Abstract
Background Revascularization of a limb following prolonged ischemia results in substantial skeletal muscle injury. Statins play a well-understood role in the treatment of hypercholesterolemia but are also known to have anti-inflammatory properties. The purpose of this study was to examine the effects of pravastatin pre-treatment in the setting of skeletal muscle ischemia reperfusion injury (IRI).
Methods Adult male Sprague Dawley rats (n = 27) were randomized into 3 groups: control group, I/R group, IR group pre-treated with pravastatin. Bilateral hind-limb ischemia was induced by rubber band application proximal to the level of the greater trochanters for 2.5 h. Treatment groups received normal saline in equal volumes prior to tourniquet release. Following 12 h reperfusion, the tibialis anterior muscle was dissected and muscle function assessed electrophysiologically by electrical field stimulation. The animals were then killed and skeletal muscle harvested for evaluation.
Results We found that pre-treatment with pravastatin reduces the tissue oxidative damage and edema associated with skeletal muscle reperfusion injury. Skeletal muscle injury, measured by edema, leucose-questration and electrical properties were significantly lower with pravastatin pre-treatment compared to the non-treated group.
Interpretation We feel that pravastatin pre-treatment may be a potential therapeutic intervention for skeletal muscle ischemia reperfusion injury in the clinical setting. ▪
Skeletal muscle ischemia reperfusion injury occurs in a number of clinical scenarios and may be associated with both local and systemic proinflammatory responses which can adversely affect patient outcome. It may occur following arterial clamping during arterial reconstructive surgery and tourniquet use during extremity procedures and opera-tions (Barry et al. Citation1997, Wakai et al. Citation2001b). Ischemia reperfusion injury also arises following vascularized composite tissue transfer for reconstructing large tissue defects and may reduce the contractile function and survival of the transplanted muscle. Reperfusion is obviously a prerequisite for recovery from ischemic injury, but paradoxically, the re-introduction of molecular oxygen is the source of the deleterious oxygen radicals which are associated with lipid peroxidation and consequently disruption of cell membrane integrity (Grace Citation1994). It is therefore valuable for the surgeon to be knowledgeable about the pathophysiology of skeletal muscle ischemia reperfusion injury to minimize the development of the inflammatory response that may well ensue in these clinical situations.
There are currently no effective interventional strategies in clinical practice for attenuation of the reperfusion injury that accompanies limb revascularization. One potential therapeutic modality that has been explored is that of ischemic preconditioning. This is a process whereby cells or tissues exposed to a sublethal stimulus are transiently protected from a subsequent normally lethal stress. However, this is a time-consuming exercise and is not routinely employed in surgical procedures. Consequently, much research has focused on pharmacological manipulation of the reperfusion injury in an attempt to reduce the potential complications associated with prolonged tourniquet application.
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors—statins—are widely prescribed drugs for the treatment of hyper-cholesterolemia, and act by competitively inhibiting the rate-limiting step in cholesterol synthesis (Goldstein and Brown Citation1990). However, it is believed that statins have anti-inflammatory properties independent of their cholesterol-lowering properties, and have indeed been shown to attenuate renal and cardiac injury in a number of models of ischemia reperfusion (Lefer et al. Citation1999, Joyce et al. Citation2001). Furthermore, statins have been shown to inhibit the generation of superoxide in activated neutrophils by reducing tyrosine phosphorylation, and to reduce endotoxin-induced leukocyte endothelial cell interactions (Kanno et al. Citation1999, Pruefer et al. Citation2002).
We investigated whether pretreatment with oral pravastatin would attenuate tourniquet-induced skeletal muscle ischemia reperfusion injury in a rat model.
Animals and methods
Hindlimb ischemia and reperfusion model
Adult male Sprague-Dawley rats (Biological Services Unit, University College Cork, Ireland) weighing 300–350 g were used in all experiments. The model of tourniquet hindlimb ischemia and reperfusion was prepared as described elsewhere (Wakai et al. Citation2001a). Briefly, under intraperitoneal thiopentone sodium anesthetic (60 mg/kg), bilateral rubber bands were applied above the greater trochanters to interrupt the arterial blood supply to the hindlimbs. The rubber bands were removed after 2.5 h, thus initiating hindlimb reperfusion. Animals used in this study were maintained in accordance with the guidelines of the Cruelty to Animals Act, 1876, of the Department of Health, Ireland, and those of the European Community Directive (86/609/EC).
Animal group
The animals were randomized blindly into 3groups (9 rats per group). Group A underwent anesthesia alone as a control for the anesthesia. Group B underwent an intravenous injection of normal saline (0.9% NaCl, 4 mL/kg) 15 min before tourniquet release, followed by 12 h of reperfusion. These animals were given 1 mL of distilled water by gavage for 5 days prior to the experiment. Rats in group C were pretreated with pravastatin for 5 days prior to the experiment and underwent a similar volume intravenous injection of normal saline (0.9% NaCl, 4 mL/kg). Pravastatin (courtesy of Bristol-Meyers Squibb Pharmaceuticals, Ireland) was gavaged in 1 mL of distilled water, in a single daily dose of 0.4 mg/kg/day for 5 days prior to the experiment. Ischemia-reperfusion was induced by application of rubber bands above the greater trochanters bilaterally. Following 12 h of reperfusion, the animals were then killed for tissue harvesting.
Functional assessment of tibialis anterior muscle
After 12 h of reperfusion, while still under IP anesthesia, the tibialis anterior muscle of each animal was exposed. The animals were fixed to an external frame in a supine position. A 2-0 silk suture was tied around the distal tendon, which was then sectioned and attached to a force transducer (AD Instruments, Europe, Oxfordshire, UK) to measure the isometric contractile force. The muscle temperature was maintained at 32–35°C using an overhead heating lamp. The in-situ muscle was stimulated directly (0.1 milliseconds duration, 10 volts) via two electrodes connected to a stimulator (AD Instruments). The length of resting muscle was adjusted to produce maximum twitch tension. The isometric twitch contractile properties were determined. Tetanic tension in response to a tetanic electrical stimulus (50 Hz) was then recorded for each muscle. Twitch and tetanic contractions were reported as N/g of muscle weight.
Wet to dry (W/D) ratio
Sections of gastrocnemius were excised and weighed (wet weight). The muscle tissue was then dried at 600°C in a convection oven for 72 h and reweighed (dry weight). Wet to dry ratios were calculated and used as an index of edema formation.
Myeloperoxidase (MPO) assay
Muscle tissue samples were homogenized in buffer A (0.021% K2HPO4, 0.663% KH2PO4 and 0.5% hexadecyltrimethyl ammonium bromide in distilled water). The homogenates were freeze-thawed 3 times and centrifuged at 2,000 rpm for 10 min. The supernatant was assayed spectrophotometrically for MPO activity by adding 1 mL of supernatant to 2 mL of freshly prepared solution B. (Solution B was prepared by dissolving 0.0105 g K2HPO4 and 0.3315 g KH2PO4 in 40 mL distilled water and adding 5 mL of a 0.017% solution of dianisidine in methanol and 5 mL of 0.006% hydrogen peroxide in distilled water). The change in absorbance with time was then measured at 460 nm. 1 unit of MPO was defined as that which degraded 1 micromole of hydrogen peroxide at 25°C and was then calculated per gram of tissue.
Data analysis
All data are presented as mean values (SD) of the mean. Statistical analysis was determined by one-way analysis of variance (ANOVA) with post-hoc Tukey test analysis. Differences were considered significant at p-values less than 0.05.
Muscle wet-to-dry (W/D) ratios. Mean (SD)
Results
Functional assessment of gastrocnemius muscle
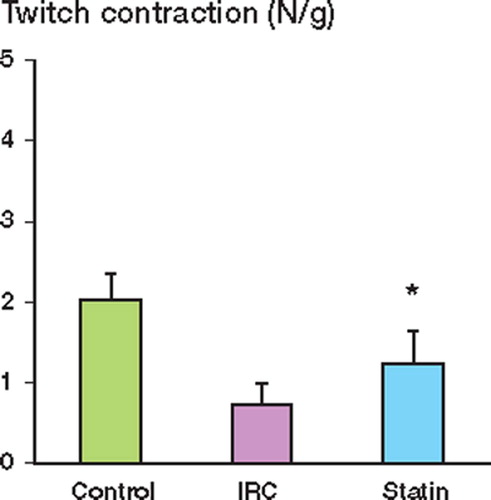
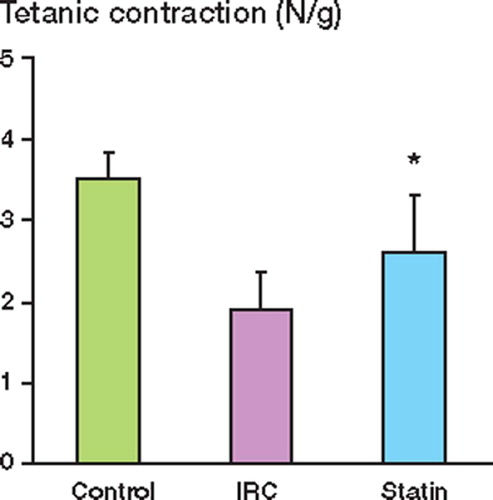
Muscle contractile function was measured after 12 h of reperfusion and expressed as the peak tension achieved for each muscle for both twitch and tetanic contractions. Ischemia reperfusion injury (IRI) impaired muscle twitch contraction as compared to the control group. Pretreatment with pravastatin preserved twitch contraction after IRI (). Muscle tetanic contraction was also significantly reduced by IRI relative to the control, and again, this reduction was attenuated in the pravastatintreated group ().
Tissue edema in gastrocnemius muscle
To assess tissue edema, tissue wet/dry (W/D) ratios were measured. The muscle W/D ratios in the pravastatin-treated group were significantly lower than those in the saline-treated group (Table).
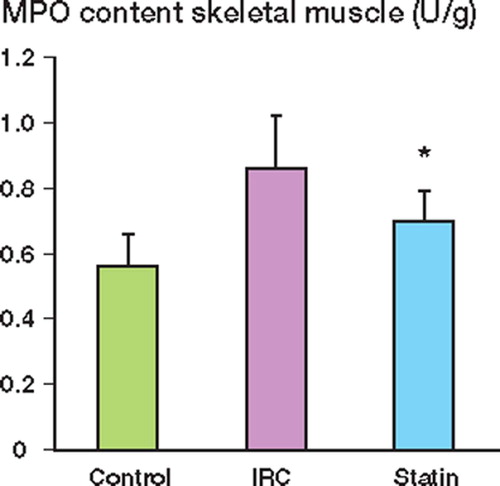
Effect of IRI on muscle neutrophil infiltration
Myeloperoxidase (MPO) activity was used as a marker of neutrophil infiltration in skeletal muscle. Ischemia-reperfusion injury resulted in a significant increase in MPO activity as compared to the control. This increase was attenuated with pravastatin pretreatment ().
Discussion
Ischemia reperfusion injury is a commonly encountered problem across many surgical disciplines, and may lead to muscle dysfunction and necrosis. Advances in molecular biology are increasing our understanding of tourniquet-induced ischemia reperfusion injury at the cellular level, and pharmacological manipulation of the inflammatory cascade may now be possible. Restoration of blood flow to a limb following tourniquet deflation initiates a cascade of cellular and biochemical events that result in muscle edema, necrosis and impaired muscle function (Kearns et al. Citation2001). Although the pathophysiology of ischemia reperfusion injury is not fully understood, an aberrantly activated immune response characterized by neutrophilmediated injury is felt to play a central role in this process. The primary target of the reperfusion injury is the microcirculation, where the leukocyte-endothelium interaction results in transendothelial migration and tissue injury from the release of reactive oxygen species and elastases (Welbourn et al. Citation1991). In addition, ischemia reperfusion directly causes dysregulation of endothelial function via accelerated endothelial cell apoptosis. This may contribute to the increased microvascular permeability and impairment in barrier function (Kohli et al. Citation1999).
Although skeletal muscle has a relatively high tolerance to ischemia, skeletal muscle dysfunction and infarction are well-recognized complications of the reperfusion injury. Our study demonstrates the beneficial effect of pravastatin pretreatment in skeletal muscle ischemia reperfusion injury. The results of the present study show that pretreatment with pravastatin reduces the tissue oxidative damage and edema associated with skeletal muscle reperfusion injury. Skeletal muscle injury, measured by edema, leucosequestration and electrical properties, were significantly lower with pravastatin pretreatment than in the untreated group. Although previous work has demonstrated the anti-inflammatory properties of pravastatin in renal and cardiac models of ischemia reperfusion injury, its effect on skeletal muscle ischemia reperfusion has not been studied previously (Lefer et al. Citation1999, Joyce et al. Citation2001).
While statins play a well-understood role in the treatment of hypercholesterolemia, their anti-inflammatory properties are less well defined. One potential mechanism of action is their ability to upregulate endothelial nitric oxide synthase. Endo-thelium-derived NO is a potent vasodilator that regulates mean arterial blood pressure, and it has also been shown to reduce leukocyte and platelet activation and aggregation (Kubes et al. Citation1991, Gaboury et al. Citation1993). Previous work has demonstrated that pretreatment with HMG CoA reductase inhibitors can reduce the extent of cerebral infarction and also attenuate renal injury in an experimental model of ischemia reperfusion injury by the upregulation of constitutive endothelial nitric oxide synthase (ecNOS) (Endres et al. Citation1998, Joyce et al. Citation2001).However, the involvement of nitric oxide in skeletal muscle ischemia reperfusion injury remains controversial, and has been shown to be both beneficial and deleterious in this setting. It has been reported that administration of an exogenous NO donor can protect against endothelial dysfunction during ischemia reperfusion injury (Hallstrom et al. Citation2002). The exogenous NO exerts a negative feedback on further production of NO by eNOS, and thus limits excessive O2 formation caused by eNOS derangementin ischemia reperfusion injury. However, Ozaki et al. have reported that overexpression of eNOS appears to reduce skeletal muscle ischemia reperfusion injury by maintaining vascular integrity, and Prorock et al. have shown that the protective effects of estrogen in skeletal muscle ischemia reperfusion are significantly reduced in eNOS-/- mice, suggesting that the underlying mechanism of estrogen vasoprotection is the induction of eNOS activity (Ozaki et al. Citation2002, Prorock et al. Citation2003). Another potential mechanism by which statins may exert their beneficial effects is through their direct antioxidant and anti-inflammatory properties. It has been shown recently that pretreatment with statins inhibits leukocyte-endo-thelial interaction and prevents neuronal death in the rat retina following ischemia reperfusion injury (Honjo et al. Citation2002). Statins have also been shown to downregulate the expression of NF-κB and AP-1 at the transcriptional level, both of which are known to have central roles in ischemia reperfusion injury (Dechend et al. Citation2002). Further work is required to elucidate the possible mechanisms by which statin pretreatment attenuates skeletal muscle ischemia reperfusion injury.
In conclusion, our study demonstrates that pre-treatment with pravastatin ameliorates tourniquet-induced skeletal muscle ischemia reperfusion injury. Although we should be cautious in extrapolating results obtained from animal experiments, the present study implies that pretreatment with statins may be a possible candidate for therapeutic manipulation of skeletal muscle reperfusion injury in the clinical setting. This may in turn permit longer and safer tourniquet times, and possibly limit complications such as ischemic contractures and compartment syndrome.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
- Barry M C, Kelly C, Burke P, Sheehan S, Redmond H P, Bouchier-Hayes D. Immunological and physiological responses to aortic surgery: effect of reperfusion on neutrophil and monocyte activation and pulmonary function. Br J Surg 1997; 84: 513–9
- Dechend R, Muller D, Park J K, Fiebeler A, Haller H, Luft F C. Statins and angiotensin II-induced vascular injury. Nephrol Dial Transplant 2002; 17: 349–53
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Mos-kowitz M, Liao J K. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA 1998; 95: 8880–5
- Gaboury J, Woodman R C, Granger D N, Reinhardt P, Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol Heart Circ Physiol 1993; 265: H862–H867
- Goldstein J L, Brown M S. Regulation of the mevalonate pathway. Nature 1990; 343: 425–30
- Grace P A. Ischaemia-reperfusion injury. Br J Surg 1994; 81: 637–47
- Hallstrom S, Gasser H, Neumayer C, Fugl A, Nanobashvili J, Jakubowski A, Huk I, Schlag G, Malinski T. S-Nitroso human serum albumin treatment reduces ischaemia/reper-fusion injury in skeletal muslce via nitric oxide release. Circulation 2002; 105: 3032–8
- Honjo M, Tanihara H, Nishijima K, Kiryu J, Honda Y, Yue B Y, Sawamura T. Statin inhibits leukocyte-endothelial interaction and prevents neuronal death induced by isch-aemia-reperfusion injury in the rat retina. Arch Ophthalmol 2002; 120: 1707–13
- Joyce M, Kelly C, Winter D C, Chen G, Leahy A, Bouchier-Hayes D. Pravastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, attenuates renal injury in an experimental model of ischaemia reperfusion. J Surg Res 2001; 101: 79–84
- Kanno T, Abe K, Yabuki M, Akiyama J, Yasuda T, Horton A A. Selective inhibition of formyl-methionyl-leucyl-phe-nylalanine (fMLF)-dependent superoxide generation in neutrophils by pravastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. Bio-chem Pharmacol 1999; 58: 1975–80
- Kearns S R, Monoley D, Murray P, Kelly C, Daly A F. Oral vitamin C attenuates acute ischaemia-reperfusion injury in skeletal muscle. J Bone Joint Surg (Br) 2001; 83: 1202–6
- Kohli V, Selzner M, Madden J F, Bentley R C, Clavien P A. Endothelial cell and hepatocyte deaths by apoptosis after ischaemia/reperfusion injury in the rat liver. Transplantation 1999; 67: 1099–105
- Kubes P, Suzuki M, Granger D N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA 1991; 88: 4651–5
- Lefer A M, Campbell B, Shin Y K, Scalia R, Hayward R, Lefer D J. Simvastatin preserves the ischaemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation 1999; 100: 178–84
- Ozaki M, Kawashime S, Hirase T, Yamashita T, Namiki M, Inoue N, Hirata K, Yokoyama M. Overexpression of endothelial nitric oxide synthase in endothelial cells is protective against ischaemia-reperfusion injury in mouse skeletal muslce. Am J Pathol 2002; 160: 1335–44
- Prorock A, Hafezi-Moghadam A, Laubach V, Liao J, Ley K. Vascular protection by estrogen in ischaemia-reperfusion injury requires endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 2003; 284: H133–H140
- Pruefer D, Makowski J, Schnell M, Buerke U, Dahm M, Oelert H, Sibelius U, Grandel U, Grimminger F, Seeger W, Meyer J, Darius H, Buerke M. Simvastatin inhibits inflammatory properties of Staphylococcus aureus toxin. Circulation 2002; 106: 2104–10
- Wakai A, Winter D C, Street J T, O’Sullivan R G, Wang J H, Redmond H P. Inosine attenuates tourniquet-induced skeletal muscle reperfusion injury. J Surg Res 2001a; 99: 311–5
- Wakai A, Wang J H, Winter D C, Street J T, O’Sullivan R G, Redmond H P. Tourniquet-induced systemic inflammatory response in extremity surgery. J. Trauma 2001b; 51: 922–6
- Welbourn C R, Goldman G, Paterson I S. Pathophysiology of ischaemia reperfusion injury: central role of the neutro-phil. Br J Surg 1991; 78: 651–5