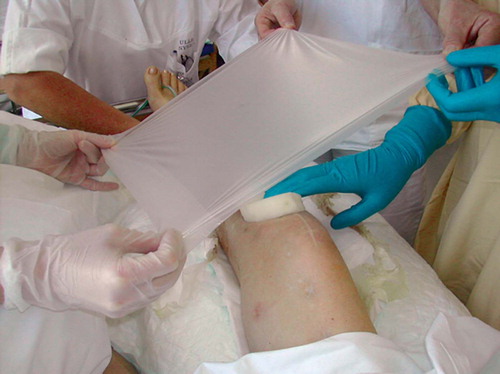
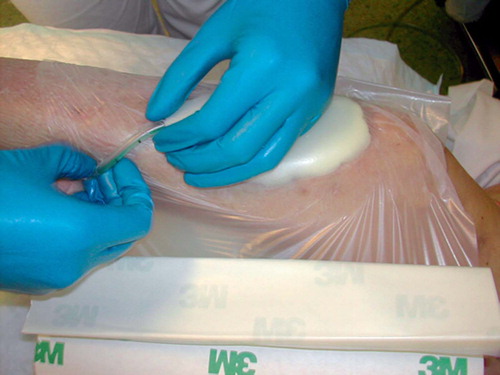
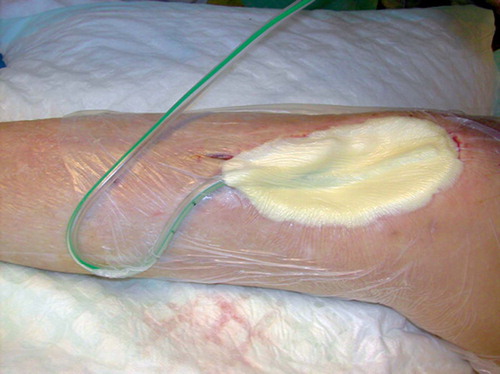
A 25-mm-thick sterile polyurethane sponge is individually trimmed () and shaped to make close contact with all wound surfaces. A suction drainage tube, Ch 18, is placed into the substance of the sponge () after greasing the spear of the drain with sterile Vaseline to ease its passage through the sponge. All holes of the suction drain are enclosed within the sponge, which is then applied to the wound and covered with an adhesive drape () at least 5 cm beyond the wound margins onto intact and dry skin. The drape must be carefully wrapped around the tube () to avoid pressure to the skin from the tubing. The Ch 18 tube is connected with a ¼” × ¼” disposable connector (Polystan) to a 7-mm Unifit tube (EMS Medical), again connected to a suction device such as a vacuum bottle with 20–60 kPa, normally 40 kPa, negative continuous suction pressure (). This induces a high-contact zone in the sponge-wound interface, resulting in efficient cleaning and conditioning of the wound. Due to the need for continuous suction, the patients have to remain close to the wall suction outlet—either in bed or in a chair. They are allowed toilet visits after clamping the tube.
The VAC is applied to the wound after regular surgical revision and debridement. Doctors or trained nurses change the sponge every second day initially, as long as further cleaning or debridement is necessary, and then every fourth or fifth day until the wound has healed or is ready for skin grafting. It is important to examine the tubing system regularly to ensure that it is not blocked by blood clots.
We have used our VAC system successfully for approximately 70 patients since August 2004—in open fractures, wounds with bone-, metal and tendon exposure, infected and acute wounds, and on split skin grafts. After split skin grafting, we have only employed limited suction and removed the sponge on the fourth postoperative day.
Complications and pitfalls
Superficial skin reactions to polyurethane have been reported in 3% of patients (Webb and Schmidt Citation2001). We have seen minor skin problems due to the adhesive drape, but only rarely.
In some early patients, we observed a pressure zone on the adjacent skin after the tube; this occurred because the tube was tightly trapped between drape and skin (). This problem was resolved when we started wrapping the drape carefully around the tube () to ensure minimal direct contact between tube and skin.
Leakage of tubes or clotting has not been a significant problem. Even so, it is important to check the system regularly.
A significant challenge of using the method is to avoid air leakage around external fixator pins. The best way of doing this is to wrap the drape closely around the pins for some 2–4 cm above the skin, and always well up on the smooth part of the pins. Loosening of the tube connections was a problem in the first few patients, before standardizing the sets with the presently used Unifit tube and disposable connector.
Case report 1
A 41-year-old man with osteogenis imperfecta was referred to our hospital after a road traffic accident with an open tibial shaft fracture. The soft tissue opening was 3 × 1 cm and located anteromedially over the middle part of the leg. 1.5 × 1 cm of the bone was stripped from the periosteum and exposed.
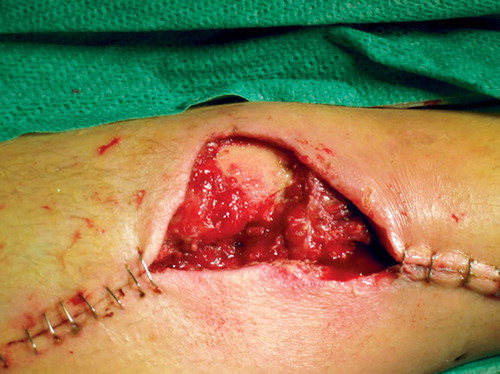
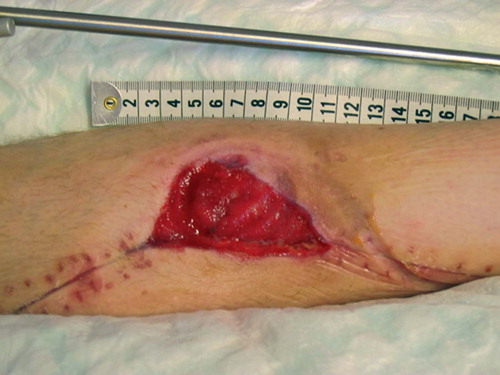
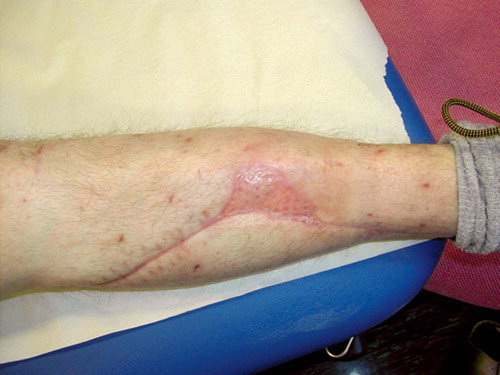
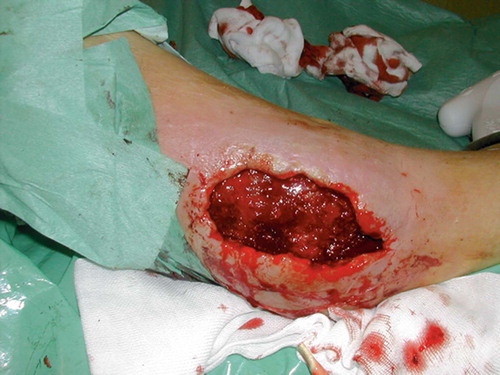
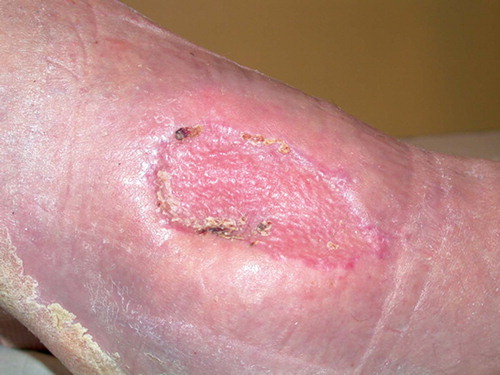
After initial fixation of the fracture with an intramedullary nail and revision of the soft tissues, the defect was covered with a soleus flap. The flap, however, went into necrosis and had to be removed after 3 days, leaving a soft tissue defect sized 7 × 5 cm with 2 × 2 cm of exposed bone (). VAC was applied over the defect and after 5 days approximately one-third of it was covered with granulation tissue. The bone was completely covered after 16 days () and ready for split skin grafting. The wound had healed completely by 2 months ().
Case report 2
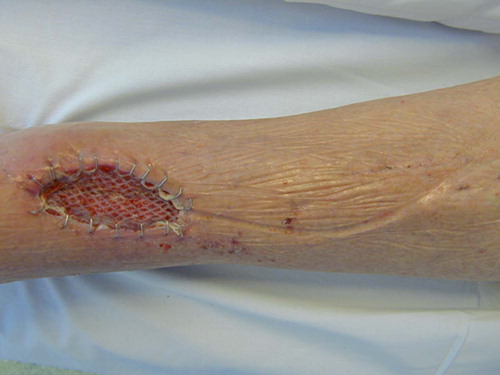
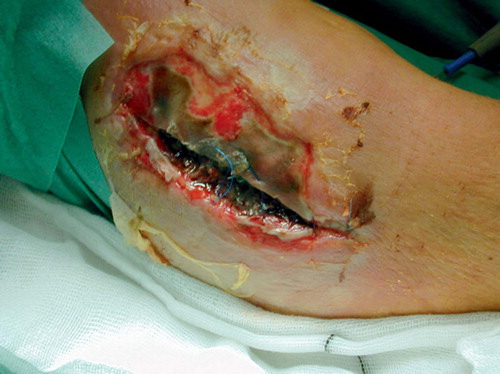
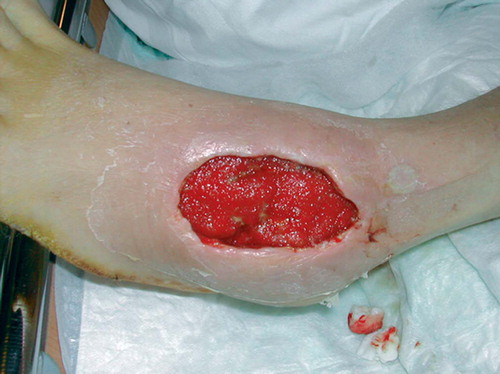
A 55-year-old healthy man with bilateral congenital cavovarus feet was operated for an acquired 4 × 5 cm ganglion on the lateral side of the dorsum of his left foot. Due to postoperative infection, the wound was surgically revised ( and ) 19 days after the initial operation and treated further with VAC and intravenous antibiotics. After 5 days of treatment (), the wound was clean and ready for split skin grafting. The patient was discharged from hospital 19 days after the revision with a nearly healed wound and the split skin graft had healed completely 1 month later ().
Discussion
Our system is based on the same principles as the expensive, commercially available VAC system. Therapy price per day on VAC ATS from KCI is 70 EUR including rental of the pump (42 EUR). The price for a pump is 17,424 EUR excluding VAT (October 2004, personal communications).
The total price of the equipment used for one change of wound dressing in our method is approximately 16 EUR. If dressings are changed twice weekly, the daily cost becomes less than 5 EUR, making the commercial method more than 10 times more expensive to use.
The disadvantage of our system is that it can only be used for hospitalized patients, and it should therefore be reserved for patients confined to hospital for reasons other than wound treatment alone. If the patient can be treated in an outpatient clinic, the commercially available mobile pump system would probably be more cost-effective than keeping the patient hospitalized.
Another weakness of our VAC method is the limited possibility of performing intermittent treatment, as advocated by Morykwas et al. (Citation1997). However, the importance of this has not been clarified in clinical studies, and intermittent treatment can often lead to increased pain. The VAC method has reduced the need for wound dressing changes in the operating theater in patients with complicated wounds. A rapid and marked proliferation of granulation tissue occurs, and the wounds seem to resolve more efficiently than with conventional treatment. The method described is inexpensive, effective and easy to use.
- Armstrong D G, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomized controlled trial. Lancet 2005; 266(9498)1704–10
- Fleischmann W, Becker U, Bischoff M, Hoekstra H. Vacuum sealing: indication, technique, and results. Euro J Orthop Surg Traumatol 1995; 5: 37–40
- Morykwas M J, Argenta L C, Shelton-Brown E I, McGuirt B S. Vacuum-assisted closure: a new method for wound closure and treatment. Animal studies and basic foundation. Ann Plast Surg 1997; 38: 553–62
- Müllner T, Mrkonjic L, Kwasny O, Vecsei V. The use of negative pressure to promote the healing of tissue defects: a clinical trial using the vacuum sealing technique. Br J Plast Surg 1997; 50: 194–9
- Webb L X. New techniques in wound management: vacuumassisted wound closure. J Am Acad Orthop Surg 2002; 10: 303–11
- Webb L X, Schmidt U. Wound management with vacuum therapy. Unfallchirurg 2001; 104(10)918–26