Abstract
Background Periprosthetic bone loss is a well-docu-mented phenomenon after uncemented total hip arthroplasty (THA); however, little is known about how bone mineral density (BMD) changes after 2 years.
Patients and methods 14 patients with hip arthrosis (group A) were operated with a proximally porous- and hydroxyapatite-coated stem and followed for 10 years with DEXA, radiographs and Harris hip score (HHS). Another group of 14 patients (group B) was evaluated at 6 and 14 years using the same prosthesis and protocol.
Results No stem was revised and all stems were well-Fixed. At final follow-up, HHS was 97 points in group A after 10 years and 94 points in group B after 14 years. Bone mineral changes in group A were greatest in Gruen zones 1 and 7, where the losses were 31% and 26%, respectively, after 2 years on the operated side. The decrease in BMD continued after 2 years and in Gruen zone 7 it was faster than the rate of bone loss on the control side. In group B, the annual change in BMD on the operated side was not significantly different from the bone loss in group A.
Interpretation Up to 14 years after implantation of a tapered uncemented stem, the BMD in the calcar region continues to decrease faster than would be expected from normal ageing.
Uncemented femoral stems of modern design have shown promising 10-year results (Keisu et al. Citation2001a, Oosterbos et al. Citation2004, Eskelinen et al. Citation2005). Good clinical outcome and persistent osseointegration have convinced many surgeons to use this concept, especially in younger patients. However, proximal femoral bone resorption due to distal stress transfer (i.e. stress shielding) seems to occur to a substantial degree. The loss of BMD occurs most rapidly during the first 3–6 months after surgery (Nishii et al. Citation1997, Kröger et al. Citation1998). Thereafter, a plateau phase appears to be reached, even though some authors have pointed out a continuous decline which is faster than the normal ageing of bone (Kiratli et al. Citation1996, Rosenthall et al. Citation1999, Aldinger et al. Citation2003). Concern has been expressed that in the long run continuous bone resorption may jeopardize the ingrowth and stability of the implant and increase the risk of periprosthetic fractures.
We determined the long-term longitudinal changes in BMD around a tapered uncemented stem. We also assessed the clinical outcome with Harris hip score (HHS), and determined the radiological changes.
Patients and methods
A consecutive group of 14 patients (10 women) with unilateral hip arthrosis (group A), planned for uncemented THA in 1994–1995, gave their informed consent for postoperative follow-up with dual-energy X-ray absorptiometry (DEXA) in addition to conventional radiographic investigation in 2 projections and clinical examination. Only patients without earlier fractures or other disabilities of the hips, which might interfere with proper assessment, were included. Mean age at operation was 57 (49–67) years. DEXA and the radiographic investigation were performed within 1 week of surgery and then at 0.5, 1, 2 and 10 years. HHS was determined at operation and after 1, 2 and 10 years. No patient was lost to follow-up.
With the purpose of prolonging the observation time, another group of 14 consecutive patients with unilateral hip arthrosis (8 men) operated on in 1990–1992 (group B) was evaluated after mean 6 (5–8) years and 14 (14–15) years postoperatively with DEXA, radiographs, and HHS. These patients were included using the same criteria as in group A. The mean age at operation was 53 (38–60) years. 1 man died during follow-up, leaving 13 patients for the final evaluation. The studies were conducted by physicians who had not been involved in the surgery.
All patients in both groups received an uncemented, collarless stem (Bi-Metric; Biomet Inc., Warsaw, IN) made of titanium alloy (Ti-6Al-4V), where the proximal one-quarter has a circumferential, plasma-sprayed, titanium alloy porous coating with a mean pore size of 300 μm. The distal part has a textured surface with a roughness of 6.9 μm. The porous part has a plasma-sprayed hydroxyapatite (HA) layer of 40–70 μm thickness, crystallinity of 50–70%, and a purity of < 95%. The stem has a straight 3° proximal-to-distal taper in 2 planes and a taper from the lateral shoulder to the medial calcar area. It articulates with a 28-mm modular cobaltchrome head of varying lengths of neck extension (–6, 0, +6 mm). The stem is available in proportional sizes from 7 to 19 mm, with corresponding lengths of 115 to 175 mm. In both groups, the stem was combined with an uncemented, porous- and HA-coated, threaded screw-in cup with a highdensity polyethylene liner. All patients were operated on by a senior surgeon through a standardized posterior approach without removal of the greater trochanter or section of the abductor muscles of the hip. No patient received prophylactic treatment against heterotopic ossification. The patients were mobilized on the day after the operation under the supervision of a physiotherapist. Postoperative weight bearing was individualized according to the preference of the surgeon.
The clinical assessment was conducted using a standardized questionnaire, including HHS. Thigh pain in particular was assessed, and it was graded as either: no pain, mild pain, or pain limiting activity.
The radiographs were assessed by the criteria of Engh et al. (Citation1990). A component was defined as having fixation by bone ingrowth if there was no subsidence and no formation of a radiolucent or radiodense (reactive) line along the porous-coated portion of the implant. A vertical migration of 5 mm or more, from the easily identified inferior border of the coating to the most medial point of the lesser trochanter, was considered to indicate definite subsidence. Furthermore, we registered the occurrence of endosteal bone bridging (spot welds), radiodense (reactive) and radiolucent lines, distal cortical hypertrophy, pedestal formation, femoral osteolysis, and calcar atrophy, defined as resorption of the calcar femoris adjacent to the implant (not round-off). Heterotopic ossification was assessed according to Brooker et al. (Citation1973). Linear wear rate was measured with a digital caliper using a modification of the method of Livermore et al. (Citation1990).
The BMD of the periprosthetic femur was measured in the coronal plane by DEXA (DPXL; Lunar Co., Madison, WI) (Mazess et al. Citation1989). During scanning, the patient was placed supine with standard knee and foot supports and with the femur in neutral rotation. The scanner was equipped with software for femoral periprosthetic bone mineral measurement. This software detected the interface between the bony part and the prosthesis stem on the basis of density changes, and simulated the stem in the form of a prosthesis mask which was superimposed on the healthy side. The healthy hip (control side) was scanned at the corresponding level and BMD was determined in 7 regions of interest (ROIs) based on Gruen zones. To avoid the effect of operation on bone mass, we performed the first measurement within the first 4 days after the operation (group A), as proposed by Kröger et al. (Citation1996) and Nishii et al. (Citation1997). The values were expressed as areal BMD, in g/cm2. The ratio between the longitudinal values on each side was calculated. In addition, to control for the natural ageing process of the bone, the ratio between the operated side and the control side was calculated. To determine the annual absolute change in BMD, the difference between the 2 latest values was divided by time in both groups, to give values in g/cm2/year. All DEXA measurements were performed with the same apparatus.
The investigation was approved by the ethics committee of Karolinska Hospital (D. no. 04-848/4). The patients gave their informed consent before being included in the study.
Precision
To estimate the precision error of the DEXA method, we have earlier made double measurements in 10 patients, with complete repositioning of the patient and the scanner. We found a precision error for the DEXA method of 1–4% at the different Gruen zones (Bodén et al. Citation2004).
Statistics
Median values of absolute and percentage changes in BMD were calculated. Wilcoxon signed-rank test (paired observations), Mann-Whitney U-test (unpaired observations) and Spearman correlations were used in the statistical analyses, which were performed using Statistica software version 7.1 (StatSoft, Tulsa, Okla.) Differences were considered significant at p-values less than 0.05.
Results
Clinical
None of the stems have been revised to date. All hips were well-functioning, with a mean HHS of 97 points at final follow-up at 10 years in group A and 94 points at 14 years in group B. There was no significant deterioration of HHS with time in either group. 1 patient in group B complained of mild thigh pain. 1 patient from each group had undergone closed reduction for dislocation. None of the patients had suffered from a fracture of the femur. No deep infection was registered. During the follow-up time, 3 patients in group A and 4 patients in group B had undergone a contralateral THA operation because of osteoarthritis. These patients were excluded from the BMD analyses after the contralateral hip operation.
Radiographical findings
None of the stems showed definite subsidence or other signs of loosening. At last follow-up, according to Engh's criteria (1990), all stems were osseointegrated with visible endosteal bone bridging (spot welds) in 12/14 patients in group A and 12/13 in group B. All structural remodeling changes found on the radiographs after 10.3 years (group A) and 14.4 years (group B) are shown in the .
Fixation and remodeling changes of the proximal femur
Bone mineral density findings
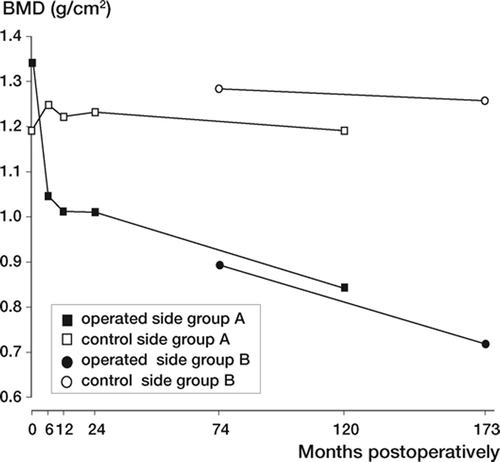
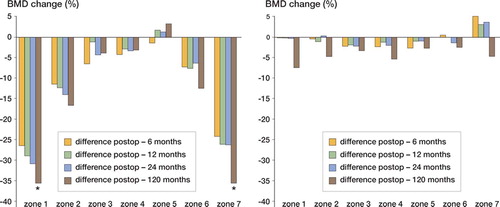
In group A, on the operated side the BMD decreased significantly in the proximal Gruen zones (zones 1, 2, 6 and 7) during the first 6 months postoperatively (, left panel). After this initial phase, a retardation of the BMD changes occurred but, in the trochanteric region (zone 1) and in the calcar region (zone 7), there was also continued reduction between 2 and 10 years postoperatively (p = 0.005 and p = 0.003, respectively). There were only small changes on the control side (, right panel).
Figure 1. BMD changes in group A. Median percent difference on the operated side (left panel) and the control side (right panel) at 4 different times.
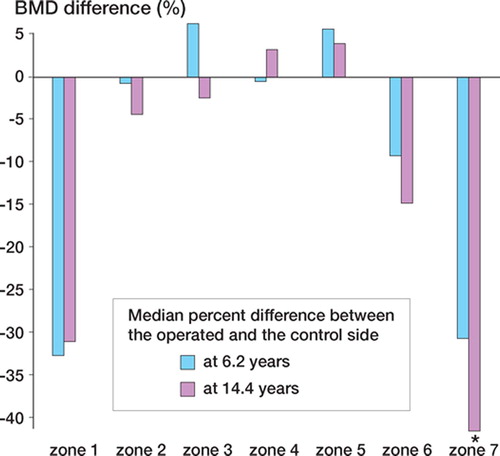
In group B, the percent differece between the BMD on the operated side and the BMD on the control side was calculated at 6 and 14 years postoperatively (). In zones 1 and 7, the median difference between the operated side and the control side after 14 years, was 31% (20–53) and 41% (33–59).The difference in BMD increased in the calcar region (zone 7) between 6 and 14 years postoperatively (p = 0.01). Distally, around the tip of the prosthesis, no significant change was noted. We calculated the annual absolute change in BMD after the plateau phase was reached (after 24 months in group A and after 6.4 years in group B). There was no difference in annual change in BMD between the groups in any zone.
After 2 years and 6 years in both groups, the annual absolute loss in BMD was greater in zone 7 on the operated side than on the control side (group A: p = 0.02; group B: p = 0.03; for group A and B together: p = 0.001) ().
No significant difference in BMD change on the operated side was detected between sexes, although a trend towards greater BMD loss in women was seen. On the control side, there was significantly greater loss of BMD in zones 3 and 5 in women. In the distal zones (zones 3, 4 and 5), where cortical hypertrophy and pedestal formation were most commonly found, BMD showed the highest values. Among the hips with distal cortical hypertrophy, a tendency towards greater loss of BMD in zone 7 was noted, compared to hips without hypertrophy. However, the difference did not reach significance in this small sample. There was no correlation between linear wear rate and BMD change at last follow-up in any zone in any group. There was no correlation between age of the patient at operation and BMD change.
Discussion
Stress shielding due to distal load transfer between the prosthetic component and the host bone is a common phenomenon on the femoral side after THA (Engh et al. Citation1992, Kilgus et al. Citation1993). It may predict adverse events such as periprosthetic fractures, stem fractures and loosening. The weakened bone may also cause complications in revison surgery. This phenomenon has been described most often with uncemented stems, but remodeling has also been observed around stable cemented stems (Cohen and Rushton Citation1995b, Venesmaa et al. Citation2003, Nygaard et al. Citation2004).
In a laboratory setting, and comparing strain patterns in proximal femurs before and after implanting cemented and uncemented stems, Djerf and Gill quist (Citation1987) observed that the high stress area was situated more distally in prosthetic specimens and that the uncemented stems had the highest stress concentration. Even so, excellent clinical results even after 10 years have been reported with uncemented stems that cause an obvious proximal bone resorption (Keisu et al. Citation2001a, Engh et al. Citation2003, Eskelinen et al. Citation2005). Assessment of bone loss on plain radiographs is, however, unreliable since the loss is not always recognized until 70% of the bone is resorbed (Engh et al. Citation2000). Even though BMD alone cannot allow prediction of bone strength and quality (Banse Citation2002), BMD is commonly used to estimate the remodeling changes around implants. By using DEXA, BMD can be determined with high precision (< 4%, Kiratli et al. (Citation1996); 1–5.3%, Kröger et al. (Citation1998)). Throughout our study, all monitoring was done with the same DEXA apparatus, using the same software and by the same medical physicist (HJL); and since the clinical outcome was good in all patients and all prostheses were radiographically well osseointegrated, these results can be regarded as normative data for the Bi-Metric HA-coated stem.
Using this prosthesis, we have previously found an average BMD loss of 20% in the proximal zones up to 2–5 years postoperatively (Bodén and Adolphson Citation2004, Sköldenberg et al. Citation2006). Since the aim of this study was to investigate whether the BMD changes continued, we used the value after the plateau phase (after 2 years) as the baseline in group A. Assuming that the change is linear, we calculated the annual change by subtracting the value obtained at last follow-up from the baseline value and divided it by the time between the observations. Since the baseline values for groups A and B were obtained after different times in situ, and thereby different degrees of host bone response, we used absolute BMD changes instead of the more commonly used relative loss from baseline value. This enabled us to compare the BMD changes between the groups. Since no significant difference was seen between the groups, we can assume that the remodeling rate is linear up to 14 years.
Many uncemented femoral implants have been evaluated by DEXA and all intramedullary stems cause bone loss proximally, predominantly in the calcar region. Caution must be exercised when interpreting and comparing results from different studies, since several influences must be considered such as: surgical approach (Perka et al. Citation2005), definition of ROIs, leg position (Cohen and Rushton Citation1995a), pre- or postoperative baseline value (Kröger et al. Citation1996), and cross-sectional or longitudinal studies (Martini et al. Citation2000). However, a tendency towards a better retained proximal BMD has been found after implantation of thinner tapered stems without porous coating. Korovessis et al. (Citation1997) and Brodner et al. (Citation2004) used the press-fit titanium Alloclassic SL stem which—after 4 years in longitudinal studies—showed only 7% and 14% loss in zone 7, respectively. Also, the tapered Spotorno CLS stem has been noted to cause only moderate BMD loss in zone 7. Sabo et al. (Citation1998) reported a 12% loss after 2 years, and Gibbons et al. (Citation2001) reported a 20% decrease after a mean of 4 years. Zerahn et al. (Citation1998) reported a 20% loss after 2 years and Roth et al. (Citation2005) reported a loss of the same magnitude (19%) after 1 year.
The important issue is whether the bone loss continues. Most authors have found that the remodeling process has ceased after 1–2 years (Nishii et al. Citation1997, Kröger et al. Citation1998). To the best of our knowledge, however, only 2 longitudinal studies covering more than 5 years have been published and they showed contradictory results. Thus, with DEXA, Aldinger et al. (Citation2003) demonstrated a continuous bone loss in the proximal zones for up to 7 years with the Spotorno CLS stem, while in a 10year comparison of 4 different stems with DEXA, Karachalios et al. (Citation2004) reported a progressive recovery in BMD for all 4 designs from 2–10 years postoperatively. Our findings support the findings of the former study, since both of our independent groups of patients (A and B) differed significantly from the contralateral side in terms of remodeling rate in zone 7. At variance with the current study, and with the study of Aldinger et al., Karachalios et al. used the preoperative value as baseline. Furthermore, they observed a significant increase in BMD over time, also in the lumbar spine and in the contralateral hip. This contrasts with the findings of Soininvaara et al. (Citation2004) who showed no recovery of BMD in any hip or in the contralateral knee after total knee arthroplasty despite good functional recovery. Another discrepancy between our study and that of Karachalios et al. (Citation2004) is the mean age of the patients, which was 18 years more in the latter study. Differences in remodeling activity between younger and older patients have been reported, both at the cellular level (Groessner-Schreiber et al. Citation1992) and at the macroscopic level (Brockstedt et al. Citation1993). These findings suggest that younger patients may have a different remodeling pattern after insertion of orthopedic implants. However, in our homogeneous groups, we found no correlation between age and BMD changes; nor did we find any ominous radiographic sign in the elderly patients. This is in accordance with other studies that have concentrated on patients with inferior bone quality. Lyback et al. (Citation2004) reported excellent results with the Bi-Metric stem in patients with juvenile arthritis, and a similar tapered stem has shown excellent results in octogenarians (Keisu et al. Citation2001b).
An advantage of HA coating has been proposed by Rosenthall et al. (Citation2000), who demonstrated that the Multiloc HA stem caused less bone loss after 2 years than the same stem with only porous coating. In a retrieval study by Coathup et al. (Citation2001), the Bi-Metric stem was analyzed with and without HA and they noted more ingrowth and more proximal attachment of bone to the HA-coated implant surface than to the plain porous-coated implant. Even so, in our study using a proximally HA-coated stem, we found a substantial degree of proximal bone loss with time, suggesting that geometric design is of greater importance. This is exemplified by the work of Rahmy et al. (Citation2004) who compared 2 proximally HA-coated designs, the anatomic (ABG) and the tapered (Mallory Head) stems, and found 10% greater bone loss in zone 7 in the anatomic group after 3 years. Several experimental studies have indicated that close geometric fit between an uncemented stem and the endosteal bone of the proximal femur is essential for physiological load transfer (Huiskes et al. Citation1989, Hua and Walker Citation1995). Nevertheless, a custom-made stem designed from CT information, with optimal proximal fit, proximal HA coating, and with a narrow tapered shaft, has been reported to cause losses of about 35% in zone 7 after 2 years (Benum and Aamodt Citation2000) and after 5 years (Müller et al. 2005). On the other hand, a low stem/cortical contact ratio was found to be predictive of stem revision in a large multicenter study of the Porous-Coated Anatomic stem (Malchau et al. Citation1997); and, in a radiographic comparison of the ABG stem with the Bi-Metric PC stem, the importance of “fit-and-fill” to prevent subsidence in the ABG group was pointed out (Laine et al. Citation2000). It may be more difficult to achieve a sufficient fit with a standard “off the shelf” anatomically designed stem—compared to a straight tapered stem—even though signs of greater distal stress transfer were obvious with the Bi-Metric stem after 5 years (Laine et al. Citation2000).
In a study of the ABG stem, Oosterbos et al. (Citation2004) described radiographic evidence for how a proximal-to-distal endosteal densification continues between 5 and 10 years after implantation. We have described the same phenomenon for the Bi-Metric HA-coated stem in a previous report (Bodén et al. Citation2006). In long-term follow-up studies of these stems, designed with a more voluminous and coated metaphyseal region, one striking observation has been the absence of radiolucent lines proximally (McNally et al. Citation2000, Oosterbos et al. Citation2004, Bodén et al. Citation2006). A more favorable stressshielding effect is generally observed in designs with a more slender geometry without coating
(i.e. press-fit stems), but the frequency of proximal radiolucencies is high, and is progressive over many years (Donnelly et al. Citation1997, Siebold et al. Citation2001, Pospischill et al. Citation2005, Vervest et al. Citation2005). This sign is a known predictor of aseptic loosening and revision (Malchau et al. Citation1997, Khalily and Whiteside Citation1998). The slow progressive proximal bone loss together with excellent osseointegration that has been demonstrated in the current study may be preferable in the long run.
Postoperative femoral periprosthetic fracture is often a severe complication and results in high morbidity (Lindahl et al. Citation2005). With only proximal osteopenia, the trochanteric region seems to be at greatest risk, but a potential fracture in this region does not necessarily imply major surgical difficulties. In a series of 887 tapered and proximally porous-coated Omnifit stems, Hsieh et al. (Citation2005) found 23 fractures of the greater trochanter (3%), of which 15 were successfully treated nonoperatively. However, much effort has been allocated to designing a femoral component that can overcome the problem of stress shielding while maintaining a secure fixation. Resurfacing (Daniel et al. Citation2004, Kishida et al. Citation2004), short stems (Morrey et al. Citation2000, Roth et al. Citation2005), stemless femoral components (Munting et al. Citation1997, Albrektsson et al. Citation1998), the Press-Fit gliding stem (Krüger et al. Citation1998), isoelastic stem by geometry (Butel and Robb Citation1988) and composite material (Kärrholm et al. Citation2002) are all examples of femoral implants that have been designed to address this problem. Promising results from BMD evaluations have been published for most concepts, but good long-term clinical results are still to be shown.
The study was kindly supported by research grants from the Ulla and Gustaf af Uggla Foundation and the Emil and Maria Palm Foundation, Sweden.
No competing interests declared.
Contributions of authors
HB examined all patients, collected the data and prepared the manuscript. OS and MS contributed with the clinical investigations and manuscript preparation. HJL performed the DEXA investigations. PA supervised the statistical analyses and proofread the manuscript.
- Albrektsson T, Carlsson L V, Jacobsson M, Macdonald W. Gothenburg osseointegrated hip arthroplasty, Experience with a novel type of hip design. Clin Orthop 1998; 352: 81–94
- Aldinger P R, Sabo D, Pritsch M, Thomsen M, Mau H, Ewerbeck V, Breusch S J. Pattern of periprosthetic bone remodeling around stable uncemented tapered hip stems:, a prospective 84-month follow-up study and a edian 156-month cross-sectional study with DXA. Calcif Tissue Int 2003; 73(2)115–21
- Banse X. When density fails to predict bone strength. Acta Orthop Scand 2002, Suppl 303: 73
- Benum P, Aamodt A. Customized uncemented femoral stems. Review of 10 years experimental and clinical research. Acta Orthop et Traumatologica Hellenica 2000; 51(4)346–57
- Bodén H, Adolphson P. No adverse effects of early weight bearing after uncemented total hip arthroplasty:, a randomized study of 20 patients. Acta Orthop Scand 2004; 75(1)21–9
- Bodén H, Adolphson P, öberg M. Unstable versus stable uncemented femoral stems:, A radiological study of periprosthetic bone changes in two types of uncemented stems with different concepts of fixation. Arch Orthop Trauma Surg 2004; 124(6)382–92
- Bodé H, Salemyr M, Sköldenberg O, Ahl T, Adolphson P. Total hip arthroplasty with an uncemented hydroxyapa-tite-coated tapered titanium stem. Excellent results at a minimum of 10-years follow-up in 104 hips. J Orthop Sci 2006; 11(2)175–9
- Brockstedt H, Kassem M, Eriksen E F, Mosekilde L, Melsen F. Age and sex related changes in iliac cortical bone mass and remodeling. Bone 1993; 14(4)681–91
- Brodner W, Bitzan P, Lomoschitz F, Krepler P, Jankovsky R, Lehr S, Kainberger F, Gottsauner-Wolf F. Changes in bone mineral density in the proximal femur after cementless total hip arthroplasty. A five-year longitudinal study. J Bone Joint Surg (Br) 2004; 86(1)20–6
- Brooker A F, Bowerman J W, Robinson R A, Riley L H, Jr. Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg (Am) 1973; 55(8)1629–32
- Butel J, Robb J E. The isoelastic hip prosthesis followed for 5 years. Acta Orthop Scand 1988; 59(3)258–62
- Coathup M J, Blunn G W, Flynn N, Williams C, Thomas N P. A comparison of bone remodelling around hydroxyap-atite-coated, porous-coated and grit-blasted hip replacements retrieved at post-mortem. J Bone Joint Surg (Br) 2001; 83(1)118–23
- Cohen B, Rushton N. Accuracy of DEXA measurement of bone mineral density after total hip arthroplasty. J Bone Joint Surg (Br) 1995a; 77(3)479–83
- Cohen B, Rushton N. Bone remodelling in the proximal femur after Charnley total hip arthroplasty. J Bone Joint Surg (Br) 1995b; 77(5)815–9
- Daniel J, Pynsent P B, McMinn D J. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg (Br) 2004; 86(2)177
- Djerf K, Gillquist J. Calcar unloading after hip replacement. A cadaver study of femoral stem designs. Acta Orthop Scand 1987; 58(2)97–103
- Donnelly W J, Kobayashi A, Freeman M A, Chin T W, Yeo H, West M, Scott G. Radiological and survival comparison of four methods of fixation of a proximal femoral stem. J Bone Joint Surg (Br) 1997; 79(3)351–60
- Engh C A, Massin P, Suthers K E. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop 1990; 257: 107–28
- Engh C A, McGovern T F, Bobyn J D, Harris W H. A quantitative evaluation of periprosthetic bone-remodeling after cementless total hip arthroplasty. J Bone Joint Surg (Am) 1992; 74(7)1009–20
- Engh C A., Jr, McAuley J P, Sychterz C J, Sacco M E, Engh C A, Sr. The accuracy and reproducibility of radiographic assessment of stress-shielding. A postmortem analysis. J Bone Joint Surg (Am) 2000; 82(10)1414–20
- Engh C A, Jr, Young A M, Engh C A, Sr, Hopper R H, Jr. Clinical consequences of stress shielding after porous-coated total hip arthroplasty. Clin Orthop 2003; 417: 157–63
- Eskelinen A, Remes V, Helenius I, Pulkkinen P, Nevalainen J, Paavolainen P. Total hip arthroplasty for primary osteoarthrosis in younger patients in the Finnish arthroplasty register. 4,661 primary replacements followed for 0-22 years. Acta Orthop 2005; 76(1)28–41
- Gibbons C E, Davies A J, Amis A A, Olearnik H, Parker B C, Scott J E. Periprosthetic bone mineral density changes with femoral components of differing design philosophy. Int Orthop 2001; 25(2)89–92
- Groessner-Schreiber B, Krukowski M, Lyons C, Osdoby P. Osteoclast recruitment in response to human bone matrix is age related. Mech Ageing Dev 1992; 62(2)143–54
- Hsieh P H, Chang Y H, Lee P C, Shih C H. Periprosthetic fractures of the greater trochanter through osteolytic cysts with uncemented MicroStructured Omnifit prosthesis: retrospective analyses pf 23 fractures in 887 hips after 514 years. Acta Orthop 2005; 76(4)538–43
- Hua J, Walker P S. Closeness of fit of uncemented stems improves the strain distribution in the femur. J Orthop Res 1995; 13(3)339–46
- Huiskes R, Weinans H, Dalstra M. Adaptive bone remodeling and biomechanical design considerations for noncemented total hip arthroplasty. Orthopedics 1989; 12(9)1255–67
- Karachalios T, Tsatsaronis C, Efraimis G, Papadelis P, Lyritis G, Diakoumopoulos G. The long-term clinical relevance of calcar atrophy caused by stress shielding in total hip arthroplasty: a 10-year, prospective, randomized study. J Arthroplasty 2004; 19(4)469–75
- Kärrholm J, Anderberg C, Snorrason F, Thanner J, Langeland N, Malchau H, Herberts P. Evaluation of a femoral stem with reduced stiffness. A randomized study with use of radiostereometry and bone densitometry. J Bone Joint Surg (Am) 2002; 84(9)1651–8
- Keisu K S, Mathiesen E B, Lindgren J U. The uncemented fully textured Lord hip prosthesis:, a 10 to 15-year followup study. Clin Orthop 2001a; 382: 133–42
- Keisu K S, Orozco F, Sharkey P F, Hozack W J, Rotman R H, McGuigan F X. Primary cementless total hip arthroplasty in octogenarians. Two to eleven-year follow-up. J Bone Joint Surg (Am) 2001b; 83(3)359–63
- Khalily C, Whiteside L A. Predictive value of early radiographic findings in cementless total hip arthroplasty femoral components: an 8 to 12-year follow-up. J Arthroplasty 1998; 13(7)768–73
- Kilgus D J, Shimaoka E E, Tipton J S, Eberle R W. Dualenergy X-ray absorptiometry measurement of bone mineral density around porous-coated cementless femoral implants. Methods and preliminary results. J Bone Joint Surg (Br) 1993; 75(2)279–87
- Kiratli B J, Checovich M M, McBeath A A, Wilson M A, Heiner J P. Measurement of bone mineral density by dualenergy x-ray absorptiometry in patients with the Wisconsin hip, an uncemented femoral stem. J Arthroplasty 1996; 11(2)184–93
- Kishida Y, Sugano N, Nishii T, Miki H, Yamaguchi K, Yoshikawa H. Preservation of the bone mineral density of the femur after surface replacement of the hip. J Bone Joint Surg (Br) 2004; 86(2)185–9
- Korovessis P, Piperos G, Michael A, Baikousis A, Stamatakis M. Changes in bone mineral density around a stable uncemented total hip arthroplasty. Int Orthop 1997; 21(1)30–4
- Krüger A, Berli B, Lampert C, Kranzlin C, Morscher E. Comparative periprosthetic bone density measurements of the proximal femur shaft using dual energy x-ray absorptiometry (DEXA) with experimental "Press Fitgliding Stem Prosthesis". Z Orthop Ihre Grenzgeb 1998; 136(2)115–25
- Kröger H, Miettinen H, Arnala I, Koski E, Rushton N, Suomalainen O. Evaluation of periprosthetic bone using dualenergy x-ray absorptiometry: precision of the method and effect of operation on bone mineral density. J Bone Miner Res 1996; 11(10)1526–30
- Kröger H, Venesmaa P, Jurvelin J, Miettinen H, Suomalainen O, Alhava E. Bone density at the proximal femur after total hip arthroplasty. Clin Orthop 1998; 352: 66–74
- Laine H J, Puolakka T J, Moilanen T, Pajamäki K J, Wirta J, Lehto M U. The effects of cementless femoral stem shape and proximal surface texture on 'fit-and-fill' characteristics and on bone remodeling. Int Orthop 2000; 24(4)184–90
- Lindahl H, Malchau H, Herberts P, Garellick G. Periprosthetic femoral fractures classification and demographics of 1049 periprosthetic femoral fractures from the Swedish National Hip Arthroplasty Register. J Arthroplasty 2005; 20(7)857–65
- Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg (Am) 1990; 72(4)518–28
- Lyback C C, Lyback C O, Kyro A, Kautiainen H J, Belt E A. Survival of Bi-Metric femoral stems in 77 total hip arthroplasties for juvenile chronic arthritis. Int Orthop 2004; 28(6)357–61
- Malchau H, Wang Y X, Kärrholm J, Herberts P. Scandinavian multicenter porous coated anatomic total hip arthroplasty study. Clinical and radiographic results with 7 to 10-year follow-up evaluation. J Arthroplasty 1997; 12(2)133–48
- Martini F, Lebherz C, Mayer F, Leichtle U, Kremling E, Sell S. Precision of the measurements of periprosthetic bone mineral density in hips with a custom-made femoral stem. J Bone Joint Surg (Br) 2000; 82(7)1065–71
- Mazess R, Collick B, Trempe J, Barden H, Hanson J. Performance evaluation of a dual-energy x-ray bone densitometer. Calcif Tissue Int 1989; 44(3)228–32
- McNally S A, Shepperd J A, Mann C V, Walczak J P. The results at nine to twelve years of the use of a hydroxyapa-tite-coated femoral stem. J Bone Joint Surg (Br) 2000; 82(3)378–82
- Morrey B F, Adams R A, Kessler M. A conservative femoral replacement for total hip arthroplasty. A prospective study. J Bone Joint Surg (Br) 2000; 82(7)952–8
- Munting E, Smitz P, Van Sante N, Nagant de Deuxchaisnes C, Vincent A, Devogelaer J P. Effect of a stemless femoral implant for total hip arthroplasty on the bone mineral density of the proximal femur. A prospective longitudinal study. J Arthroplasty 1997; 12(4)373–9
- Müller S, Irgens F, Aamodt A. A quantitative and qualitative analysis of bone remodelling around custom uncemented femoral stems:, a five-year DEXA follow-up. Clin Biomech 2005;. 20(3)277–82
- Nishii T, Sugano N, Masuhara K, Shibuya T, Ochi T, Tamura S. Longitudinal evaluation of time related bone remodeling after cementless total hip arthroplasty. Clin Orthop 1997; 339: 121–31
- Nygaard M, ZerahnB, Bruce C, Søballe K, Borgwardt A. Early periprosthetic femoral bone remodelling using different bearing material combinations in total hip arthroplasties:, a prospective randomised study. Eur Cell Mater 2004; 8: 65–73
- Oosterbos C J, Rahmy A I, Tonino A J, Witpeerd W. High survival rate of hydroxyapatite-coated hip prostheses: 100 consecutive hips followed for 10 years. Acta Orthop Scand 2004; 75(2)127–33
- Perka C, Heller M, Wilke K, Taylor W R, Haas N P, Zippel H, Duda G N. Surgical approach influences periprosthetic femoral bone density. Clin Orthop 2005; 432: 153–9
- Pospischill M, Knahr K. Cementless total hip arthroplasty using a threaded cup and a rectangular tapered stem. Follow-up for ten to 17 years. J Bone Joint Surg (Br) 2005; 87(9)1210–5
- Rahmy A I, Gosens T, Blake G M, Tonino A, Fogeman I. Periprosthetic bone remodelling of two types of uncemented femoral implant with proximal hydroxyapatite coating: a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss. Osteoporos Int 2004; 15(4)281–9
- Rosenthall L, Bobyn J D, Brooks C E. Temporal changes of periprosthetic bone density in patients with a modular noncemented femoral prosthesis. J Arthroplasty 1999; 14(1)71–6
- Rosenthall L, Bobyns D J, Tanzer M. Periprosthetic bone densitometry of the hip: influence of prosthetic design and hydroxyapatite coating on regional bone remodelling. J Musculoskelet Neuronal Interact 2000; 1(1)57–60
- Roth A, Richartz G, Sander K, Sachse A, Fuhrmann R, Wagner A, Venbrocks R A. Periprosthetic bone loss after total hip endoprosthesis. Dependence on the type of prosthesis and preoperative bone configuration. Orthopäde 2005; 34(4)334–44
- Sabo D, Reiter A, Simank H G, Thomsen M, Lukoschek M, Ewerbeck V. Periprosthetic mineralization around cementless total hip endoprosthesis:, longitudinal study and cross-sectional study on titanium threaded acetabular cup and cementless Spotorno stem with DEXA. Calcif Tissue Int 1998; 62(2)177–82
- Siebold R, Scheller G, Schreiner U, Jani L. Long-term results with the cement-free Spotorno CLS shaft. Orthopäde 2001; 30(5)317–22
- Sköldenberg O G, Bodén H SG, Salemyr M OF, Ahl T E, Adolphson P Y. Periprosthetic proximal bone loss after uncemented hip arthroplasty is related to stem size. DXA measurements in 138 patients followed for 2–7 years. Acta Orthop 2006; 77: 386–92
- Soininvaara T A, Miettinen H J, Jurvelin J S, Alhava E M, Kröger H P. Bone mineral density in the proximal femur and contralateral knee after total knee arthroplasty. J Clin Densitom 2004; 7(4)424–31
- Venesmaa P K, Kröger H P, Jurvelin J S, Miettinen H J, Suomalainen O T, Alhava E M. Periprosthetic bone loss after cemented total hip arthroplasty:, a prospective 5year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand 2003; 74(1)31–6
- Vervest T M, Anderson P G, Van Hout F, Wapstra F H, Louwerse R T, Koetsier J W. Ten to twelve-year results with the Zweymüller cementless total hip prosthesis. J Arthroplasty 2005; 20(3)362–8
- Zerahn B, Storgaard M, Johansen T, Olsen C, Lausten G, Kanstrup I L. Changes in bone mineral density adjacent to two biomechanically different types of cementless femoral stems in total hip arthroplasty. Int Orthop 1998; 22(4)225–9